Abstract
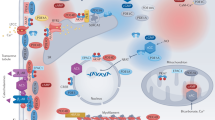
G protein-coupled receptors (GPCRs) are critical cellular sensors that mediate numerous physiological processes. In the heart, multiple GPCRs are expressed on various cell types, where they coordinate to regulate cardiac function by modulating critical processes such as contractility and blood flow. Under pathological settings, these receptors undergo aberrant changes in expression levels, localization and capacity to couple to downstream signalling pathways. Conventional therapies for heart failure work by targeting GPCRs, such as β-adrenergic receptor and angiotensin II receptor antagonists. Although these treatments have improved patient survival, heart failure remains one of the leading causes of mortality worldwide. GPCR kinases (GRKs) are responsible for GPCR phosphorylation and, therefore, desensitization and downregulation of GPCRs. In this Review, we discuss the GPCR signalling pathways and the GRKs involved in the pathophysiology of heart disease. Given that increased expression and activity of GRK2 and GRK5 contribute to the loss of contractile reserve in the stressed and failing heart, inhibition of overactive GRKs has been proposed as a novel therapeutic approach to treat heart failure.
Key points
-
G protein-coupled receptors (GPCRs) mediate a range of physiological responses in various cardiovascular cell types.
-
β-Adrenergic receptors (β-ARs) regulate cardiomyocyte contractility in response to sympathetic nervous system stimulation.
-
In the failing heart, increased levels of GPCR kinases (GRKs) phosphorylate, and thereby desensitize and downregulate, β-ARs, resulting in a loss of cardiomyocyte contractile reserve.
-
GRK2 and GRK5 can be therapeutically targeted to protect the heart against injury and failure using novel small-molecule inhibitors.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Benjamin, E. J. et al. Heart disease and stroke statistics — 2018 update: a report from the American Heart Association. Circulation 137, e67–e492 (2018).
Lusis, A. J. Atherosclerosis. Nature 407, 233–241 (2000).
Sequeira, V. & van der Velden, J. Historical perspective on heart function: the Frank–Starling Law. Biophys. Rev. 7, 421–447 (2015).
Perlman, R. L. & Chalfie, M. Catecholamine release from the adrenal medulla. Clin. Endocrinol. Metab. 6, 551–576 (1977).
Madamanchi, A. β-Adrenergic receptor signaling in cardiac function and heart failure. McGill J. Med. 10, 99–104 (2007).
Hakak, Y., Shrestha, D., Goegel, M. C., Behan, D. P. & Chalmers, D. T. Global analysis of G-protein-coupled receptor signaling in human tissues. FEBS Lett. 550, 11–17 (2003).
Fredriksson, R., Lagerström, M. C., Lundin, L.-G. & Schiöth, H. B. The G-protein-coupled receptors in the human genome form five main families. Phylogenetic analysis, paralogon groups, and fingerprints. Mol. Pharmacol. 63, 1256–1272 (2003).
Schertler, G. F. X., Villa, C. & Henderson, R. Projection structure of rhodopsin. Nature 362, 770–772 (1993).
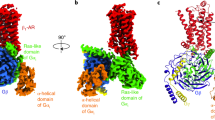
Oldham, W. M. & Hamm, H. E. Heterotrimeric G protein activation by G-protein-coupled receptors. Nat. Rev. Mol. Cell. Biol. 9, 60–71 (2008).
Kristiansen, K. Molecular mechanisms of ligand binding, signaling, and regulation within the superfamily of G-protein-coupled receptors: molecular modeling and mutagenesis approaches to receptor structure and function. Pharmacol. Ther. 103, 21–80 (2004).
Lambright, D. G., Noel, J. P., Hamm, H. E. & Sigler, P. B. Structural determinants for activation of the α-subunit of a heterotrimeric G protein. Nature 369, 621–628 (1994).
Rall, T. W., Sutherland, E. W., Maxwell, A. M. & Davis, J. W. II. The enzymatically catalyzed formation of adenosine 3ʹ,5ʹ-phosphate and inorganic pyrophosphate from adenosine triphosphate. J. Biol. Chem. 237, 1228–1232 (1962).
Rosenbaum, D. M., Rasmussen, S. G. F. & Kobilka, B. K. The structure and function of G-protein-coupled receptors. Nature 459, 356–363 (2009).
Wu, J., Brown, S. H. J., von Daake, S. & Taylor, S. S. PKA type IIα holoenzyme reveals a combinatorial strategy for isoform diversity. Science 318, 274–279 (2007).
Chen, J. & Iyengar, R. Inhibition of cloned adenylyl cyclases by mutant-activated Gi-α and specific suppression of type 2 adenylyl cyclase inhibition by phorbolester treatment. J. Biol. Chem. 268, 12253–12256 (1993).
Taussig, R., Iñiguez-Lluhi, J. A. & Gilman, A. G. Inhibition of adenylyl cyclase by Gi alpha. Science 261, 218–221 (1993).
Lee, C. H., Park, D., Wu, D., Rhee, S. G. & Simon, M. I. Members of the Gq α subunit gene family activate phospholipase C β isozymes. J. Biol. Chem. 267, 16044–16047 (1992).
Harden, T. K., Waldo, G. L., Hicks, S. N. & Sondek, J. Mechanism of activation and inactivation of Gq/phospholipase C-β signaling nodes. Chem. Rev. 111, 6120–6129 (2011).
Berridge, M. J. & Irvine, R. F. Inositol trisphosphate, a novel second messenger in cellular signal transduction. Nature 312, 315–321 (1984).
Kaibuchi, K., Takai, Y. & Nishizuka, Y. Cooperative roles of various membrane phospholipids in the activation of calcium-activated, phospholipid-dependent protein kinase. J. Biol. Chem. 256, 7146–7149 (1981).
Kozasa, T. et al. p115 RhoGEF, a GTPase activating protein for Gα12 and Gα13. Science 280, 2109–2111 (1998).
Capote, L. A., Mendez Perez, R. & Lymperopoulos, A. GPCR signaling and cardiac function. Eur. J. Pharmacol. 763, 143–148 (2015).
Salazar, N. C., Chen, J. & Rockman, H. A. Cardiac GPCRs: GPCR signaling in healthy and failing hearts. Biochim. Biophys. Acta 1768, 1006–1018 (2007).
Berwick, Z. C. et al. Contribution of adenosine A(2A) and A(2B) receptors to ischemic coronary dilation: role of K(V) and K(ATP) channels. Microcirculation 17, 600–607 (2010).
Gustafsson, F. & Holstein-Rathlou, N. H. Angiotensin II modulates conducted vasoconstriction to norepinephrine and local electrical stimulation in rat mesenteric arterioles. Cardiovasc. Res. 44, 176–184 (1999).
Yoshida, M., Suzuki, A. & Itoh, T. Mechanisms of vasoconstriction induced by endothelin-1 in smooth muscle of rabbit mesenteric artery. J. Physiol. 477, 253–265 (1994).
Gericke, A. et al. Role of the M3 muscarinic acetylcholine receptor subtype in murine ophthalmic arteries after endothelial removal. Invest. Ophthalmol. Vis. Sci. 55, 625–631 (2014).
McMurdo, L., Thiemermann, C. & Vane, J. R. The endothelin ETB receptor agonist, IRL 1620, causes vasodilatation and inhibits ex vivo platelet aggregation in the anaesthetised rabbit. Eur. J. Pharmacol. 259, 51–55 (1994).
Bristow, M. R., Hershberger, R. E., Port, J. D., Minobe, W. & Rasmussen, R. Beta 1- and beta 2-adrenergic receptor-mediated adenylate cyclase stimulation in nonfailing and failing human ventricular myocardium. Mol. Pharmacol. 35, 295–303 (1989).
Fischmeister, R. & Hartzell, H. C. Mechanism of action of acetylcholine on calcium current in single cells from frog ventricle. J. Physiol. 376, 183–202 (1986).
Méry, P. F. et al. Muscarinic regulation of the L-type calcium current in isolated cardiac myocytes. Life Sci. 60, 1113–1120 (1997).
Xiao, R. P. et al. Coupling of β2-adrenoceptor to Gi proteins and its physiological relevance in murine cardiac myocytes. Circ. Res. 84, 43–52 (1999).
Dorn, G. W., Tepe, N. M., Lorenz, J. N., Koch, W. J. & Liggett, S. B. Low- and high-level transgenic expression of β2-adrenergic receptors differentially affect cardiac hypertrophy and function in Gαq-overexpressing mice. Proc. Natl Acad. Sci. USA 96, 6400–6405 (1999).
Morisco, C., Zebrowski, D. C., Vatner, D. E., Vatner, S. F. & Sadoshima, J. β-adrenergic cardiac hypertrophy is mediated primarily by the β1-subtype in the rat heart. J. Mol. Cell. Cardiol. 33, 561–573 (2001).
Yamazaki, T., Kurihara, H., Kurihara, Y., Komuro, I. & Yazaki, Y. Endothelin-1 regulates normal cardiovascular development and cardiac cellular hypertrophy. J. Card. Fail. 2, S7–S12 (1996).
Piascik, M. T. et al. Immunocytochemical localization of the alpha-1B adrenergic receptor and the contribution of this and the other subtypes to vascular smooth muscle contraction: analysis with selective ligands and antisense oligonucleotides. J. Pharmacol. Exp. Ther. 283, 854–868 (1997).
Myagmar, B. E. et al. Adrenergic receptors in individual ventricular myocytes: the beta-1 and alpha-1B are in all cells, the alpha-1A is in a subpopulation, and the beta-2 and beta-3 are mostly absent. Circ. Res. 120, 1103–1115 (2017).
Lorenz, W. et al. Expression of three alpha 2-adrenergic receptor subtypes in rat tissues: implications for alpha 2 receptor classification. Mol. Pharmacol. 38, 599–603 (1990).
Krief, S. et al. Tissue distribution of beta 3-adrenergic receptor mRNA in man. J. Clin. Invest. 91, 344–349 (1993).
Molinoff, P. B. Alpha- and beta-adrenergic receptor subtypes properties, distribution and regulation. Drugs 28 (Suppl. 2), 1–15 (1984).
Rockman, H. A., Koch, W. J. & Lefkowitz, R. J. Seven-transmembrane-spanning receptors and heart function. Nature 415, 206–212 (2002).
Gauthier, C. et al. The negative inotropic effect of beta3-adrenoceptor stimulation is mediated by activation of a nitric oxide synthase pathway in human ventricle. J. Clin. Invest. 102, 1377–1384 (1998).
Dessy, C. & Balligand, J.-L. Beta3-adrenergic receptors in cardiac and vascular tissues emerging concepts and therapeutic perspectives. Adv. Pharmacol. 59, 135–163 (2010).
Kamp, T. J. & Hell, J. W. Regulation of cardiac L-type calcium channels by protein kinase A and protein kinase C. Circ. Res. 87, 1095–1102 (2000).
Lai, Y., Seagar, M. J., Takahashi, M. & Catterall, W. A. Cyclic AMP-dependent phosphorylation of two size forms of alpha 1 subunits of L-type calcium channels in rat skeletal muscle cells. J. Biol. Chem. 265, 20839–20848 (1990).
Zhang, R., Zhao, J., Mandveno, A. & Potter, J. D. Cardiac troponin I phosphorylation increases the rate of cardiac muscle relaxation. Circ. Res. 76, 1028–1035 (1995).
Mesirca, P., Torrente, A. G. & Mangoni, M. E. Functional role of voltage gated Ca(2+) channels in heart automaticity. Front. Physiol. 6, 19 (2015).
Nikolaev, V. O. et al. Beta2-adrenergic receptor redistribution in heart failure changes cAMP compartmentation. Science 327, 1653–1657 (2010).
Brodde, O. E. et al. Myocardial beta-adrenoceptor changes in heart failure: concomitant reduction in beta 1- and beta 2-adrenoceptor function related to the degree of heart failure in patients with mitral valve disease. J. Am. Coll. Cardiol. 14, 323–331 (1989).
Neumann, J. et al. Increase in myocardial Gi-proteins in heart failure. Lancet 2, 936–937 (1988).
Chen, C. K. et al. Characterization of human GRK7 as a potential cone opsin kinase. Mol. Vis. 7, 305–313 (2001).
Premont, R. T. et al. Characterization of the G protein-coupled receptor kinase GRK4. Identification of four splice variants. J. Biol. Chem. 271, 6403–6410 (1996).
Somers, R. L. & Klein, D. C. Rhodopsin kinase activity in the mammalian pineal gland and other tissues. Science 226, 182–184 (1984).
Dzimiri, N., Muiya, P., Andres, E. & Al-Halees, Z. Differential functional expression of human myocardial G protein receptor kinases in left ventricular cardiac diseases. Eur. J. Pharmacol. 489, 167–177 (2004).
Montó, F. et al. Different expression of adrenoceptors and GRKs in the human myocardium depends on heart failure etiology and correlates to clinical variables. Am. J. Physiol. Heart Circ. Physiol. 303, H368–H376 (2012).
Sato, P. Y., Chuprun, J. K., Schwartz, M. & Koch, W. J. The evolving impact of g protein-coupled receptor kinases in cardiac health and disease. Physiol. Rev. 95, 377–404 (2015).
Bownds, D., Dawes, J., Miller, J. & Stahlman, M. Phosphorylation of frog photoreceptor membranes induced by light. Nature New Biol. 237, 125–127 (1972).
Hisatomi, O. et al. A novel subtype of G-protein-coupled receptor kinase, GRK7, in teleost cone photoreceptors. FEBS Lett. 424, 159–164 (1998).
Inglese, J., Koch, W. J., Caron, M. G. & Lefkowitz, R. J. Isoprenylation in regulation of signal transduction by G-protein-coupled receptor kinases. Nature 359, 147–150 (1992).
Carman, C. V. et al. Mutational analysis of Gbetagamma and phospholipid interaction with G protein-coupled receptor kinase 2. J. Biol. Chem. 275, 10443–10452 (2000).
Jiang, X., Benovic, J. L. & Wedegaertner, P. B. Plasma membrane and nuclear localization of G protein–coupled receptor kinase 6A. Mol. Biol. Cell 18, 2960–2969 (2007).
Pronin, A. N., Carman, C. V. & Benovic, J. L. Structure-function analysis of G protein-coupled receptor kinase-5. Role of the carboxyl terminus in kinase regulation. J. Biol. Chem. 273, 31510–31518 (1998).
Kühn, H., Hall, S. W. & Wilden, U. Light-induced binding of 48-kDa protein to photoreceptor membranes is highly enhanced by phosphorylation of rhodopsin. FEBS Lett. 176, 473–478 (1984).
Krupnick, J. G., Gurevich, V. V. & Benovic, J. L. Mechanism of quenching of phototransduction. Binding competition between arrestin and transducin for phosphorhodopsin. J. Biol. Chem. 272, 18125–18131 (1997).
Goodman, O. B., Krupnick, J. G., Gurevich, V. V., Benovic, J. L. & Keen, J. H. Arrestin/clathrin interaction. Localization of the arrestin binding locus to the clathrin terminal domain. J. Biol. Chem. 272, 15017–15022 (1997).
Baugher, P. J. & Richmond, A. The carboxyl-terminal PDZ ligand motif of chemokine receptor CXCR2 modulates post-endocytic sorting and cellular chemotaxis. J. Biol. Chem. 283, 30868–30878 (2008).
Nobles, K. N. et al. Distinct phosphorylation sites on the β2-adrenergic receptor establish a barcode that encodes differential functions of β-arrestin. Sci. Signal. 4, ra51 (2011).
Hollinger, S. & Hepler, J. R. Cellular regulation of RGS proteins: modulators and integrators of G protein signaling. Pharmacol. Rev. 54, 527–559 (2002).
Koch, W. J. et al. Cardiac function in mice overexpressing the beta-adrenergic receptor kinase or a beta ARK inhibitor. Science 268, 1350–1353 (1995).
Rockman, H. A. et al. Receptor-specific in vivo desensitization by the G protein-coupled receptor kinase-5 in transgenic mice. Proc. Natl Acad. Sci. USA 93, 9954–9959 (1996).
Rockman, H. A. et al. Control of myocardial contractile function by the level of β-adrenergic receptor kinase 1 in gene-targeted mice. J. Biol. Chem. 273, 18180–18184 (1998).
Chuang, T. T., LeVine, H. & Blasi, A. D. Phosphorylation and activation of β-adrenergic receptor kinase by protein kinase C. J. Biol. Chem. 270, 18660–18665 (1995).
Cong, M. et al. Regulation of membrane targeting of the G protein-coupled receptor kinase 2 by protein kinase A and its anchoring protein AKAP79. J. Biol. Chem. 276, 15192–15199 (2001).
Krasel, C. et al. Phosphorylation of GRK2 by protein kinase C abolishes its inhibition by calmodulin. J. Biol. Chem. 276, 1911–1915 (2001).
Penela, P., Elorza, A., Sarnago, S. & Mayor, F. β-Arrestin- and c-Src-dependent degradation of G-protein-coupled receptor kinase 2. EMBO J. 20, 5129–5138 (2001).
Pitcher, J. A. et al. Feedback inhibition of G protein-coupled receptor kinase 2 (GRK2) activity by extracellular signal-regulated kinases. J. Biol. Chem. 274, 34531–34534 (1999).
Whalen, E. J. et al. Regulation of beta-adrenergic receptor signaling by S-nitrosylation of G-protein-coupled receptor kinase 2. Cell 129, 511–522 (2007).
Pronin, A. N. & Benovic, J. L. Regulation of the G protein-coupled receptor kinase GRK5 by protein kinase C. J. Biol. Chem. 272, 3806–3812 (1997).
Carman, C. V., Lisanti, M. P. & Benovic, J. L. Regulation of G protein-coupled receptor kinases by caveolin. J. Biol. Chem. 274, 8858–8864 (1999).
Pronin, A. N., Satpaev, D. K., Slepak, V. Z. & Benovic, J. L. Regulation of G protein-coupled receptor kinases by calmodulin and localization of the calmodulin binding domain. J. Biol. Chem. 272, 18273–18280 (1997).
Vinge, L. E. et al. Myocardial distribution and regulation of GRK and beta-arrestin isoforms in congestive heart failure in rats. Am. J. Physiol. Heart Circ. Physiol. 281, H2490–H2499 (2001).
Huang, Z. M. et al. Convergence of G protein-coupled receptor and S-nitrosylation signaling determines the outcome to cardiac ischemic injury. Sci. Signal. 6, ra95 (2013).
Luo, J. & Benovic, J. L. G. Protein-coupled receptor kinase interaction with Hsp90 mediates kinase maturation. J. Biol. Chem. 278, 50908–50914 (2003).
Chen, M. et al. Prodeath signaling of G protein-coupled receptor kinase 2 in cardiac myocytes after ischemic stress occurs via extracellular signal-regulated kinase-dependent heat shock protein 90-mediated mitochondrial targeting. Circ. Res. 112, 1121–1134 (2013).
Sato, P. Y. et al. GRK2 compromises cardiomyocyte mitochondrial function by diminishing fatty acid-mediated oxygen consumption and increasing superoxide levels. J. Mol. Cell. Cardiol. 89, 360–364 (2015).
Gold, J. I. et al. Nuclear translocation of cardiac G protein-Coupled Receptor kinase 5 downstream of select Gq-activating hypertrophic ligands is a calmodulin-dependent process. PLOS ONE 8, e57324 (2013).
Hullmann, J. E. et al. GRK5-mediated exacerbation of pathological cardiac hypertrophy involves facilitation of nuclear NFAT activity. Circ. Res. 115, 976–985 (2014).
Pitcher, J. A. et al. The G protein-coupled receptor kinase 2 is a microtubule-associated protein kinase that phosphorylates tubulin. J. Biol. Chem. 273, 12316–12324 (1998).
Peregrin, S. et al. Phosphorylation of p38 by GRK2 at the docking groove unveils a novel mechanism for inactivating p38MAPK. Curr. Biol. CB 16, 2042–2047 (2006).
Rose, B. A., Force, T. & Wang, Y. Mitogen-activated protein kinase signaling in the heart: angels versus demons in a heart-breaking tale. Physiol. Rev. 90, 1507–1546 (2010).
Weinbrenner, C., Liu, G. S., Cohen, M. V. & Downey, J. M. Phosphorylation of tyrosine 182 of p38 mitogen-activated protein kinase correlates with the protection of preconditioning in the rabbit heart. J. Mol. Cell. Cardiol. 29, 2383–2391 (1997).
Ciccarelli, M. et al. GRK2 activity impairs cardiac glucose uptake and promotes insulin resistance following myocardial ischemia. Circulation 123, 1953–1962 (2011).
DiNicolantonio, J. J. et al. β-Blockers in hypertension, diabetes, heart failure and acute myocardial infarction: a review of the literature. Open Heart 2, e000230 (2015).
Bristow, M. R. β-adrenergic receptor blockade in chronic heart failure. Circulation 101, 558–569 (2000).
Flesch, M. et al. Differential effects of carvedilol and metoprolol on isoprenaline-induced changes in beta-adrenoceptor density and systolic function in rat cardiac myocytes. Cardiovasc. Res. 49, 371–380 (2001).
Leineweber, K. et al. G-protein-coupled receptor kinase activity in human heart failure: effects of beta-adrenoceptor blockade. Cardiovasc. Res. 66, 512–519 (2005).
Doi, M. et al. Propranolol prevents the development of heart failure by restoring FKBP12.6-mediated stabilization of ryanodine receptor. Circulation 105, 1374–1379 (2002).
Reiken, S. et al. Beta-blockers restore calcium release channel function and improve cardiac muscle performance in human heart failure. Circulation 107, 2459–2466 (2003).
Bartholomeu, J. B. et al. Intracellular mechanisms of specific β-adrenoceptor antagonists involved in improved cardiac function and survival in a genetic model of heart failure. J. Mol. Cell. Cardiol. 45, 240–249 (2008).
Babick, A., Elimban, V., Zieroth, S. & Dhalla, N. S. Reversal of cardiac dysfunction and subcellular alterations by metoprolol in heart failure due to myocardial infarction. J. Cell. Physiol. 228, 2063–2070 (2013).
Koch, W. J., Inglese, J., Stone, W. C. & Lefkowitz, R. J. The binding site for the βγ subunits of heterotrimeric G proteins on the β-adrenergic receptor kinase. J. Biol. Chem. 268, 8256–8260 (1993).
Ostrom, R. S. et al. Receptor number and caveolar co-localization determine receptor coupling efficiency to adenylyl cyclase. J. Biol. Chem. 276, 42063–42069 (2001).
Akhter, S. A. et al. In vivo inhibition of elevated myocardial β-adrenergic receptor kinase activity in hybrid transgenic mice restores normal β-adrenergic signaling and function. Circulation 100, 648–653 (1999).
Korzick, D. H. et al. Transgenic manipulation of β-adrenergic receptor kinase modifies cardiac myocyte contraction to norepinephrine. Am. J. Physiol. Heart Circ. Physiol. 272, H590–H596 (1997).
Eckhart, A. D. & Koch, W. J. Expression of a β-adrenergic receptor kinase inhibitor reverses dysfunction in failing cardiomyocytes. Mol. Ther. 5, 74–79 (2002).
Rockman, H. A. et al. Expression of a β-adrenergic receptor kinase 1 inhibitor prevents the development of myocardial failure in gene-targeted mice. Proc. Natl Acad. Sci. USA 95, 7000–7005 (1998).
Suzuki, Y., Nakano, K., Sugiyama, M. & Imagawa, J. βARK1 inhibition improves survival in a mouse model of heart failure induced by myocardial infarction. J. Cardiovasc. Pharmacol. 44, 329–334 (2004).
Tachibana, H., Naga Prasad, S. V., Lefkowitz, R. J., Koch, W. J. & Rockman, H. A. Level of β-adrenergic receptor kinase 1 inhibition determines degree of cardiac dysfunction after chronic pressure overload-induced heart failure. Circulation 111, 591–597 (2005).
Harding, V. B., Jones, L. R., Lefkowitz, R. J., Koch, W. J. & Rockman, H. A. Cardiac βARK1 inhibition prolongs survival and augments β blocker therapy in a mouse model of severe heart failure. Proc. Natl Acad. Sci. USA 98, 5809–5814 (2001).
Brinks, H. et al. Level of G protein-coupled receptor kinase-2 determines myocardial ischemia/reperfusion injury via pro- and anti-apoptotic mechanisms. Circ. Res. 107, 1140–1149 (2010).
Choi, D.-J., Koch, W. J., Hunter, J. J. & Rockman, H. A. Mechanism of β-adrenergic receptor desensitization in cardiac hypertrophy is increased β-adrenergic receptor kinase. J. Biol. Chem. 272, 17223–17229 (1997).
Drazner, M. H. et al. Potentiation of beta-adrenergic signaling by adenoviral-mediated gene transfer in adult rabbit ventricular myocytes. J. Clin. Invest. 99, 288–296 (1997).
Tevaearai, H. T., Eckhart, A. D., Shotwell, K. F., Wilson, K. & Koch, W. J. Ventricular dysfunction after cardioplegic arrest is improved after myocardial gene transfer of a beta-adrenergic receptor kinase inhibitor. Circulation 104, 2069–2074 (2001).
Tevaearai, H. T., Walton, G. B., Eckhart, A. D., Keys, J. R. & Koch, W. J. Donor heart contractile dysfunction following prolonged ex vivo preservation can be prevented by gene-mediated beta-adrenergic signaling modulation. Eur. J. Cardiothorac. Surg. 22, 733–737 (2002).
Tevaearai, H. T., Walton, G. B., Keys, J. R., Koch, W. J. & Eckhart, A. D. Acute ischemic cardiac dysfunction is attenuated via gene transfer of a peptide inhibitor of the β-adrenergic receptor kinase (βARK1). J. Gene Med. 7, 1172–1177 (2005).
White, D. C. et al. Preservation of myocardial beta-adrenergic receptor signaling delays the development of heart failure after myocardial infarction. Proc. Natl Acad. Sci. USA 97, 5428–5433 (2000).
Raake, P. W. J. et al. AAV6.βARKct cardiac gene therapy ameliorates cardiac function and normalizes the catecholaminergic axis in a clinically relevant large animal heart failure model. Eur. Heart J. 34, 1437–1447 (2013).
Rengo, G. et al. Myocardial adeno-associated virus serotype 6-βARKct gene therapy improves cardiac function and normalizes the neurohormonal axis in chronic heart failure. Circulation 119, 89–98 (2009).
Emani, S. M., Shah, A. S., White, D. C., Glower, D. D. & Koch, W. J. Right ventricular gene therapy with a beta-adrenergic receptor kinase inhibitor improves survival after pulmonary artery banding. Ann. Thorac. Surg. 72, 1657–1661 (2001).
Shang, D. et al. Adenoviral βARKct cardiac gene transfer ameliorates post-resuscitation myocardial injury in a porcine model of cardiac arrest. Shock https://doi.org/10.1097/SHK.0000000000001320 (2019).
Swain, J. D. et al. MCARD-mediated gene transfer of GRK2 inhibitor in ovine model of acute myocardial infarction. J. Cardiovasc. Transl Res. 6, 253–262 (2013).
Katz, M. G. et al. AAV6-βARKct gene delivery mediated by molecular cardiac surgery with recirculating delivery (MCARD) in sheep results in robust gene expression and increased adrenergic reserve. J. Thorac. Cardiovasc. Surg. 143, 720–726 (2012).
Gupta, D. et al. Adenoviral beta-adrenergic receptor kinase inhibitor gene transfer improves exercise capacity, cardiac contractility, and systemic inflammation in a model of pressure overload hypertrophy. Cardiovasc. Drugs Ther. 22, 373–381 (2008).
Molina, E. J., Gupta, D., Palma, J., Gaughan, J. P. & Macha, M. Right ventricular beneficial effects of beta adrenergic receptor kinase inhibitor (ßARKct) gene transfer in a rat model of severe pressure overload. Biomed. Pharmacother. 63, 331–336 (2009).
Shah, A. S. et al. In vivo ventricular gene delivery of a beta-adrenergic receptor kinase inhibitor to the failing heart reverses cardiac dysfunction. Circulation 103, 1311–1316 (2001).
Woodard, G. E., Jardín, I., Berna-Erro, A., Salido, G. M. & Rosado, J. A. Regulators of G-protein-signaling proteins: negative modulators of G-protein-coupled receptor signaling. Int. Rev. Cell. Mol. Biol. 317, 97–183 (2015).
Carman, C. V. et al. Selective regulation of Gαq/11 by an RGS domain in the G protein-coupled receptor kinase, GRK2. J. Biol. Chem. 274, 34483–34492 (1999).
Day, P. W. et al. Characterization of the GRK2 binding site of Gαq. J. Biol. Chem. 279, 53643–53652 (2004).
Tesmer, V. M., Kawano, T., Shankaranarayanan, A., Kozasa, T. & Tesmer, J. J. G. Snapshot of activated G proteins at the membrane: the Gαq-GRK2-Gßγ complex. Science 310, 1686–1690 (2005).
Schumacher, S. M. et al. A peptide of the RGS domain of GRK2 binds and inhibits Gα(q) to suppress pathological cardiac hypertrophy and dysfunction. Sci. Signal. 9, ra30 (2016).
Sorriento, D. et al. The G-protein-coupled receptor kinase 5 inhibits NFκB transcriptional activity by inducing nuclear accumulation of IκBα. Proc. Natl Acad. Sci. USA 105, 17818–17823 (2008).
Sorriento, D. et al. Intracardiac injection of AdGRK5-NT reduces left ventricular hypertrophy by inhibiting NF-κB-dependent hypertrophic gene expression. Hypertension 56, 696–704 (2010).
Sorriento, D. et al. The amino-terminal domain of GRK5 inhibits cardiac hypertrophy through the regulation of calcium-calmodulin dependent transcription factors. Int. J. Mol. Sci. 19, E861 (2018).
Bonacci, T. M. et al. Differential targeting of Gßγ-subunit signaling with small molecules. Science 312, 443–446 (2006).
Casey, L. M. et al. Small molecule disruption of Gβγ signaling inhibits the progression of heart failure. Circ. Res. 107, 532–539 (2010).
Kamal, F. A. et al. Simultaneous adrenal and cardiac G-protein–coupled receptor-Gβγ inhibition halts heart failure progression. J. Am. Coll. Cardiol. 63, 2549–2557 (2014).
Travers, J. G. et al. Pharmacological and activated fibroblast targeting of Gβγ-GRK2 after myocardial ischemia attenuates heart failure progression. J. Am. Coll. Cardiol. 70, 958–971 (2017).
Thal, D. M. et al. Paroxetine is a direct inhibitor of g protein-coupled receptor kinase 2 and increases myocardial contractility. ACS Chem. Biol. 7, 1830–1839 (2012).
Guo, S., Carter, R. L., Grisanti, L. A., Koch, W. J. & Tilley, D. G. Impact of paroxetine on proximal β-adrenergic receptor signaling. Cell. Signal. 38, 127–133 (2017).
Schumacher, S. M. et al. Paroxetine-mediated GRK2 inhibition reverses cardiac dysfunction and remodeling after myocardial infarction. Sci. Transl Med. 7, 277ra31 (2015).
Tian, X. et al. Effects of paroxetine-mediated inhibition of GRK2 expression on depression and cardiovascular function in patients with myocardial infarction. Neuropsychiatr. Dis. Treat. 12, 2333–2341 (2016).
Mayer, G. et al. An RNA molecule that specifically inhibits G-protein-coupled receptor kinase 2 in vitro. RNA 14, 524–534 (2008).
Waldschmidt, H. V. et al. Structure-based design of highly selective and potent G protein-coupled receptor kinase 2 inhibitors based on paroxetine. J. Med. Chem. 60, 3052–3069 (2017).
Waldschmidt, H. V. et al. Structure-based design, synthesis, and biological evaluation of highly selective and potent G protein-coupled receptor kinase 2 inhibitors. J. Med. Chem. 59, 3793–3807 (2016).
Homan, K. T., Wu, E., Cannavo, A., Koch, W. J. & Tesmer, J. J. G. Identification and characterization of amlexanox as a G protein-coupled receptor kinase 5 inhibitor. Molecules 19, 16937–16949 (2014).
Park, C. H. et al. A novel role of G protein-coupled receptor kinase 5 in urotensin II-stimulated cellular hypertrophy in H9c2UT cells. Mol. Cell. Biochem. 422, 151–160 (2016).
Cho, S. Y. et al. Design and synthesis of novel 3-(benzo[d]oxazol-2-yl)-5-(1-(piperidin-4-yl)-1H-pyrazol-4-yl)pyridin-2-amine derivatives as selective G-protein-coupled receptor kinase-2 and -5 inhibitors. Bioorg. Med. Chem. Lett. 23, 6711–6716 (2013).
Homan, K. T. et al. Crystal structure of G protein-coupled receptor kinase 5 in complex with a rationally designed inhibitor. J. Biol. Chem. 290, 20649–20659 (2015).
Homan, K. T. et al. Identification and structure-function analysis of subfamily selective G protein-coupled receptor kinase inhibitors. ACS Chem. Biol. 10, 310–319 (2015).
Toma, M. & Starling, R. C. Inotropic therapy for end-stage heart failure patients. Curr. Treat. Opt. Cardiovasc. Med. 12, 409–419 (2010).
Eckhart, A. D. et al. Inhibition of βARK1 restores impaired biochemical β-adrenergic receptor responsiveness but does not rescue CREBA133 induced cardiomyopathy. J. Mol. Cell. Cardiol. 34, 669–677 (2002).
Bauer, R. et al. Various effects of AAV9-mediated βARKct gene therapy on the heart in dystrophin-deficient (mdx) mice and δ-sarcoglycan-deficient (Sgcd-/-) mice. Neuromuscul. Disord. 29, 231–241 (2018).
Jafferjee, M. et al. GRK2 up-regulation creates a positive feedback loop for catecholamine production in chromaffin cells. Mol. Endocrinol. 30, 372–381 (2016).
Lymperopoulos, A., Rengo, G., Zincarelli, C., Soltys, S. & Koch, W. J. Modulation of adrenal catecholamine secretion by in vivo gene transfer and manipulation of G protein-coupled receptor kinase-2 activity. Mol. Ther. 16, 302–307 (2008).
Cannavo, A. et al. GRK2 regulates α2-adrenergic receptor-dependent catecholamine release in human adrenal chromaffin cells. J. Am. Coll. Cardiol. 69, 1515–1517 (2017).
Rengo, G. et al. Adrenal GRK2 lowering is an underlying mechanism for the beneficial sympathetic effects of exercise training in heart failure. Am. J. Physiol. Heart Circ. Physiol. 298, H2032–H2038 (2010).
Lymperopoulos, A. et al. Reduction of sympathetic activity via adrenal-targeted GRK2 gene deletion attenuates heart failure progression and improves cardiac function after myocardial infarction. J. Biol. Chem. 285, 16378–16386 (2010).
Lymperopoulos, A., Rengo, G., Funakoshi, H., Eckhart, A. D. & Koch, W. J. Adrenal GRK2 upregulation mediates sympathetic overdrive in heart failure. Nat. Med. 13, 315–323 (2007).
Liu, P. P. Cardiorenal syndrome in heart failure: a cardiologist’s perspective. Can. J. Cardiol. 24, 25B–29B (2008).
Polhemus, D. J. et al. Radiofrequency renal denervation protects the ischemic heart via inhibition of GRK2 and increased nitric oxide signaling. Circ. Res. 119, 470–480 (2016).
Kamal, F. A. et al. G protein-coupled receptor-G-protein βγ-subunit signaling mediates renal dysfunction and fibrosis in heart failure. J. Am. Soc. Nephrol. 28, 197–208 (2017).
Liggett, S. B. Pharmacogenomics of β1-adrenergic receptor polymorphisms in heart failure. Heart Fail. Clin. 6, 27–33 (2010).
Dorn, G. W. Adrenergic signaling polymorphisms and their impact on cardiovascular disease. Physiol. Rev. 90, 1013–1062 (2010).
Sofowora, G. G. et al. A common β1-adrenergic receptor polymorphism (Arg389Gly) affects blood pressure response to β-blockade. Clin. Pharmacol. Ther. 73, 366–371 (2003).
Lee, H.-Y. et al. Impact of the β-1 adrenergic receptor polymorphism on tolerability and efficacy of bisoprolol therapy in Korean heart failure patients: association between β adrenergic receptor polymorphism and bisoprolol therapy in heart failure (ABBA) study. Korean J. Intern. Med. 31, 277–287 (2016).
Small, K. M., Brown, K. M., Forbes, S. L. & Liggett, S. B. Polymorphic deletion of three intracellular acidic residues of the α2B-adrenergic receptor decreases G protein-coupled receptor kinase-mediated phosphorylation and desensitization. J. Biol. Chem. 276, 4917–4922 (2001).
Nguyen, K., Kassimatis, T. & Lymperopoulos, A. Impaired desensitization of a human polymorphic α2B-adrenergic receptor variant enhances its sympatho-inhibitory activity in chromaffin cells. Cell Commun. Signal. 9, 5 (2011).
Wang, W. C. H., Mihlbachler, K. A., Bleecker, E. R., Weiss, S. T. & Liggett, S. B. A. Polymorphism of GRK5 alters agonist-promoted desensitization of β2-adrenergic receptors. Pharmacogenet. Genom. 18, 729–732 (2008).
Liggett, S. B. et al. A GRK5 polymorphism that inhibits beta-adrenergic receptor signaling is protective in heart failure. Nat. Med. 14, 510–517 (2008).
Li, Y. et al. Association between polymorphisms of ADRBK1 gene and plasma renin activity in hypertensive patients: a case-control study. Med. Sci. Monit. 22, 2981–2988 (2016).
Author information
Authors and Affiliations
Contributions
J.P. and K.G. researched data and wrote the article. All the authors contributed to reviewing and editing the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Pfleger, J., Gresham, K. & Koch, W.J. G protein-coupled receptor kinases as therapeutic targets in the heart. Nat Rev Cardiol 16, 612–622 (2019). https://doi.org/10.1038/s41569-019-0220-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41569-019-0220-3
This article is cited by
-
Thrombin receptor activating peptide-6 decreases acute graft-versus-host disease through activating GPR15
Leukemia (2024)
-
The mechanism of 25-hydroxycholesterol-mediated suppression of atrial β1-adrenergic responses
Pflügers Archiv - European Journal of Physiology (2024)
-
GRK5 Deficiency in the Hippocampus Leads to Cognitive Impairment via Abnormal Microglial Alterations
Molecular Neurobiology (2023)
-
Pepducin ICL1-9-Mediated β2-Adrenergic Receptor-Dependent Cardiomyocyte Contractility Occurs in a Gi Protein/ROCK/PKD-Sensitive Manner
Cardiovascular Drugs and Therapy (2023)
-
A non-coding GWAS variant impacts anthracycline-induced cardiotoxic phenotypes in human iPSC-derived cardiomyocytes
Nature Communications (2022)