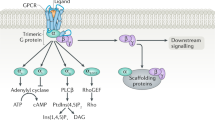
Abstract
Cyclic nucleotide phosphodiesterases (PDEs) modulate the neurohormonal regulation of cardiac function by degrading cAMP and cGMP. In cardiomyocytes, multiple PDE isozymes with different enzymatic properties and subcellular localization regulate local pools of cyclic nucleotides and specific functions. This organization is heavily perturbed during cardiac hypertrophy and heart failure (HF), which can contribute to disease progression. Clinically, PDE inhibition has been considered a promising approach to compensate for the catecholamine desensitization that accompanies HF. Although PDE3 inhibitors, such as milrinone or enoximone, have been used clinically to improve systolic function and alleviate the symptoms of acute HF, their chronic use has proved to be detrimental. Other PDEs, such as PDE1, PDE2, PDE4, PDE5, PDE9 and PDE10, have emerged as new potential targets to treat HF, each having a unique role in local cyclic nucleotide signalling pathways. In this Review, we describe cAMP and cGMP signalling in cardiomyocytes and present the various PDE families expressed in the heart as well as their modifications in pathological cardiac hypertrophy and HF. We also appraise the evidence from preclinical models as well as clinical data pointing to the use of inhibitors or activators of specific PDEs that could have therapeutic potential in HF.
Key points
-
Cyclic nucleotide phosphodiesterases (PDEs) hydrolyse cAMP and cGMP to modulate the neurohormonal regulation of cardiac function.
-
Multiple PDE isozymes with various enzymatic properties and subcellular localizations finely tune local pools of cyclic nucleotides and control specific cardiac functions.
-
Changes in PDE expression, activity and subcellular localization during cardiac hypertrophy and heart failure can contribute to disease progression.
-
Despite limited success in the clinical arena, evidence in the literature continues to point to inhibitors or activators of specific PDEs as promising therapies for heart failure.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
McDonagh, T. A. et al. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 24, 4–131 (2022).
Hartupee, J. & Mann, D. L. Neurohormonal activation in heart failure with reduced ejection fraction. Nat. Rev. Cardiol. 14, 30–38 (2017).
Cohn, J. N. et al. Plasma norepinephrine as a guide to prognosis in patients with chronic congestive heart failure. N. Engl. J. Med. 311, 819–823 (1984).
El-Armouche, A. & Eschenhagen, T. β-Adrenergic stimulation and myocardial function in the failing heart. Heart Fail. Rev. 14, 225–241 (2009).
McMurray, J. J. et al. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N. Engl. J. Med. 371, 993–1004 (2014).
Armstrong, P. W. et al. Vericiguat in patients with heart failure and reduced ejection fraction. N. Engl. J. Med. 382, 1883–1893 (2020).
Petraina, A. et al. Cyclic GMP modulating drugs in cardiovascular diseases: Mechanism-based network pharmacology. Cardiovasc. Res. https://doi.org/10.1093/cvr/cvab240 (2021).
Anton, S. E. et al. Receptor-associated independent cAMP nanodomains mediate spatiotemporal specificity of GPCR signaling. Cell 185, 1130–1142 (2022).
Bock, A. et al. Optical mapping of cAMP signaling at the nanometer scale. Cell 182, 1519–1530 (2020).
Kokkonen, K. & Kass, D. A. Nanodomain regulation of cardiac cyclic nucleotide signaling by phosphodiesterases. Annu. Rev. Pharmacol. Toxicol. 57, 455–479 (2017).
Lohse, M. J., Engelhardt, S. & Eschenhagen, T. What is the role of β-adrenergic signaling in heart failure? Circ. Res. 93, 896–906 (2003).
Nikolaev, V. O. et al. β2-Adrenergic receptor redistribution in heart failure changes cAMP compartmentation. Science 327, 1653–1657 (2010).
Katz, S. D. et al. Vascular endothelial dysfunction and mortality risk in patients with chronic heart failure. Circulation 111, 310–314 (2005).
Dickey, D. M., Dries, D. L., Margulies, K. B. & Potter, L. R. Guanylyl cyclase (GC)-A and GC-B activities in ventricles and cardiomyocytes from failed and non-failed human hearts: GC-A is inactive in the failed cardiomyocyte. J. Mol. Cell Cardiol. 52, 727–732 (2012).
Packer, M. et al. Effect of oral milrinone on mortality in severe chronic heart failure. The PROMISE Study Research Group. N. Engl. J. Med. 325, 1468–1475 (1991).
Redfield, M. M. et al. Effect of phosphodiesterase-5 inhibition on exercise capacity and clinical status in heart failure with preserved ejection fraction: a randomized clinical trial. JAMA 309, 1268–1277 (2013).
Preedy, M. E. J. Cardiac cyclic nucleotide phosphodiesterases: roles and therapeutic potential in heart failure. Cardiovasc. Drugs Ther. 34, 401–417 (2020).
Chen, S. & Yan, C. An update of cyclic nucleotide phosphodiesterase as a target for cardiac diseases. Expert Opin. Drug Discov. 16, 183–196 (2021).
Boivin, B. et al. Functional β-adrenergic receptor signalling on nuclear membranes in adult rat and mouse ventricular cardiomyocytes. Cardiovasc. Res. 71, 69–78 (2006).
Nash, C. A., Wei, W., Irannejad, R. & Smrcka, A. V. Golgi localized β1-adrenergic receptors stimulate Golgi PI4P hydrolysis by PLCe to regulate cardiac hypertrophy. Elife 8, e48167 (2019).
Wang, Y. et al. Intracellular β1-adrenergic receptors and organic cation transporter 3 mediate phospholamban phosphorylation to enhance cardiac contractility. Circ. Res. 128, 246–261 (2021).
Guellich, A., Mehel, H. & Fischmeister, R. Cyclic AMP synthesis and hydrolysis in the normal and failing heart. Pflügers Arch. 466, 1163–1175 (2014).
Xiang, Y., Rybin, V. O., Steinberg, S. F. & Kobilka, B. Caveolar localization dictates physiologic signaling of β2-adrenoceptors in neonatal cardiac myocytes. J. Biol. Chem. 277, 34280–34286 (2002).
Ostrom, R. S. et al. Receptor number and caveolar co-localization determine receptor coupling efficiency to adenylyl cyclase. J. Biol. Chem. 276, 42063–42069 (2001).
Rybin, V. O., Xu, X., Lisanti, M. P. & Steinberg, S. F. Differential targeting of β-adrenergic receptor subtypes and adenylyl cyclase to cardiomyocyte caveolae: a mechanism to functionally regulate the cAMP signaling pathway. J. Biol. Chem. 275, 41447–41457 (2000).
Timofeyev, V. et al. Adenylyl cyclase subtype-specific compartmentalization: differential regulation of L-type Ca2+ current in ventricular myocytes. Circ. Res. 112, 1567–1576 (2013).
Wang, Z. et al. A cardiac mitochondrial cAMP signaling pathway regulates calcium accumulation, permeability transition and cell death. Cell Death Dis. 7, e2198 (2016).
Di Benedetto, G., Scalzotto, E., Mongillo, M. & Pozzan, T. Mitochondrial Ca2+ uptake induces cyclic AMP generation in the matrix and modulates organelle ATP levels. Cell Metab. 17, 965–975 (2013).
Zhang, Y. et al. Cardiomyocyte PKA ablation enhances basal contractility while eliminates cardiac β-adrenergic response without adverse effects on the heart. Circ. Res. 12, 1760–1777 (2019).
Liu, G. et al. Mechanism of adrenergic CaV1.2 stimulation revealed by proximity proteomics. Nature 577, 695–700 (2020).
Leroy, J. & Fischmeister, R. β-Adrenergic regulation of the L-type Ca2+ current: the missing link eventually discovered. Med. Sci. 36, 569–572 (2020).
Bers, D. M. Cardiac excitation-contraction coupling. Nature 415, 198–205 (2002).
Hayes, J. S., Brunton, L. L., Brown, J. H., Reese, J. B. & Mayer, S. E. Hormonally specific expression of cardiac protein kinase activity. Proc. Natl Acad. Sci. USA 76, 1570–1574 (1979).
Lehmann, L. H. et al. A proteolytic fragment of histone deacetylase 4 protects the heart from failure by regulating the hexosamine biosynthetic pathway. Nat. Med. 24, 62–72 (2018).
Chang, C. W. et al. Acute β-adrenergic activation triggers nuclear import of histone deacetylase 5 and delays Gq-induced transcriptional activation. J. Biol. Chem. 288, 192–204 (2013).
Ha, C. H. et al. PKA phosphorylates histone deacetylase 5 and prevents its nuclear export, leading to the inhibition of gene transcription and cardiomyocyte hypertrophy. Proc. Natl Acad. Sci. USA 107, 15467–15472 (2010).
Backs, J. et al. Selective repression of MEF2 activity by PKA-dependent proteolysis of HDAC4. J. Cell Biol. 195, 403–415 (2011).
Antos, C. L. et al. Dilated cardiomyopathy and sudden death resulting from constitutive activation of protein kinase A. Circ. Res. 89, 997–1004 (2001).
Zhang, X. et al. Cardiotoxic and cardioprotective features of chronic β-adrenergic signaling. Circ. Res. 112, 498–509 (2013).
Tomita, H. et al. Inducible cAMP early repressor (ICER) is a negative-feedback regulator of cardiac hypertrophy and an important mediator of cardiac myocyte apoptosis in response to β-adrenergic receptor stimulation. Circ. Res. 93, 12–22 (2003).
Bedioune, I. et al. PDE4 and mAKAPβ are nodal organizers of β2-AR nuclear PKA signaling in cardiac myocytes. Cardiovasc. Res. 114, 1499–1511 (2018).
DiFrancesco, D. A brief history of pacemaking. Front. Physiol. 10, 1599 (2019).
Brand, T. The popeye domain containing genes and their function as cAMP effector proteins in striated muscle. J. Cardiovasc. Dev. Dis. 5, 18 (2018).
Robichaux, W. G. III & Cheng, X. Intracellular cAMP sensor EPAC: physiology, pathophysiology, and therapeutics development. Physiol. Rev. 98, 919–1053 (2018).
Fujita, T., Umemura, M., Yokoyama, U., Okumura, S. & Ishikawa, Y. The role of Epac in the heart. Cell. Mol. Life Sci. 74, 591–606 (2017).
Lezoualc’h, F., Fazal, L., Laudette, M. & Conte, C. Cyclic AMP sensor EPAC proteins and their role in cardiovascular function and disease. Circ. Res. 118, 881–897 (2016).
Morel, E. et al. cAMP-binding protein Epac induces cardiomyocyte hypertrophy. Circ. Res. 97, 1296–1304 (2005).
Métrich, M. et al. Epac mediates β-adrenergic receptor-induced cardiomyocyte hypertrophy. Circ. Res. 102, 959–965 (2008).
Pereira, L. et al. Epac2 mediates cardiac β1-adrenergic dependent SR Ca2+ leak and arrhythmia. Circulation 127, 913–922 (2013).
Zhang, L. et al. Phospholipase Cε hydrolyzes perinuclear phosphatidylinositol 4-phosphate to regulate cardiac hypertrophy. Cell 153, 216–227 (2013).
Zhang, L., Malik, S., Kelley, G. G., Kapiloff, M. S. & Smrcka, A. V. Phospholipase C epsilon scaffolds to muscle-specific A kinase anchoring protein (mAKAPβ) and integrates multiple hypertrophic stimuli in cardiac myocytes. J. Biol. Chem. 286, 23012–23021 (2011).
Dodge-Kafka, K. L. et al. The protein kinase A anchoring protein mAKAP co-ordinates two integrated cAMP effector pathways. Nature 437, 574–578 (2005).
Fazal, L. et al. The multifunctional mitochondrial Epac1 controls myocardial cell death. Circ. Res. 120, 645–657 (2017).
Feil, R., Lehners, M., Stehle, D. & Feil, S. Visualising and understanding cGMP signals in the cardiovascular system. Br. J. Pharmacol. 179, 2394–2412 (2022).
Manfra, O. et al. CNP regulates cardiac contractility and increases cGMP near both SERCA and TnI - difference from BNP visualized by targeted cGMP biosensors. Cardiovasc. Res. 118, 1506–1519 (2022).
Subramanian, H. et al. Distinct submembrane localisation compartmentalises cardiac NPR1 and NPR2 signalling to cGMP. Nat. Commun. 9, 2446 (2018).
Moltzau, L. R. et al. Different compartmentation of responses to brain natriuretic peptide and C-type natriuretic peptide in failing rat ventricle. J. Pharmacol. Exp. Ther. 350, 681–690 (2014).
Méry, P.-F., Lohmann, S. M., Walter, U. & Fischmeister, R. Ca2+ current is regulated by cyclic GMP-dependent protein kinase in mammalian cardiac myocytes. Proc. Natl Acad. Sci. USA 88, 1197–1201 (1991).
Raeymaekers, L., Hofmann, F. & Casteels, R. Cyclic GMP-dependent protein kinase phosphorylates phospholamban in isolated sarcoplasmic reticulum from cardiac and smooth muscle. Biochem. J. 252, 269–273 (1988).
Lee, D. I. et al. PDE5A suppression of acute β-adrenergic activation requires modulation of myocyte beta-3 signaling coupled to PKG-mediated troponin I phosphorylation. Basic Res. Cardiol. 105, 337–347 (2010).
Thoonen, R. et al. Molecular screen identifies cardiac myosin-binding protein-C as a protein kinase G-Ia substrate. Circ. Heart Fail. 8, 1115–1122 (2015).
Kruger, M. et al. Protein kinase G modulates human myocardial passive stiffness by phosphorylation of the titin springs. Circ. Res. 104, 87–94 (2009).
Fiedler, B. et al. Inhibition of calcineurin-NFAT hypertrophy signaling by cGMP-dependent protein kinase type I in cardiac myocytes. Proc. Natl Acad. Sci. USA 99, 11363–11368 (2002).
Takimoto, E. et al. Regulator of G protein signaling 2 mediates cardiac compensation to pressure overload and antihypertrophic effects of PDE5 inhibition in mice. J. Clin. Invest. 119, 408–420 (2009).
Tokudome, T. et al. Regulator of G-protein signaling subtype 4 mediates antihypertrophic effect of locally secreted natriuretic peptides in the heart. Circulation 117, 2329–2339 (2008).
Kinoshita, H. et al. Inhibition of TRPC6 channel activity contributes to the antihypertrophic effects of natriuretic peptides-guanylyl cyclase-A signaling in the heart. Circ. Res. 106, 1849–1860 (2010).
Ranek, M. J. et al. PKG1-modified TSC2 regulates mTORC1 activity to counter adverse cardiac stress. Nature 566, 264–269 (2019).
Ranek, M. J., Terpstra, E. J., Li, J., Kass, D. A. & Wang, X. Protein kinase G positively regulates proteasome-mediated degradation of misfolded proteins. Circulation 128, 365–376 (2013).
Ranek, M. J. et al. CHIP phosphorylation by protein kinase G enhances protein quality control and attenuates cardiac ischemic injury. Nat. Commun. 11, 5237 (2020).
Frantz, S. et al. Stress-dependent dilated cardiomyopathy in mice with cardiomyocyte-restricted inactivation of cyclic GMP-dependent protein kinase I. Eur. Heart J. 34, 1233–1244 (2013).
Lukowski, R. et al. Cardiac hypertrophy is not amplified by deletion of cGMP-dependent protein kinase I in cardiomyocytes. Proc. Natl Acad. Sci. USA 107, 5646–5651 (2010).
Patrucco, E. et al. Roles of cGMP-dependent protein kinase I (cGKI) and PDE5 in the regulation of Ang II-induced cardiac hypertrophy and fibrosis. Proc. Natl Acad. Sci. USA 111, 12925–12929 (2014).
Bolger, G. B. The PDE-opathies: diverse phenotypes produced by a functionally related multigene family. Trends Genet. 37, 669–681 (2021).
Gardner, C., Robas, N., Cawkill, D. & Fidock, M. Cloning and characterization of the human and mouse PDE7B, a novel cAMP-specific cyclic nucleotide phosphodiesterase. Biochem. Biophys. Res. Commun. 272, 186–192 (2000).
Han, P., Zhu, X. Y. & Michaeli, T. Alternative splicing of the high affinity cAMP-specific phosphodiesterase (PDE7A) mRNA in human skeletal muscle and heart. J. Biol. Chem. 272, 16152–16157 (1997).
Michaeli, T. et al. Isolation and characterization of a previously undetected human cAMP phosphodiesterase by complementation of cAMP phosphodiesterase-deficient Saccharomyces cerevisiae. J. Biol. Chem. 268, 12925–12932 (1993).
Loughney, K., Taylor, J. & Florio, V. A. 3′,5′-Cyclic nucleotide phosphodiesterase 11A: localization in human tissues. Int. J. Impot. Res. 17, 320–325 (2005).
Aravind, L. & Ponting, C. P. The GAF domain: an evolutionary link between diverse phototransducing proteins. Trends Biochem. Sci. 22, 458–459 (1997).
Martinez, S. E. et al. The two GAF domains in phosphodiesterase 2A have distinct roles in dimerization and in cGMP binding. Proc. Natl Acad. Sci. USA 99, 13260–13265 (2002).
Turko, I. V., Francis, S. H. & Corbin, J. D. Binding of cGMP to both allosteric sites of cGMP-binding cGMP-specific phosphodiesterase (PDE5) is required for its phosphorylation. Biochem. J. 329, 505–510 (1998).
Soderling, S. H., Bayuga, S. J. & Beavo, J. A. Isolation and characterization of a dual-substrate phosphodiesterase gene family: PDE10A. Proc. Natl Acad. Sci. USA 96, 7071–7076 (1999).
Omori, K. & Kotera, J. Overview of PDEs and their regulation. Circ. Res. 100, 309–327 (2007).
Baillie, G. S., Tejeda, G. S. & Kelly, M. P. Therapeutic targeting of 3′,5′-cyclic nucleotide phosphodiesterases: inhibition and beyond. Nat. Rev. Drug Discov. 18, 770–796 (2019).
Keravis, T. & Lugnier, C. Cyclic nucleotide phosphodiesterase (PDE) isozymes as targets of the intracellular signalling network: benefits of PDE inhibitors in various diseases and perspectives for future therapeutic developments. Br. J. Pharmacol. 165, 1288–1305 (2012).
Francis, S. H., Blount, M. A. & Corbin, J. D. Mammalian cyclic nucleotide phosphodiesterases: molecular mechanisms and physiological functions. Physiol. Rev. 91, 651–690 (2011).
Conti, M. & Beavo, J. A. Biochemistry and physiology of cyclic nucleotide phosphodiesterases: essential components in cyclic nucleotide signaling. Ann. Rev. Biochem. 76, 481–511 (2007).
Shi, Q. et al. Heterologous desensitization of cardiac β-adrenergic signal via hormone-induced bAR/arrestin/PDE4 complexes. Cardiovasc. Res. 113, 656–670 (2017).
Mika, D., Richter, W., Westenbroek, R. E., Catterall, W. A. & Conti, M. PDE4B mediates local feedback regulation of b1-adrenergic cAMP signaling in a sarcolemmal compartment of cardiac myocytes. J. Cell Sci. 127, 1033–1042 (2014).
Berthouze-Duquesnes, M. et al. Specific interactions between Epac1, β-arrestin2 and PDE4D5 regulate β-adrenergic receptor subtypes differential effects on cardiac hypertrophic signaling. Cell. Signal. 25, 970–980 (2013).
De Arcangelis, V., Liu, R., Soto, D. & Xiang, Y. Differential association of phosphodiesterase 4D isoforms with β2-adrenoceptor in cardiac myocytes. J. Biol. Chem. 284, 33824–33832 (2009).
Richter, W. et al. Signaling from β1-and β2-adrenergic receptors is defined by differential interactions with PDE4. EMBO J. 27, 384–393 (2008).
Rochais, F. et al. A specific pattern of phosphodiesterases controls the cAMP signals generated by different Gs-coupled receptors in adult rat ventricular myocytes. Circ. Res. 98, 1081–1088 (2006).
Baillie, G. S. et al. β-Arrestin-mediated PDE4 cAMP phosphodiesterase recruitment regulates β-adrenoceptor switching from Gs to Gi. Proc. Natl Acad. Sci. USA 100, 941–945 (2003).
Perry, S. J. et al. Targeting of cyclic AMP degradation to b2-adrenergic receptors by β-arrestins. Science 298, 834–836 (2002).
Liu, S. et al. Phosphodiesterases coordinate cAMP propagation induced by two stimulatory G protein-coupled receptors in hearts. Proc. Natl Acad. Sci. USA 109, 6578–6583 (2012).
Zhang, Y., Knight, W., Chen, S., Mohan, A. & Yan, C. Multiprotein complex with TRPC (transient receptor potential-canonical) channel, PDE1C (phosphodiesterase 1C), and A2R (adenosine A2 receptor) plays a critical role in regulating cardiomyocyte cAMP and survival. Circulation 138, 1988–2002 (2018).
Lee, D. I. et al. Phosphodiesterase 9A controls nitric-oxide-independent cGMP and hypertrophic heart disease. Nature 519, 472–476 (2015).
Castro, L. R. V., Schittl, J. & Fischmeister, R. Feedback control through cGMP-dependent protein kinase contributes to differential regulation and compartmentation of cGMP in rat cardiac myocytes. Circ. Res. 107, 1232–1240 (2010).
Castro, L. R., Verde, I., Cooper, D. M. F. & Fischmeister, R. Cyclic guanosine monophosphate compartmentation in rat cardiac myocytes. Circulation 113, 2221–2228 (2006).
Leroy, J. et al. Phosphodiesterase 4B in the cardiac L-type Ca2+ channel complex regulates Ca2+ current and protects against ventricular arrhythmias. J. Clin. Invest. 121, 2651–2661 (2011).
Terrenoire, C., Houslay, M. D., Baillie, G. S. & Kass, R. S. The cardiac IKs potassium channel macromolecular complex includes the phosphodiesterase PDE4D3. J. Biol. Chem. 284, 9140–9146 (2009).
Bastug-Ozel, Z. et al. Heart failure leads to altered b2-adrenoceptor/cAMP dynamics in the sarcolemmal phospholemman/Na,K ATPase microdomain. Cardiovasc. Res. 115, 546–555 (2019).
Beca, S. et al. Phosphodiesterase type 3A regulates basal myocardial contractility through interacting with SERCA2a-signaling complexes in mouse heart. Circ. Res. 112, 289–297 (2013).
Beca, S. et al. Phosphodiesterase 4D regulates baseline sarcoplasmic reticulum Ca2+ release and cardiac contractility, independently of L-type Ca2+ current. Circ. Res. 109, 1024–1030 (2011).
Lehnart, S. E. et al. Phosphodiesterase 4D deficiency in the ryanodine receptor complex promotes heart failure and arrhythmias. Cell 123, 23–35 (2005).
Lugnier, C. et al. Characterization of cyclic nucleotide phosphodiesterase isoforms associated to isolated cardiac nuclei. Biochim. Biophys. Acta 1472, 431–446 (1999).
Barbagallo, F. et al. Genetically encoded biosensors reveal PKA hyperphosphorylation on the myofilaments in rabbit heart failure. Circ. Res. 119, 931–943 (2016).
Verde, I. et al. Myomegalin is a novel protein of the Golgi/centrosome that interacts with a cyclic nucleotide phosphodiesterase. J. Biol. Chem. 276, 11189–11198 (2001).
Dodge, K. L. et al. mAKAP assembles a protein kinase A/PDE4 phosphodiesterase cAMP signaling module. EMBO J. 20, 1921–1930 (2001).
Liu, D. et al. PDE2 regulates membrane potential, respiration and permeability transition of rodent subsarcolemmal cardiac mitochondria. Mitochondrion 47, 64–75 (2019).
Monterisi, S. et al. PDE2A2 regulates mitochondria morphology and apoptotic cell death via local modulation of cAMP/PKA signalling. Elife 6, e21374 (2017).
Takimoto, E. et al. cGMP catabolism by phosphodiesterase 5A regulates cardiac adrenergic stimulation by NOS3-dependent mechanism. Circ. Res. 96, 100–109 (2005).
Fischmeister, R. et al. Compartmentation of cyclic nucleotide signaling in the heart: the role of cyclic nucleotide phosphodiesterases. Circ. Res. 99, 816–828 (2006).
Zaccolo, M., Zerio, A. & Lobo, M. J. Subcellular organization of the cAMP signaling pathway. Pharmacol. Rev. 73, 278–309 (2021).
Cuello, F. & Nikolaev, V. O. Cardiac cGMP signaling in health and disease: location, location, location. J. Cardiovasc. Pharmacol. 75, 399–409 (2020).
Ghigo, A. & Mika, D. cAMP/PKA signaling compartmentalization in cardiomyocytes: lessons from FRET-based biosensors. J. Mol. Cell Cardiol. 131, 112–121 (2019).
Chen, S., Knight, W. E. & Yan, C. Roles of PDE1 in pathological cardiac remodeling and dysfunction. J. Cardiovasc. Dev. Dis. 5, 22 (2018).
Hambleton, R. et al. Isoforms of cyclic nucleotide phosphodiesterase PDE3 and their contribution to cAMP-hydrolytic activity in subcellular fractions of human myocardium. J. Biol. Chem. 280, 39168–39174 (2005).
Lugnier, C., Gauthier, C., Lebec, A. & Soustre, H. Cyclic nucleotide phosphodiesterases from frog atrial fibers: isolation and drug sensitivities. Am. J. Physiol. 262, H654–H660 (1992).
Vandeput, F. et al. Cyclic nucleotide phosphodiesterase PDE1C1in human cardiac myocytes. J. Biol. Chem. 282, 32749–32757 (2007).
Bode, D. C., Kanter, J. R. & Brunton, L. L. Cellular distribution of phosphodiesterase isoforms in rat cardiac tissue. Circ. Res. 68, 1070–1079 (1991).
Miller, C. L. et al. Cyclic nucleotide phosphodiesterase 1A: a key regulator of cardiac fibroblast activation and extracellular matrix remodeling in the heart. Basic Res. Cardiol. 106, 1023–1039 (2011).
Knight, W. et al. PDE1C deficiency antagonizes pathological cardiac remodeling and dysfunction. Proc. Natl Acad. Sci. USA 113, E7116–E7125 (2016).
Miller, C. L. et al. Role of Ca2+/calmodulin-stimulated cyclic nucleotide phosphodiesterase 1 in mediating cardiomyocyte hypertrophy. Circ. Res. 105, 956–964 (2009).
Wu, M. P. et al. Vinpocetine attenuates pathological cardiac remodeling by inhibiting cardiac hypertrophy and fibrosis. Cardiovasc. Drugs Ther. 31, 157–166 (2017).
Zhang, H. et al. PDE1 inhibition facilitates proteasomal degradation of misfolded proteins and protects against cardiac proteinopathy. Sci. Adv. 5, eaaw5870 (2019).
Hashimoto, T. et al. Acute enhancement of cardiac function by phosphodiesterase type 1 inhibition - a translational study in the dog and rabbit. Circulation 138, 1974–1987 (2018).
Muller, G. K. et al. PDE1 inhibition modulates Cav1.2 channel to stimulate cardiomyocyte contraction. Circ. Res. 129, 872–886 (2021).
Gilotra, N. A. et al. Acute hemodynamic effects and tolerability of phosphodiesterase-1 inhibition with ITI-214 in human systolic heart failure. Circ. Heart Fail. 14, e008236 (2021).
Martins, T. J., Mumby, M. C. & Beavo, J. A. Purification and characterization of a cyclic GMP-stimulated cyclic nucleotide phosphodiesterase from bovine tissues. J. Biol. Chem. 257, 1973–1979 (1982).
Hartzell, H. C. & Fischmeister, R. Opposite effects of cyclic GMP and cyclic AMP on Ca2+ current in single heart cells. Nature 323, 273–275 (1986).
Dittrich, M. et al. Local response of L-type Ca2+ current to nitric oxide in frog ventricular myocytes. J. Physiol. 534, 109–121 (2001).
Vandecasteele, G., Verde, I., Rucker-Martin, C., Donzeau-Gouge, P. & Fischmeister, R. Cyclic GMP regulation of the L-type Ca2+ channel current in human atrial myocytes. J. Physiol. 533, 329–340 (2001).
Rosman, G. J. et al. Isolation and characterization of human cDNAs encoding a cGMP-stimulated 3′,5′-cyclic nucleotide phosphodiesterase. Gene 191, 89–95 (1997).
Yang, Q. et al. A novel cyclic GMP stimulated phosphodiesterase from rat brain. Biochem. Biophys. Res. Commun. 205, 1850–1858 (1994).
Sonnenburg, W. K., Mullaney, P. J. & Beavo, J. A. Molecular cloning of a cyclic GMP-stimulated cyclic nucleotide phosphodiesterase cDNA -identification and distribution of isozyme variants. J. Biol. Chem. 266, 17655–17661 (1991).
Pyne, N. J., Cooper, M. E. & Houslay, M. D. Identification and characterization of both the cytosolic and particulate forms of cyclic GMP-stimulated cyclic AMP phosphodiesterase from rat liver. Biochem. J. 234, 325–334 (1986).
Vettel, C. et al. PDE2-mediated cAMP hydrolysis accelerates cardiac fibroblast to myofibroblast conversion and is antagonized by exogenous activation of cGMP signaling pathways. Am. J. Physiol. Heart Circ. Physiol. 306, H1246–H1252 (2014).
Chen, W. et al. Endothelial actions of ANP enhance myocardial inflammatory infiltration in the early phase after acute infarction. Circ. Res. 119, 237–248 (2016).
Favot, L., Keravis, T. & Lugnier, C. Modulation of VEGF-induced endothelial cell cycle protein expression through cyclic AMP hydrolysis by PDE2 and PDE4. Thromb. Haemost. 92, 634–645 (2004).
Keravis, T., Komas, N. & Lugnier, C. Cyclic nucleotide hydrolysis in bovine aortic endothelial cells in culture: differential regulation in cobblestone and spindle phenotypes. J. Vasc. Res. 37, 235–249 (2000).
Mika, D. et al. Differential regulation of cardiac excitation-contraction coupling by cAMP phosphodiesterase subtypes. Cardiovasc. Res. 100, 336–346 (2013).
Aye, T. T. et al. Reorganized PKA-AKAP associations in the failing human heart. J. Mol. Cell Cardiol. 52, 511–518 (2012).
Mehel, H. et al. Phoshodiesterase-2 is upregulated in human failing hearts and blunts β-adrenergic responses in cardiomyocytes. J. Am. Coll. Cardiol. 62, 1596–1606 (2013).
Galindo-Tovar, A., Vargas, M. L. & Kaumann, A. J. Phosphodiesterase PDE2 activity, increased by isoprenaline, does not reduce β-adrenoceptor-mediated chronotropic and inotropic effects in rat heart. Naunyn Schmiedebergs Arch. Pharmacol. 391, 571–585 (2018).
Sprenger, J. U. et al. In vivo model with targeted cAMP biosensor reveals changes in receptor-microdomain communication in cardiac disease. Nat. Commun. 6, 6965 (2015).
Zoccarato, A. et al. Cardiac hypertrophy is inhibited by a local pool of cAMP regulated by phosphodiesterase 2. Circ. Res. 117, 707–719 (2015).
Baliga, R. S. et al. Phosphodiesterase 2 inhibition preferentially promotes NO/guanylyl cyclase/cGMP signaling to reverse the development of heart failure. Proc. Natl Acad. Sci. USA 115, E7428–E7437 (2018).
Liu, K. et al. Phosphodiesterase 2A as a therapeutic target to restore cardiac neurotransmission during sympathetic hyperactivity. JCI Insight 3, 98694 (2018).
Vettel, C. et al. Phosphodiesterase 2 protects against catecholamine-induced arrhythmias and preserves contractile function after myocardial infarction. Circ. Res. 120, 120–132 (2017).
Wagner, M. et al. Cellular mechanisms of the anti-arrhythmic effect of cardiac PDE2 overexpression. Int. J. Mol. Sci. 22, 4816 (2021).
Ahmad, F. et al. Regulation of sarcoplasmic reticulum Ca2+ ATPase 2 (SERCA2) activity by phosphodiesterase 3A (PDE3A) in human myocardium: phosphorylation-dependent interaction of PDE3A1 with SERCA2. J. Biol. Chem. 290, 6763–6776 (2015).
Wechsler, J. et al. Isoforms of cyclic nucleotide phosphodiesterase PDE3A in cardiac myocytes. J. Biol. Chem. 277, 38072–38078 (2002).
Sun, B. et al. Role of phosphodiesterase type 3A and 3B in regulating platelet and cardiac function using subtype-selective knockout mice. Cell. Signal. 19, 1765–1771 (2007).
Movsesian, M., Ahmad, F. & Hirsch, E. Functions of PDE3 isoforms in cardiac muscle. J. Cardiovasc. Dev. Dis. 5, 10 (2018).
Chung, Y. W. et al. Targeted disruption of PDE3B, but not PDE3A, protects murine heart from ischemia/reperfusion injury. Proc. Natl Acad. Sci. USA 112, E2253–E2262 (2015).
Leroy, J. et al. Spatiotemporal dynamics of β-adrenergic cAMP signals and L-type Ca2+ channel regulation in adult rat ventricular myocytes: role of phosphodiesterases. Circ. Res. 102, 1091–1100 (2008).
Verde, I., Vandecasteele, G., Lezoualc’h, F. & Fischmeister, R. Characterization of the cyclic nucleotide phosphodiesterase subtypes involved in the regulation of the L-type Ca2+ current in rat ventricular myocytes. Br. J. Pharmacol. 127, 65–74 (1999).
Ono, K. & Trautwein, W. Potentiation by cyclic GMP of β-adrenergic effect on Ca2+ current in guinea-pig ventricular cells. J. Physiol. 443, 387–404 (1991).
Fischmeister, R. & Hartzell, H. C. Regulation of calcium current by low-Km cyclic AMP phosphodiesterases in cardiac cells. Mol. Pharmacol. 38, 426–433 (1990).
Yano, M. et al. Effect of milrinone on left ventricular relaxation and Ca2+ uptake function of cardiac sarcoplasmic reticulum. Am. J. Physiol. Heart Circ. Physiol. 279, H1898–H1905 (2000).
Malecot, C., Bers, D. M. & Katzung, B. G. Biphasic contractions induced by milrinone at low temperature in ferret ventricular muscle: role of the sarcoplasmic reticulum and transmembrane calcium influx. Circ. Res. 59, 151–162 (1986).
Lugnier, C., Muller, B., Lebec, A., Beaudry, C. & Rousseau, E. Characterization of indolidan-sensitive and rolipram-sensitive cyclic nucleotide phosphodiesterases in canine and human cardiac microsomal fractions. J. Pharmacol. Exp. Ther. 265, 1142–1151 (1993).
Crambert, G., Fuzesi, M., Garty, H., Karlish, S. & Geering, K. Phospholemman (FXYD1) associates with Na,K-ATPase and regulates its transport properties. Proc. Natl Acad. Sci. USA 99, 11476–11481 (2002).
Movsesian, M. A., Smith, C. J., Krall, J., Bristow, M. R. & Manganiello, V. C. Sarcoplasmic reticulum-associated cyclic adenosine 5′-monophosphate phosphodiesterase activity in normal and failing human hearts. J. Clin. Invest. 88, 15–19 (1991).
von der Leyen, H. et al. Mechanism underlying the reduced positive inotropic effects of the phosphodiesterase III inhibitors pimobendan, adibendan and saterinone in failing as compared to nonfailing human cardiac muscle preparations. Naunyn Schmiedebergs Arch. Pharmacol. 344, 90–100 (1991).
Ding, B. et al. Functional role of phosphodiesterase 3 in cardiomyocyte apoptosis: implication in heart failure. Circulation 111, 2469–2476 (2005).
Mika, D. et al. Synergic PDE3 and PDE4 control intracellular cAMP and cardiac excitation-contraction coupling in a porcine model. J. Mol. Cell Cardiol. 133, 57–66 (2019).
Abi-Gerges, A. et al. Decreased expression and activity of cAMP phosphodiesterases in cardiac hypertrophy and its impact on β-adrenergic cAMP signals. Circ. Res. 105, 784–792 (2009).
Ding, B. et al. A positive feedback loop of phosphodiesterase 3 (PDE3) and inducible cAMP early repressor (ICER) leads to cardiomyocyte apoptosis. Proc. Natl Acad. Sci. USA 102, 14771–14776 (2005).
Smith, C. J. et al. Downregulation of right ventricular phosphodiesterase PDE-3A mRNA and protein before the development of canine heart failure. Cell Biochem. Biophys. 29, 67–88 (1998).
Smith, C. J. et al. Development of decompensated dilated cardiomyopathy is associated with decreased gene expression and activity of the milrinone-sensitive cAMP phosphodiesterase PDE3A. Circulation 96, 3116–3123 (1997).
Hanna, R. et al. Cardiac phosphodiesterases are differentially increased in diabetic cardiomyopathy. Life Sci. 283, 119857 (2021).
Polidovitch, N. et al. Phosphodiesterase type 3A (PDE3A), but not type 3B (PDE3B), contributes to the adverse cardiac remodeling induced by pressure overload. J. Mol. Cell Cardiol. 132, 60–70 (2019).
Li, E. A., Xi, W., Han, Y. S. & Brozovich, F. V. Phosphodiesterase expression in the normal and failing heart. Arch. Biochem. Biophys. 662, 160–168 (2019).
Takahashi, K., Osanai, T., Nakano, T., Wakui, M. & Okumura, K. Enhanced activities and gene expression of phosphodiesterase types 3 and 4 in pressure-induced congestive heart failure. Heart Vessel. 16, 249–256 (2002).
Harrison, S. A., Reifsnyder, D. H., Gallis, B., Cadd, G. G. & Beavo, J. A. Isolation and characterization of bovine cardiac muscle cGMP-inhibited phosphodiesterase: a receptor for new cardiotonic drugs. Mol. Pharmacol. 29, 506–514 (1986).
Holmes, J. R., Kubo, S. H., Cody, R. J. & Kligfield, P. Milrinone in congestive heart failure: observations on ambulatory ventricular arrhythmias. Am. Heart J. 110, 800–806 (1985).
DiBianco, R. et al. A comparison of oral milrinone, digoxin, and their combination in the treatment of patients with chronic heart failure. N. Engl. J. Med. 320, 677–683 (1989).
Sucharov, C. C. et al. A PDE3A promoter polymorphism regulates cAMP-induced transcriptional activity in failing human myocardium. J. Am. Coll. Cardiol. 73, 1173–1184 (2019).
Amsallem, E., Kasparian, C., Haddour, G., Boissel, J. P. & Nony, P. Phosphodiesterase III inhibitors for heart failure. Cochrane Database Syst. Rev. 2005, CD002230 (2005).
Sanada, S. et al. Cardioprotective effect afforded by transient exposure to phosphodiesterase III inhibitors — the role of protein kinase A and p38 mitogen-activated protein kinase. Circulation 104, 705–710 (2001).
Nakata, T. M., Suzuki, K., Uemura, A., Shimada, K. & Tanaka, R. Contrasting effects of inhibition of phosphodiesterase 3 and 5 on cardiac function and interstitial fibrosis in rats with isoproterenol-induced cardiac dysfunction. J. Cardiovasc. Pharmacol. 73, 195–205 (2019).
Yan, C., Miller, C. L. & Abe, J. Regulation of phosphodiesterase 3 and inducible cAMP early repressor in the heart. Circ. Res. 100, 489–501 (2007).
Nash, C. A., Brown, L. M., Malik, S., Cheng, X. & Smrcka, A. V. Compartmentalized cyclic nucleotides have opposing effects on regulation of hypertrophic phospholipase Cepsilon signaling in cardiac myocytes. J. Mol. Cell Cardiol. 121, 51–59 (2018).
Oikawa, M. et al. Cyclic nucleotide phosphodiesterase 3A1 protects the heart against ischemia-reperfusion injury. J. Mol. Cell Cardiol. 64, 11–19 (2013).
Conti, M. et al. Cyclic AMP-specific PDE4 phosphodiesterases as critical components of cyclic AMP signaling. J. Biol. Chem. 278, 5493–5496 (2003).
Cedervall, P., Aulabaugh, A., Geoghegan, K. F., McLellan, T. J. & Pandit, J. Engineered stabilization and structural analysis of the autoinhibited conformation of PDE4. Proc. Natl Acad. Sci. USA 112, E1414–E1422 (2015).
Richter, W. & Conti, M. Dimerization of the type 4 cAMP-specific phosphodiesterases is mediated by the upstream conserved regions (UCRs). J. Biol. Chem. 277, 40212–40221 (2002).
Beard, M. B. et al. UCR1 and UCR2 domains unique to the cAMP-specific phosphodiesterase family form a discrete module via electrostatic interactions. J. Biol. Chem. 275, 10349–10358 (2000).
Hoffmann, R., Baillie, G. S., MacKenzie, S. J., Yarwood, S. J. & Houslay, M. D. The MAP kinase ERK2 inhibits the cyclic AMP-specific phosphodiesterase HSPDE4D3 by phosphorylating it at Ser579. EMBO J. 18, 893–903 (1999).
Ghigo, A. et al. Phosphoinositide 3-KinaseY protects against catecholamine-induced ventricular arrhythmia through PKA-mediated regulation of distinct phosphodiesterases. Circulation 126, 2073–2083 (2012).
Rochais, F. et al. Negative feedback exerted by PKA and cAMP phosphodiesterase on subsarcolemmal cAMP signals in intact cardiac myocytes. An in vivo study using adenovirus-mediated expression of CNG channels. J. Biol. Chem. 279, 52095–52105 (2004).
Mika, D., Richter, W. & Conti, M. A CaMKII/PDE4D negative feedback regulates cAMP signaling. Proc. Natl Acad. Sci. USA 112, 2023–2028 (2015).
Abi-Gerges, A. et al. Selective changes in cytosolic β-adrenergic cAMP signals and L-type calcium channel regulation by phosphodiesterases during cardiac hypertrophy. J. Mol. Cell Cardiol. 150, 109–121 (2021).
Qvigstad, E. et al. Natriuretic peptides increase β1-adrenoceptor signalling in failing hearts through phosphodiesterase 3 inhibition. Cardiovasc. Res. 85, 763–772 (2010).
Mika, D., Leroy, J., Vandecasteele, G. & Fischmeister, R. PDEs create local domains of cAMP signaling. J. Mol. Cell Cardiol. 52, 323–329 (2012).
Berisha, F. et al. cAMP Imaging at ryanodine receptors reveals β2-adrenoceptor driven arrhythmias. Circ. Res. 129, 81–94 (2021).
Schwinger, R. H. et al. Reduced Ca2+-sensitivity of SERCA 2a in failing human myocardium due to reduced serin-16 phospholamban phosphorylation. J. Mol. Cell Cardiol. 31, 479–491 (1999).
Sande, J. B. et al. Reduced level of serine(16) phosphorylated phospholamban in the failing rat myocardium: a major contributor to reduced SERCA2 activity. Cardiovasc. Res. 53, 382–391 (2002).
Minamisawa, S. et al. Chronic phospholamban-sarcoplasmic reticulum calcium ATPase interaction is the critical calcium cycling defect in dilated cardiomyopathy. Cell 99, 313–322 (1999).
Schwinger, R. H. G. et al. Unchanged protein levels of SERCA II and phospholamban but reduced Ca2+ uptake and Ca2+-ATPase activity of cardiac sarcoplasmic reticulum from dilated cardiomyopathy patients compared with patients with nonfailing hearts. Circulation 92, 3220–3228 (1995).
Meyer, M. et al. Alterations of sarcoplasmic reticulum proteins in failing human dilated cardiomyopathy. Circulation 92, 778–784 (1995).
Yang, J. H., Polanowska-Grabowska, R. K., Smith, J. S., Shields, C. W. T. & Saucerman, J. J. PKA catalytic subunit compartmentation regulates contractile and hypertrophic responses to β-adrenergic signaling. J. Mol. Cell Cardiol. 66C, 83–93 (2013).
Haj Slimane, Z. et al. Control of cytoplasmic and nuclear protein kinase A activity by phosphodiesterases and phosphatases in cardiac myocytes. Cardiovasc. Res. 102, 97–106 (2014).
Wang, L. et al. UCR1C is a novel activator of phosphodiesterase 4 (PDE4) long isoforms and attenuates cardiomyocyte hypertrophy. Cell. Signal. 27, 908–922 (2015).
Sin, Y. Y. et al. Disruption of the cyclic AMP phosphodiesterase-4 (PDE4)-HSP20 complex attenuates the β-agonist induced hypertrophic response in cardiac myocytes. J. Mol. Cell Cardiol. 50, 872–883 (2011).
Karam, S. et al. Cardiac overexpression of PDE4B blunts β-adrenergic response and maladaptive remodeling in heart failure. Circulation 142, 161–174 (2020).
Omar, F. et al. Small-molecule allosteric activators of PDE4 long form cyclic AMP phosphodiesterases. Proc. Natl Acad. Sci. USA 116, 13320–13329 (2019).
Rybalkin, S. D., Rybalkina, I. G., Shimizu-Albergine, M., Tang, X. B. & Beavo, J. A. PDE5 is converted to an activated state upon cGMP binding to the GAF A domain. EMBO J. 22, 469–478 (2003).
Zoraghi, R., Bessay, E. P., Corbin, J. D. & Francis, S. H. Structural and functional features in human PDE5A1 regulatory domain that provide for allosteric cGMP binding, dimerization, and regulation. J. Biol. Chem. 280, 12051–12063 (2005).
Blount, M. A. et al. A 46-amino acid segment in phosphodiesterase-5 GAF-B domain provides for high vardenafil potency over sildenafil and tadalafil and is involved in phosphodiesterase-5 dimerization. Mol. Pharmacol. 70, 1822–1831 (2006).
Corbin, J. D., Turko, I. V., Beasley, A. & Francis, S. H. Phosphorylation of phosphodiesterase-5 by cyclic nucleotide-dependent protein kinase alters its catalytic and allosteric cGMP-binding activities. Eur. J. Biochem. 267, 2760–2767 (2000).
Wallis, R. M., Corbin, J. D., Francis, S. H. & Ellis, P. Tissue distribution of phosphodiesterase families and the effects of sildenafil on tissue cyclic nucleotides, platelet function, and the contractile responses of trabeculae carneae and aortic rings in vitro. Am. J. Cardiol. 83, 3C–12C (1999).
Corbin, J. et al. Sildenafil citrate does not affect cardiac contractility in human or dog heart. Curr. Med. Res. Opin. 19, 747–752 (2003).
Degen, C. V. et al. The emperor’s new clothes: PDE5 and the heart. PLoS One 10, e0118664 (2015).
Borlaug, B. A., Melenovsky, V., Marhin, T., Fitzgerald, P. & Kass, D. A. Sildenafil inhibits β-adrenergic-stimulated cardiac contractility in humans. Circulation 112, 2642–2649 (2005).
Mokni, W. et al. Concerted regulation of cGMP and cAMP phosphodiesterases in early cardiac hypertrophy induced by angiotensin II. PLoS One 5, e14227 (2010).
Pokreisz, P. et al. Ventricular phosphodiesterase-5 expression is increased in patients with advanced heart failure and contributes to adverse ventricular remodeling after myocardial infarction in mice. Circulation 119, 408–416 (2009).
Nagendran, J. et al. Phosphodiesterase type 5 is highly expressed in the hypertrophied human right ventricle, and acute inhibition of phosphodiesterase type 5 improves contractility. Circulation 116, 238–248 (2007).
Shan, X. et al. Differential expression of PDE5 in failing and nonfailing human myocardium. Circ. Heart Fail. 5, 79–86 (2012).
Garcia, A. M. et al. Phosphodiesterase-5 is elevated in failing single ventricle myocardium and affects cardiomyocyte remodeling in vitro. Circ. Heart Fail. 11, e004571 (2018).
Senzaki, H. et al. Cardiac phosphodiesterase 5 (cGMP-specific) modulates β-adrenergic signaling in vivo and is down-regulated in heart failure. FASEB J. 15, 1718–1726 (2001).
Pofi, R. et al. Everything you ever wanted to know about phosphodiesterase 5 inhibitors and the heart (but never dared ask): how do they work? J. Endocrinol. Invest. 39, 131–142 (2016).
Kumar, P., Francis, G. S. & Tang, W. H. Phosphodiesterase 5 inhibition in heart failure: mechanisms and clinical implications. Nat. Rev. Cardiol. 6, 349–355 (2009).
Takimoto, E. et al. Chronic inhibition of cyclic GMP phosphodiesterase 5A prevents and reverses cardiac hypertrophy. Nat. Med. 11, 214–222 (2005).
Zhang, M. et al. Expression, activity, and pro-hypertrophic effects of PDE5A in cardiac myocytes. Cell. Signal. 20, 2231–2236 (2008).
Koitabashi, N. et al. Cyclic GMP/PKG-dependent inhibition of TRPC6 channel activity and expression negatively regulates cardiomyocyte NFAT activation: Novel mechanism of cardiac stress modulation by PDE5 inhibition. J. Mol. Cell Cardiol. 48, 713–724 (2010).
Nishida, M. et al. Phosphorylation of TRPC6 channels at Thr69 is required for anti-hypertrophic effects of phosphodiesterase 5 inhibition. J. Biol. Chem. 285, 13244–13253 (2010).
Zhang, M. et al. Myocardial remodeling is controlled by myocyte-targeted gene regulation of phosphodiesterase type 5. J. Am. Coll. Cardiol. 56, 2021–2030 (2010).
Blanton, R. M. et al. Protein kinase G Ia inhibits pressure overload–induced cardiac remodeling and is required for the cardioprotective effect of sildenafil in vivo. J. Am. Heart Assoc. 1, e003731 (2012).
Salloum, F. N. et al. Sildenafil (Viagra) attenuates ischemic cardiomyopathy and improves left ventricular function in mice. Am. J. Physiol. Heart Circ. Physiol. 294, H1398–H1406 (2008).
Ockaili, R., Salloum, F., Hawkins, J. & Kukreja, R. C. Sildenafil (Viagra) induces powerful cardioprotective effect via opening of mitochondrial KATP channels in rabbits. Am. J. Physiol. Heart Circ. Physiol. 283, H1263–H1269 (2002).
Salloum, F. N., Ockaili, R. A., Wittkamp, M., Marwaha, V. R. & Kukreja, R. C. Vardenafil: a novel type 5 phosphodiesterase inhibitor reduces myocardial infarct size following ischemia/reperfusion injury via opening of mitochondrial KATP channels in rabbits. J. Mol. Cell Cardiol. 40, 405–411 (2006).
Das, A., Xi, L. & Kukreja, R. C. Protein kinase G dependent cardioprotective mechanism of phosphodiesterase-5 inhibition involves phosphorylation of ERK and GSK3b. J. Biol. Chem. 283, 29572–29585 (2008).
Lawless, M. et al. Phosphodiesterase 5 inhibition improves contractile function and restores transverse tubule loss and catecholamine responsiveness in heart failure. Sci. Rep. 9, 6801 (2019).
Hutchings, D. C. et al. PDE5 inhibition suppresses ventricular arrhythmias by reducing SR Ca2+ content. Circ. Res. 129, 650–665 (2021).
Sasaki, H. et al. PDE5 inhibitor efficacy is estrogen dependent in female heart disease. J. Clin. Invest. 124, 2464–2471 (2014).
Fukuma, N. et al. Estrogen receptor-a non-nuclear signaling confers cardioprotection and is essential to cGMP-PDE5 inhibition efficacy. JACC Basic Transl. Sci. 5, 282–295 (2020).
Geelen, P. et al. Sildenafil (Viagra) prolongs cardiac repolarization by blocking the rapid component of the delayed rectifier potassium current. Circulation 102, 275–277 (2000).
Andersen, M. J. et al. Sildenafil and diastolic dysfunction after acute myocardial infarction in patients with preserved ejection fraction: the Sildenafil and Diastolic Dysfunction After Acute Myocardial Infarction (SIDAMI) trial. Circulation 127, 1200–1208 (2013).
De Vecchis, R., Cesaro, A. & Ariano, C. Differential effects of the phosphodiesterase inhibition in chronic heart failure depending on the echocardiographic phenotype (HFREF or HFpEF): a meta-analysis. Minerva Cardioangiol. 66, 659–670 (2018).
Soderling, S. H., Bayuga, S. J. & Beavo, J. A. Cloning and characterization of a cAMP-specific cyclic nucleotide phosphodiesterase. Proc. Natl Acad. Sci. USA 95, 8991–8996 (1998).
Wang, H. et al. Kinetic and structural studies of phosphodiesterase-8A and implication on the inhibitor selectivity. Biochemistry 47, 12760–12768 (2008).
Brown, K. M., Lee, L. C., Findlay, J. E., Day, J. P. & Baillie, G. S. Cyclic AMP-specific phosphodiesterase, PDE8A1, is activated by protein kinase A-mediated phosphorylation. FEBS Lett. 586, 1631–1637 (2012).
Patrucco, E., Albergine, M. S., Santana, L. F. & Beavo, J. A. Phosphodiesterase 8A (PDE8A) regulates excitation-contraction coupling in ventricular myocytes. J. Mol. Cell Cardiol. 49, 330–333 (2010).
Garnier, A. et al. Mapping genetic changes in the cAMP-signaling cascade in human atria. J. Mol. Cell Cardiol. 155, 10–20 (2021).
Wang, P., Wu, P., Egan, R. W. & Billah, M. M. Human phosphodiesterase 8A splice variants: cloning, gene organization, and tissue distribution. Gene 280, 183–194 (2001).
Fisher, D. A., Smith, J. F., Pillar, J. S., St Denis, S. H. & Cheng, J. B. Isolation and characterization of PDE9A, a novel human cGMP-specific phosphodiesterase. J. Biol. Chem. 273, 15559–15564 (1998).
Rentero, C., Monfort, A. & Puigdomenech, P. Identification and distribution of different mRNA variants produced by differential splicing in the human phosphodiesterase 9A gene. Biochem. Biophys. Res. Commun. 301, 686–692 (2003).
Wang, P., Wu, P., Egan, R. W. & Billah, M. M. Identification and characterization of a new human type 9 cGMP-specific phosphodiesterase splice variant (PDE9A5). Differential tissue distribution and subcellular localization of PDE9A variants. Gene 314, 15–27 (2003).
Onody, A. et al. Effect of classic preconditioning on the gene expression pattern of rat hearts: a DNA microarray study. FEBS Lett. 536, 35–40 (2003).
Kokkonen-Simon, K. M. et al. Marked disparity of microRNA modulation by cGMP-selective PDE5 versus PDE9 inhibitors in heart disease. JCI Insight 3, e121739 (2018).
Scott, N. J. A., Rademaker, M. T., Charles, C. J., Espiner, E. A. & Richards, A. M. Hemodynamic, hormonal, and renal actions of phosphodiesterase-9 inhibition in experimental heart failure. J. Am. Coll. Cardiol. 74, 889–901 (2019).
Methawasin, M. et al. Phosphodiesterase 9a inhibition in mouse models of diastolic dysfunction. Circ. Heart Fail. 13, e006609 (2020).
Richards, D. A. et al. CRD-733, a novel PDE9 (phosphodiesterase 9) inhibitor, reverses pressure overload-induced heart failure. Circ. Heart Fail. 14, e007300 (2021).
US National Library of Medicine. ClinicalTrials.gov https://www.clinicaltrials.gov/ct2/show/NCT02038868?id=NCT02038868&draw=2&rank=1 (2022).
Kotera, J., Fujishige, K., Yuasa, K. & Omori, K. Characterization and phosphorylation of PDE10A2, a novel alternative splice variant of human phosphodiesterase that hydrolyzes cAMP and cGMP. Biochem. Biophys. Res. Commun. 261, 551–557 (1999).
Fujishige, K., Kotera, J. & Omori, K. Striatum- and testis-specific phosphodiesterase PDE10A isolation and characterization of a rat PDE10A. Eur. J. Biochem. 266, 1118–1127 (1999).
Fujishige, K. et al. Cloning and characterization of a novel human phosphodiesterase that hydrolyzes both cAMP and cGMP (PDE10A). J. Biol. Chem. 274, 18438–18445 (1999).
Jager, R. et al. Activation of PDE10 and PDE11 phosphodiesterases. J. Biol. Chem. 287, 1210–1219 (2012).
Chen, S. et al. A novel role of cyclic nucleotide phosphodiesterase 10A in pathological cardiac remodeling and dysfunction. Circulation 141, 217–233 (2020).
Zhao, C. Y., Greenstein, J. L. & Winslow, R. L. Roles of phosphodiesterases in the regulation of the cardiac cyclic nucleotide cross-talk signaling network. J. Mol. Cell Cardiol. 91, 215–227 (2016).
Rivet-Bastide, M. et al. cGMP-stimulated cyclic nucleotide phosphodiesterase regulates the basal calcium current in human atrial myocytes. J. Clin. Invest. 99, 2710–2718 (1997).
Kirstein, M. et al. Nitric oxide regulates the calcium current in isolated human atrial myocytes. J. Clin. Invest. 95, 794–802 (1995).
Méry, P.-F., Pavoine, C., Belhassen, L., Pecker, F. & Fischmeister, R. Nitric oxide regulates cardiac Ca2+ current -Involvement of cGMP-inhibited and cGMP-stimulated phosphodiesterases through guanylyl cyclase activation. J. Biol. Chem. 268, 26286–26295 (1993).
Mongillo, M. et al. Compartmentalized phosphodiesterase-2 activity blunts β-adrenergic cardiac inotropy via an NO/cGMP-dependent pathway. Circ. Res. 98, 226–234 (2006).
Schobesberger, S. et al. b3-Adrenoceptor redistribution impairs NO/cGMP/PDE2 signalling in failing cardiomyocytes. Elife 9, e52221 (2020).
Takimoto, E. et al. Compartmentalization of cardiac β-adrenergic inotropy modulation by phosphodiesterase type 5. Circulation 115, 2159–2167 (2007).
Stangherlin, A. et al. cGMP Signals modulate cAMP levels in a compartment-specific manner to regulate catecholamine-dependent signaling in cardiac myocytes. Circ. Res. 108, 929–939 (2011).
Meier, S. et al. PDE3 inhibition by C-type natriuretic peptide-induced cGMP enhances cAMP-mediated signaling in both non-failing and failing hearts. Eur. J. Pharmacol. 812, 174–183 (2017).
Perera, R. K. et al. Microdomain switch of cGMP-regulated phosphodiesterases leads to ANP-induced augmentation of β-adrenoceptor-stimulated contractility in early cardiac hypertrophy. Circ. Res. 116, 1304–1311 (2015).
Zhao, C. Y., Greenstein, J. L. & Winslow, R. L. Interaction between phosphodiesterases in the regulation of the cardiac β-adrenergic pathway. J. Mol. Cell Cardiol. 88, 29–38 (2015).
Blair, C. M. & Baillie, G. S. Reshaping cAMP nanodomains through targeted disruption of compartmentalised phosphodiesterase signalosomes. Biochem. Soc. Trans. 47, 1405–1414 (2019).
Rybalkin, S. D., Hinds, T. R. & Beavo, J. A. Enzyme assays for cGMP hydrolyzing phosphodiesterases. Methods Mol. Biol. 1020, 51–62 (2013).
Shakur, Y. et al. Regulation and function of the cyclic nucleotide phosphodiesterase (PDE3) gene family. Prog. Nucleic Acid. Res. Mol. Biol. 66, 241–277 (2001).
Grant, P. G. & Colman, R. W. Purification and characterization of a human platelet cyclic nucleotide phosphodiesterase. Biochemistry 23, 1801–1807 (1984).
Wang, P. et al. Expression, purification, and characterization of human cAMP-specific phosphodiesterase (PDE4) subtypes A, B, C, and D. Biochem. Biophys. Res. Commun. 234, 320–324 (1997).
Beavo, J. A., Francis, S. H. & Houslay, M. D. Cyclic Nucleotide Phosphodiesterases in Health and Disease 1–713 (CRC Press, 2007).
Johnson, W. B., Katugampola, S., Able, S., Napier, C. & Harding, S. E. Profiling of cAMP and cGMP phosphodiesterases in isolated ventricular cardiomyocytes from human hearts: comparison with rat and guinea pig. Life Sci. 90, 328–336 (2012).
Richter, W. et al. Conserved expression and functions of PDE4 in rodent and human heart. Basic Res. Cardiol. 106, 249–262 (2011).
Lin, C. S., Lau, A., Tu, R. & Lue, T. F. Expression of three isoforms of cGMP-binding cGMP-specific phosphodiesterase (PDE5) in human penile cavernosum. Biochem. Biophys. Res. Commun. 268, 628–635 (2000).
Kotera, J. et al. Genomic origin and transcriptional regulation of two variants of cGMP-binding cGMP-specific phosphodiesterases. Eur. J. Biochem. 262, 866–873 (1999).
Jaski, B. E., Fifer, M. A., Wright, R. F., Braunwald, E. & Colucci, W. S. Positive inotropic and vasodilator actions of milrinone in patients with severe congestive heart failure. Dose-response relationships and comparison to nitroprusside. J. Clin. Invest. 75, 643–649 (1985).
Cuffe, M. S. et al. Short-term intravenous milrinone for acute exacerbation of chronic heart failure: a randomized controlled trial. JAMA 287, 1541–1547 (2002).
Kaye, D. M., Nanayakkara, S., Vizi, D., Byrne, M. & Mariani, J. A. Effects of milrinone on rest and exercise hemodynamics in heart failure with preserved ejection fraction. J. Am. Coll. Cardiol. 67, 2554–2556 (2016).
Nanayakkara, S., Mak, V., Crannitch, K., Byrne, M. & Kaye, D. M. Extended release oral milrinone, CRD-102, for advanced heart failure. Am. J. Cardiol. 122, 1017–1020 (2018).
Nanayakkara, S. et al. Extended-release oral milrinone for the treatment of heart failure with preserved ejection fraction. J. Am. Heart Assoc. 9, e015026 (2020).
Metra, M. et al. Effects of low-dose oral enoximone administration on mortality, morbidity, and exercise capacity in patients with advanced heart failure: the randomized, double-blind, placebo-controlled, parallel group ESSENTIAL trials. Eur. Heart J. 30, 3015–3026 (2009).
Giannetta, E. et al. Chronic Inhibition of cGMP phosphodiesterase 5A improves diabetic cardiomyopathy: a randomized, controlled clinical trial using magnetic resonance imaging with myocardial tagging. Circulation 125, 2323–2333 (2012).
Guazzi, M., Tumminello, G., Di Marco, F., Fiorentini, C. & Guazzi, M. D. The effects of phosphodiesterase-5 inhibition with sildenafil on pulmonary hemodynamics and diffusion capacity, exercise ventilatory efficiency, and oxygen uptake kinetics in chronic heart failure. J. Am. Coll. Cardiol. 44, 2339–2348 (2004).
Guazzi, M., Samaja, M., Arena, R., Vicenzi, M. & Guazzi, M. D. Long-term use of sildenafil in the therapeutic management of heart failure. J. Am. Coll. Cardiol. 50, 2136–2144 (2007).
Hryniewicz, K. et al. Inhibition of angiotensin-converting enzyme and phosphodiesterase type 5 improves endothelial function in heart failure. Clin. Sci. 108, 331–338 (2005).
Lewis, G. D. et al. Sildenafil improves exercise hemodynamics and oxygen uptake in patients with systolic heart failure. Circulation 115, 59–66 (2007).
Lewis, G. D. et al. Sildenafil improves exercise capacity and quality of life in patients with systolic heart failure and secondary pulmonary hypertension. Circulation 116, 1555–1562 (2007).
Behling, A. et al. Effects of 5′-phosphodiesterase four-week long inhibition with sildenafil in patients with chronic heart failure: a double-blind, placebo-controlled clinical trial. J. Card. Fail. 14, 189–197 (2008).
Guazzi, M., Vicenzi, M., Arena, R. & Guazzi, M. D. PDE5 inhibition with sildenafil improves left ventricular diastolic function, cardiac geometry, and clinical status in patients with stable systolic heart failure: results of a 1-year, prospective, randomized, placebo-controlled study. Circ. Heart Fail. 4, 8–17 (2011).
Fernandes, A. M. et al. The immediate effect of sildenafil on right ventricular function in patients with heart failure measured by cardiac magnetic resonance: a randomized control trial. PLoS One 10, e0119623 (2015).
Guazzi, M., Vicenzi, M., Arena, R. & Guazzi, M. D. Pulmonary hypertension in heart failure with preserved ejection fraction: a target of phosphodiesterase-5 inhibition in a 1-year study. Circulation 124, 164–174 (2011).
Hoendermis, E. S. et al. Effects of sildenafil on invasive haemodynamics and exercise capacity in heart failure patients with preserved ejection fraction and pulmonary hypertension: a randomized controlled trial. Eur. Heart J. 36, 2565–2573 (2015).
Al-Hesayen, A., Floras, J. S. & Parker, J. D. The effects of intravenous sildenafil on hemodynamics and cardiac sympathetic activity in chronic human heart failure. Eur. J. Heart Fail. 8, 864–868 (2006).
Liu, L. C. et al. Effects of sildenafil on cardiac structure and function, cardiopulmonary exercise testing and health-related quality of life measures in heart failure patients with preserved ejection fraction and pulmonary hypertension. Eur. J. Heart Fail. 19, 116–125 (2017).
Kim, K. H. et al. PDE 5 inhibition with udenafil improves left ventricular systolic/diastolic functions and exercise capacity in patients with chronic heart failure with reduced ejection fraction; A 12-week, randomized, double-blind, placebo-controlled trial. Am. Heart J. 169, 813–822 (2015).
Santos, R. C. et al. Tadalafil-induced improvement in left ventricular diastolic function in resistant hypertension. Eur. J. Clin. Pharmacol. 70, 147–154 (2014).
Acknowledgements
UMR-S1180 is a member of the Laboratory of Excellence LERMIT supported by the French National Research Agency (ANR-10-LABX-33) under the programme “Investissements d’Avenir” ANR-11-IDEX-0003-01. The authors are also funded by grants from the Leducq Foundation for Cardiovascular Research (19CVD02), ERA-CVD “PDE4HEART” and ANR-16-ECVD-0007-01 to R.F. and ANR-19-CE14-0038-02 to G.V. R.K. was supported by postdoctoral fellowships from ERA-CVD and Fondation Lefoulon-Delalande.
Author information
Authors and Affiliations
Contributions
The authors contributed substantially to all aspects of the article.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Cardiology thanks Joseph Beavo and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kamel, R., Leroy, J., Vandecasteele, G. et al. Cyclic nucleotide phosphodiesterases as therapeutic targets in cardiac hypertrophy and heart failure. Nat Rev Cardiol 20, 90–108 (2023). https://doi.org/10.1038/s41569-022-00756-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41569-022-00756-z
This article is cited by
-
Abnormal phosphorylation / dephosphorylation and Ca2+ dysfunction in heart failure
Heart Failure Reviews (2024)
-
Astragaloside IV derivative HHQ16 ameliorates infarction-induced hypertrophy and heart failure through degradation of lncRNA4012/9456
Signal Transduction and Targeted Therapy (2023)