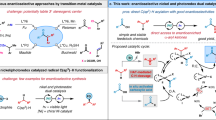
Abstract
Development of multicatalytic approach consisting of two or more mechanistically distinct catalytic steps using a single-site catalyst for rapid and straightforward access of structurally complex molecules under eco-benign conditions has significance in contemporary science. We have developed herein a rhodium-catalysed C–H activation strategy which uses an unprotected anilines and an electron-deficient alkynes to C–C bonded products as a potential intermediate in contrast to the archetypical C–N bonded products with high levels of regioselectivity. This is followed by carbonylation of C–H bond activated intermediate and subsequent annulation into quinolines has been described. This rhodium-catalysed auto-tandem reaction operates under mild, environmentally benign conditions using water as the solvent and CO surrogates as the carbonyl source with the concomitant generation of hydrogen gas. The strategy may facilitate the development of new synthetic protocols for the efficient and sustainable production of chemicals in an atom-economic way from simple, abundant starting materials.
Similar content being viewed by others
Introduction
New chemical approaches that enable rapid and straightforward access to synthetically important molecules by sequential transformations, which enhances the synthetic efficiency is one of the prime focuses in chemical science. Many enzymes are often capable of selectively processing a single substrate through a multiple sequential reactions and are often an inspiration for the development of new synthetic transformations1. Recently, ‘auto-tandem catalysis’, a strategy of association of two or more fundamentally different, that is, mechanistically distinct reactions promoted by a single-site catalyst, all of which occur under the same reaction conditions in a concurrent manner, is very attractive and highly desirable in organic synthesis2,3,4. In such tandem processes, potentially difficult work-up and product associated with the isolation and purification of the intermediates in multistep sequences can be avoided and the generation of chemical waste is also minimized. Development of such auto-tandem catalytic processes and a practically viable catalytic system is very challenging and sporadically mentioned due to the difficulty in the optimization of the separated processes independently5.
More recent, transition-metal-catalysed C–H bond activation approach has provided a direct and an atom-economical synthetic strategy to achieve structurally complex molecules from simple, pre-functionalised substrates and this has implication in developing more efficient synthetic methodologies6,7,8,9,10,11. The pre-installed directing group assisted ortho-C–H activation has been explored by several research groups. In recent times, a rare examples of challenging meta-C–H activation using the template strategy is also reported12,13. However, transition-metal-catalysed direct oxidative coupling between simple arenes and activated alkenes via the non-directed C–H bond activation strategy (electrophilic metallation) is of great interest and reported by research groups of Fujiwara14,15, Glorious16,17, Yu18,19 and others20,21. A gold-catalysed ethynylation of ‘deactivated’ arenes with electron-deficient alkynes is also documented22. Despite using pre-functionalised substrates for the C–H bond activation event, a regio- and stereoselectivity and use of excess arenes for improved reactivity towards transition metals are of potential concern. Hence, selective C–H functionalization of arenes through the non-directed C–H activation strategy is highly challenging and synthetically demanding.
On the other hand, anilines serve as versatile precursors for the synthesis of N-heterocyclic scaffolds. In general, an aniline undergoes the Micheal-type 1,4-conjugate addition reaction with an electron-deficient alkyne and leads to usual carbon–nitrogen bonded product23. Transition-metal-catalysed hydroamination of alkynes (a formal addition of a N–H bond across a carbon–carbon multiple bond) for the synthesis of organo-nitrogen molecules is well documented (Fig. 1a)24,25. However, transition-metal-catalysed C–H bond activation of unprotected or ‘directing group-free’ amines is extremely rare26,27,28,29,30,31. Typically it proceeds via the formation of a five- or a six-membered metallacyclic intermediate and notably, in all such reports, either benzylic amines or ortho-aryl/vinyl anilines were used as the effective directing groups26,27,28,29,30. Recently, transition-metal-catalysed oxidative C–H activation/annulation of anilines with alkynes to indole derivatives using molecular oxygen as an oxidant is also reported (Fig. 1b)32,33. The reaction proceeded through either in situ formation of NH(OAc) or the Michael-type intermediate. However, to the best of our knowledge, there is no example of non-directed catalytic C–H bond activation of unprotected primary anilines34,35.
(a) Transition-metal-catalysed hydroamination of alkynes (a formal addition of a N–H bond across a carbon–carbon multiple bond). (b) Previous work involving transition-metal (TM)-catalysed oxidative C–H bond activation/annulation of anilines with alkynes to indole derivatives (DG, directing group; E, electron-withdrawing group). (c) In this report, reversed reactivity of anilines with alkynes in the rhodium-catalysed C–H activation/carbonylation tandem to quinoline derivatives.
Here we report a rhodium-catalysed C–H bond activation of unprotected (electron-rich primary) anilines with electron-deficient alkynes leading to formation of carbon–carbon bonded products as a potential intermediate in contrast to the archetypical carbon–nitrogen bonded products with high regio control. This is followed by carbonylation of C–H bond activated intermediate using CO surrogates and subsequent annulation reaction into the synthetically versatile 3-substituted quinolines is described (Fig. 1c). This rhodium-catalysed auto-tandem reaction operates under mild, environmentally benign conditions using water as a solvent and paraformaldehyde as a carbonyl source with the concomitant generation of hydrogen gas.
Results
Rhodium-catalysed C-H bond activation of anilines
3,4-(Methylenedioxy)aniline (1a) and methyl propiolate (2a) were selected as a benchmark substrates for the non-directed C–H bond activation strategy. Treatment of 3,4-(methylenedioxy)aniline 1a with methyl propiolate 2a in the presence of catalytic amount of rhodium catalyst resulted in 27% yield of C–H bond activated product, ethyl-3-(6-aminobenzo[d][1,3]dioxol-5-yl)acrylate (3a) with the recovery of unreacted 1a in 49% (Fig. 2a). To gain more insight into this unobserved reactivity of anilines with alkynes, H/D exchange experiment was performed (Fig. 2c). When 3,4-(methylenedioxy)aniline (1a) and p-toluidine (1a′) were separately treated with D2O in the presence of Rh(I)/dppm (1,1-bis(diphenylphosphino)methane) catalytic system, 77% of 1a was selectively deuterated to give [D]1a with high regio control (Supplementary Figs 1 and 2) and whereas no H/D exchange was observed for 1a′. These results imply that the carbon–carbon bond formation might begin with the ortho-C–H activation of 1a and can be proceeded via the electrophilic metalation (that is, non-directed strategy)16,17,18,19,22, and a resonance effect of substituents (+R group) is responsible for the regioselective C–H activation of anilines.
C-H bond activation/carbonylation tandem
Recently, Gulías et al.36 reported a rhodium-catalysed [5+1] cycloaddition of ortho-vinylphenols with carbon monoxide to lead a coumarin derivatives. Inspired by this result, we were encouraged to explore the possibility of utilization of the carbon–carbon bonded product (ortho-vinylanilines) for further transformation. Thus, we planned to integrate rhodium-catalysed non-directed C–H activation strategy with the carbonylation reaction in an auto-tandem manner37.
Development and scope
After an extensive evaluation of combination of metal–ligand complexes, carbonyl sources, solvents and temperature (Table 1; Supplementary Tables 1–6), we have optimized the finest reaction conditions for this auto-tandem catalysis. For example, the treatment of 3,4-(methylenedioxy)aniline (1a) with methyl propiolate (2a, 1.1 equiv.) in the presence of catalytic amount of [Rh(cod)Cl]2:dppm (1:10 mol%) in tetrahydrofuran at 100 °C under 3 atm of CO for 12 h cleanly produced 4a in 49% yield with the recovery of the unreacted 1a in 42% (Table 1, entry 1). The reaction operates with double C–H bond activation under mild conditions with the formation of water as the by-product. To our delight, the same reaction was conducted using water as a solvent and yielded 4a in 46% (Table 1, entry 2) with the concomitant generation of hydrogen gas. The liberation of molecular hydrogen was qualitatively analysed by gas chromatography (Supplementary Fig. 96).
Interestingly, when aqueous formaldehyde was used as a carbon monoxide surrogate38,39,40 in our auto-tandem approach, it showed a slight improvement in the product yield (Table 1, entry 3). Remarkably, excellent yields (up to 87%) were obtained when paraformaldehyde was used as a carbonyl source in CH3CN (Table 1, entry 6). Notably, addition of trace amount of water accelerated the reaction41 and smoothly yielded the desired product (Table 1, entries 4–6). These results highlight the importance of water as a reaction medium, which may increase the solubility of paraformaldehyde and thus provide a slow release of CO gas available for the carbonylation reaction. Hence, water was used as an optimal solvent for this one-pot operation to afford a green synthetic protocol to the 3-substituted quinolines42 (1 mol% of [Rh] yielded 3a in 93% and 2.5% mol% of [Rh] yielded 3a in 95%; entries 7–8). However, a three-component reaction of an aniline, an alkyne and an (aromatic) aldehyde to yield 2-substituted quinoline derivatives is a matured transformation in organic synthesis via typical Micheal-type 1,4-conjugate addition42,43. A multicomponent reaction of electron-deficient alkynes with amines and formaldehyde leads to polysubstituted pyrimidine derivatives were reported by Jiang and his co-workers and such type of product was not detected in our system44. It is very important to note that, in our reaction, paraformaldehyde and/or formalin solution were used as a carbonyl source (without using poisonous CO gas) and the reaction operates in an auto-tandem manner. The ‘auto-tandem’ catalysis consisting of a non-directed C–H bond activation of free anilines leads to C–C bonded intermediate, generation of carbon monoxide from CO surrogates, and carbonylation of C–H activated product followed by annulation reaction to yield 3-substituted quinolines with the generation of hydrogen gas. Indeed, there are no reports describing the direct syntheses of biologically important 3-substituted quinoline derivatives from simple, feedstock chemicals and usually accessed in a multistep synthetic procedures45.
With the optimized reaction conditions in hand, we evaluated the substrate scope of the reaction with respect to the arene component and probed the generality of the auto-tandem process by generating a library of 3-substituted quinolines. As depicted in Fig. 3, variety of electron-rich anilines was found to be the good commodity for this auto-tandem reaction. This is attributed to the electronic nature of the substituents (+R effect), and may facilitate the electrophilic metallation step much easier (Fig. 2b). Thus, a tool box of electron-rich anilines (alkoxy, crown-ether type, amide, alkyl, N-allyl and N-propargyl) were reacted smoothly with 2a via C–H bond activation and yielded the C–C coupled product as a potential intermediate (in contrast to the archetypical C–N bonded products) and followed by subsequent carbonylation using CO surrogates and annulation reaction to the 3-substituted quinolines in good to excellent yields (up to 95% isolated yield). The reaction is highly regio- and chemoselective and water and hydrogen gas is the only by-products. Remarkably, both allylic and propargylic groups were well tolerated under our reaction conditions. Alkyl substituted aniline (4j) suffered from suppressed reactivity and observed poorer yield (∼6% by 1H NMR). We presume this lower reactivity of aniline to be a consequence of decreased arene electron density: less η2 coordination and more Lewis basic coordination to the metal centre.
Reaction conditions: anilines 1a–j (0.25 mmol), methyl propiolate 2a (0.275 mmol), [Rh(cod)Cl]2 (2.5 mol%), dppm (10 mol%), (HCHO)n (2.5 equiv.) and 250 μl of H2O were heated at 100 °C under closed viol for specified time and depicted yields are isolated yields (yields in parentheses are recovery of the starting material). *Yield based on 1H NMR of crude reaction mixture using toluene as an internal standard (using 1 mol% of the rhodium catalyst).
Subsequently, we have also investigated the substrate scope with regard to the alkynes (Fig. 4). Both carboxyl ester (entries 4k–4m) and keto substituted (4n–4o) terminal alkynes gave expected quinoline derivatives in excellent yields (up to 90% isolated yields). To our delight, dialkyl dicarboxylates smoothly underwent intermolecular annulation with aniline (1a) and paraformaldehyde as a carbonyl source in water leading to the corresponding 3,4-disubstitutedquinoline derivatives (4p–4q) in good yields. Thus various electron-deficient alkynes including internal and terminal alkynylesters, linear and branched alkynylesters, and aryl ketoalkynes were shown good reactivity and yielded the desired 3- and 3,4-substituted quinolines in good to excellent yields with high regio- and chemioselectivity under mild, environmentally benign conditions with the liberation of molecular hydrogen. Importantly, in the reaction of 3,4-(methylenedioxy)aniline (1a) with dimethyl acetylenedicarboxylate under standard reaction conditions with a shortened reaction time (6 h) yielded a mixture of the intermediate 3c (15%) and the desired product 4p with the yield of 13% (Fig. 5). This result evidently proved that the auto-tandem approach proceeds through the C–C bonded intermediate.
Reaction conditions: 0.25 mmol of 3,4-(methylenedioxy)aniline (1a) or 3,4-domethoxyaniline (1m), alkynes (0.275 mmol), [Rh(cod)Cl]2 (2.5 mol%), dppm (10 mol%), (HCHO)n (2.5 equiv.) and 250 μl of H2O were heated at 100 °C under closed viol for specified time and depicted yields are isolated yields (yields in parentheses are recovery of the starting material).
We have also designed a three-step synthetic protocol for 6,7-dihydroxyquinoline-3-carboxamide (8), a tyrosine kinase inhibitors (Fig. 6); which resulted in a 54% (overall) yield using our stragety45.
Carbonylation of ortho-vinylanilines using CO surrogates
In addition to the one-pot operation, we speculated on the possibility where conditions could be developed to convert various ortho-vinylanilines to the corresponding quinoline derivatives via sequential carbonylation and intramolecular imination reactions using CO surrogates as the carbonyl source (Fig. 7). However, performing carbonylation reactions both in industry and academia without the use of carbon monoxide (gasesous form, highly toxic, flammable and need of special high pressure equipment) is highly desired39,40,46. As described previously, we were hopeful with the Rh(I)/dppm catalytic system and CO surrogates in water as a carbonyl source to accomplish the carbonylation reaction of ortho-vinylanilines. This is followed by the trapping of aldehydic intermediate by the amine counterpart may lead to 3,4-disubstituted quinolines in one-pot operation. Alper et al.47 reported Pd-catalysed oxidative cyclocarbonylation of ortho-vinylanilines to 2(1H)-quinolinones. Indeed, as far as we know, carbonylation of ortho-vinylanilines using CO surrogates has never been reported. Thus, treatment of (E)-2-(1,2-diphenylvinyl)-5-methoxyaniline, 5a (0.25 mmol) with paraformaldehyde (0.75 mmol) at 100 °C for 24 h with a catalytic amount of [Rh(cod)Cl]2 (2.5 mol%) and dppm (10 mol%), using water as the solvent, resulted 7-methoxy-3,4-diphenylquinoline in 82% isolated yield (6a) with the concomitant generation of hydrogen gas. Notably, the same reaction under 3 atm pressure of carbon monoxide yielded 6a in 16% (water as the only by-product). The reaction is general and a variety of ortho-vinylanilines were compatible with this transformation. Thus, electron-donating groups proceeded smoothly to provide the corresponding carboannulated products 6a–6b (up to 86% isolated yield), wherein electron-withdrawing groups (p-NO2 and m-CF3) were found to be ineffective under standard conditions. This may be attributed to more Lewis basic coordination to the rhodium centre. However, it is noteworthy that halide substituents (5e–5f) were well tolerated and yielded the desired products 6e–6f (80% of 6e and 79% of 6f, respectively), as this is advantageous for further synthetic elaborations with transition-metal catalysis thereby broadening the diversity of the products.
Reaction conditions: ortho-vinylanilines 5a–g (0.25 mmol), [Rh(cod)Cl]2 (2.5 mol%), dppm. (10 mol%), (HCHO)n (3 equiv.) and 250 μl of H2O were heated at 100 °C under closed viol for specified time and the yields in parenthesis are recovery of unreacted starting material. *Yields based on 1H NMR of the reaction mixture (using 1 mol% of the rhodium catalyst).
Mechanistic investigation
To gain insight into the reaction mechanism, a series of control experiments, and deuterium-labelling studies were performed (Fig. 8).
(a) Irreversibility of regioselective C–H bond activation of 1a. (b) Isolation of intermediates (C–C cross-coupled product) followed by carbonylation reaction. (c) Reaction of 1c with methyl propiolate (2a) in the absence of CO source. (d) Rhodium-catalysed auto-tandem reaction with labelled compounds. (e) Rhodium-catalysed CO formation from paraformaldehyde. (f) Kinetic isotopic experiments. (Yields in parentheses are isolated yields).
Deuterium-labelling studies clearly confirmed the ortho-C(sp2)–H bond cleavage of the aniline is irreversible. Thus, the reaction of 1a with 2c using the standard reaction conditions in D2O was stopped before completion (3 h). Compounds 1a and 4p were isolated and their deuterium content was analysed by 1H NMR. With both recovered compounds, no deuterium incorporation was observed suggesting that electrophilic metalation is irreversible in presence of 2c. However, it was observed that the cyclometalation of 1a is reversible in the absence of 2c (Fig. 2c). Under standard reaction conditions, the C–C bonded intermediate methyl-3-(6-aminobenzo[d][1,3]dioxol-5-yl)acrylate (3a) was isolated in 27% from the reaction of 3,4-(methylenedioxy)aniline (1a) with methyl propiolate 2a (Fig. 8b). Further, treatment of methyl-3-(6-aminobenzo[d][1,3]dioxol-5-yl)acrylate (3a) with a catalytic amount of [Rh] under 3 atm of carbon monoxide cleanly produced 4a in 46% isolated yield. Notably, reaction of 3-methoxyaniline (1c) and methyl propiolate 2a with a catalytic amount of [Rh] in the absence of carbonyl source with the prolonged time yielded, methyl 7-methoxy-2-(2-methoxy-2-oxoethyl)quinoline-3-carboxylate (8). This result strongly support that this auto-tandem catalysis is proceeding via the C–C bond formation (Fig. 8c; Supplementary Fig. 98). On the basis of the experimental details, other plausible pathways such as reaction via formation of –NHCHO, Michael addition intermediate, imine intermediate and [3,3] rearrangement have been discarded completely (Supplementary Methods). To understand the carbonylation process involving CO surrogates several labelling experiments were performed using 13C-labelled paraformaldehyde. Indeed, labelling experiments unambiguously illustrated the formation of carbon monoxide from paraformaldehyde and therefore, utilized as a carbonyl source. Thus, under standard reaction conditions, using 13C-paraformaldehyde as a carbonyl source both ortho-vinylaniline ((E)-2-(1,2-diphenylvinyl)-5-methoxyaniline (5a) and methyl-3-(6-aminobenzo[d][1,3]dioxol-5-yl)acrylate (3a) (in situ formation by the reaction of 3,4-(methylenedioxy)aniline, 1a with methyl propiolate, 2a) predominantly yielded the corresponding 13C-labelled quinoline derivatives (Fig. 8d). In addition, the reactivity of 13C-paraformaldehyde in the presence of the rhodium catalyst under standard condition was investigated (Fig. 8e). Indeed, after 8 h, the formation of 13C-labelled carbon monoxide and dihydrogen was qualitatively analysed on gas chromatography (GC) and GC–mass spectrometry (GC–MS) studies, and thus demonstrating the slow release of CO. However, in the presence of water as a reaction medium 13CO2 and H2 were detected on GC and GC–MS (Supplementary Figs 95 and 96). This is probably due to water–gas shift reaction48.
On the basis of the above experimental findings and literature the precedent, we have proposed a plausible mechanism for the auto-tandem catalysis consisting of three mechanically distinct reactions, such as C–C cross-coupling via non-directed C–H activation, CO generation from CO surrogates and sequential carbonylation followed by annulation reaction catalysed by the single-site rhodium catalyst (Fig. 9). The electrophilic metalation of aniline can provide the ortho-C–H bond activated product A (Supplementary Fig. 97). Interaction of an alkyne with intermediate A can lead to intermediate B and subsequently alkyne insertion can lead to intermediate C. However, intermediate C can undergo proto-demetalation to provide the ortho-vinylaniline (II) in the absence of carbonyl source. Reaction of intermediate C with carbon monoxide (in situ generated from CO surrogates) can lead to E via intermediate D. This is followed by the proto-demetalation of intermediate E and intramolecular imination to provide the expected quinoline moiety III.
Discussion
Reversed reactivity of an aniline with an electron-deficient alkyne in the rhodium catalysis to lead to the formation of C–C coupled product as a potential intermediate in contrast to the archetypical C–N bonded products is disclosed. The product from this complementary approach (non-directed C–H activation strategy of free anilines) is integrated with sequential carbonylation and annulation reaction in an auto-tandem manner to lead to the 3-substituted quinolines with high rigio- and chemioselectivity is reported. This auto-tandem reaction operates under mild, environmentally benign conditions using water as a solvent and CO surrogates as a carbonyl source with extremely good atom-efficiency (only H2 and H2O as by-products). Beyond the unique reactivity of this strategy, we anticipate that this auto-tandem catalysis will open a new avenue in the designing of new catalytic process for an efficient and a sustainable production of valuable targeted scaffolds from simple, feedstock chemicals in an atom-economic way.
Methods
General procedure for this rhodium-catalysed auto-tandem reaction
To a 10-ml clean, oven-dried screw cap reaction tube was added [Rh(cod)Cl]2 (2.5 mol%), dppm 1,1-bis(diphenylphosphino)methane) (10 mol%), an aniline (0.25 mmol), CO surrogate (paraformaldehyde) (0.75 mmol), an alkyne (0.275 mmol) and water (250 μl) under argon atm. The reaction mixture was kept for heating at 100 °C for a specified time. After cooling to room temperature, reaction mixture was diluted with water (6 ml) and extracted with ethyl acetate (3 × 5 ml). The resultant organic layer was dried over anhydrous Na2SO4 and the solvent was evaporated under reduced pressure. The crude mixture was purified by silica gel column chromatography (230–400 mesh size) using petroleum-ether/ethyl acetate as an eluting system.
General procedure for rhodium-catalysed carbonylation of ortho-vinylanilines
To a 10-ml clean, oven-dried screw cap reaction tube was added [Rh(cod)Cl]2 (2.5 mol%), dppm (10 mol%), ortho-vinylaniline (5a–g) (0.25 mmol), paraformaldehyde (0.75 mmol, 2.5 equiv.) and water (250 μl) under argon atm. The reaction mixture was heated at 100 °C for specified hours. After cooling at room temperature, reaction mixture was diluted with water (6 ml) and extracted with ethyl acetate (3 × 5 ml). The combined organic layer was dried over anhydrous Na2SO4 and the solvent was evaporated. The crude product was purified by silica gel column chromatography (230–400 mesh size) using petroleum-ether/ethyl acetate as an eluent. All new compounds were fully characterized. For NMR and high-resolution mass spectrometry analysis in this article, see Supplementary Figs 3–93. General information, materials, synthesis and characterization of compounds in this article (D[1a], 3a, 3c, 4a–4q, 6a–6g and 7–9), and experimental part for mechanistic investigations see Supplementary Methods.
Additional information
How to cite this article: Midya, S. P. et al. Reversed reactivity of anilines with alkynes in the rhodium-catalysed C–H activation/carbonylation tandem. Nat. Commun. 6:8591 doi: 10.1038/ncomms9591 (2015).
References
Bornscheuer, U. T. & Kazlauskas, R. J. Catalytic promiscuity in biocatalysis: using old enzymes to form new bonds and follow new pathways. Angew. Chem. Int. Ed. 43, 6032–6040 (2004) .
Shindoh, N., Takemoto, Y. & Takasu, K. Auto-tandem catalysis: A single catalyst activating mechanistically distinct reactions in a single reactor. Chem. Euro. J. 15, 12168–12179 (2009) .
Fogg, D. E. & dos Santos, E. N. Tandem catalysis: a taxonomy and illustrative review. Coord. Chem. Rev. 248, 2365–2379 (2004) .
Nitin, T. P., Valmik, S. S. & Balakrishna, G. A one-pot catalysis: the strategic classification with some recent examples. Org. Biomol. Chem. 10, 211–224 (2012) .
Li, L. & Herzon, S. B. Temporal separation of catalytic activities allows anti-Markovnikov reductive functionalization of terminal alkynes. Nat. Chem. 6, 22–27 (2014) .
Wencel-Delord, J. & Glorius, F. C-H bond activation enables the rapid construction and late-stage diversification of functional molecules. Nat. Chem. 5, 369–375 (2013) .
McMurray, L., O’Hara, F. & Gaunt, M. J. Recent developments in natural product synthesis using metal-catalysed C-H bond functionalisation. Chem. Soc. Rev. 40, 1885–1898 (2011) .
Godula, K. & Sames, D. C-H bond functionalization in complex organic synthesis. Science 312, 67–72 (2006) .
Yamaguchi, J., Yamaguchi, A. D. & Itami, K. C-H bond functionalization: Emerging synthetic tools for natural products and pharmaceuticals. Angew. Chem. Int. Ed. 51, 8960–9009 (2012) .
Gutekunst, W. & Baran, P. S. C-H functionalization logic in total synthesis. Chem. Soc. Rev. 40, 1976–1991 (2011) .
Liu, Y.-J. et al. Overcoming the limitations of directed C-H functionalizations of heterocycles. Nature 515, 389–393 (2014) .
Tang, R.-Y., Li, G. & Yu, J.-Q. Conformation-induced remote meta-C-H activation of amines. Nature 507, 215–220 (2014) .
Wang, X.-C. et al. Ligand-enabled meta-C-H activation using a transient mediator. Nature 519, 334–338 (2015) .
Jia, C. et al. Efficient activation of aromatic C-H bonds for addition to C-C multiple bonds. Science 287, 1992–1995 (2000) .
Jia, C., Kitamura, T. & Fujiwara, Y. Catalytic functionalization of arenes and alkanes via C-H bond activation. Acc. Chem. Res. 34, 633–639 (2001) .
Kuhl, N., Hopkinson, M. N., Wencel-Delord, J. & Glorius, F. Beyond directing groups: Transition-metal-catalyzed C-H activation of simple arenes. Angew. Chem. Int. Ed. 51, 10236–10254 (2012) .
Patureau, F. W., Nimphius, C. & Glorius, F. Rh catalyzed C-H activation and oxidative olefination without chelate assistance: On the reactivity of bromoarenes. Org. Lett. 13, 6346–6349 (2011) .
Zhang, Y.-H., Shi, B.-F. & Yu, J.-Q. Pd(II)-catalyzed olefination of electron-deficient arenes using 2,6-dialkylpyridine ligands. J. Am. Chem. Soc. 131, 5072–5074 (2009) .
Vora, H. U., Silvestri, A. P., Engelin, C. J. & Yu, J.-Q. Rhodium(II)-catalyzed nondirected oxidative alkenylation of arenes: Arene loading at one equivalent. Angew. Chem. Int. Ed. 53, 2683–2686 (2014) .
Weissman, H., Song, X. & Milstein, D. Ru-catalyzed oxidative coupling of arenes with olefins using O2 . J. Am. Chem. Soc. 123, 337–338 (2001) .
Kubota, A., Emmert, M. H. & Sanford, M. S. Pyridine ligands as promoters in PdII/0-catalyzed C-H olefination reactions. Org. Lett. 14, 1760–1763 (2012) .
Haro, T. & Nevado, C. Gold-catalyzed ethynylation of arenes. J. Am. Chem. Soc. 132, 1512–1513 (2010) .
Perlmutter, P. Conjugate Addition Reaction In Organic Synthesis Pergamon (1992) .
Severin, R. & Doye, S. The catalytic hydroamination of alkynes. Chem. Soc. Rev. 36, 1407–1420 (2007) .
Müller, T. E. & Beller, M. Metal-initiated amination of alkenes and alkynes. Chem. Rev. 98, 675–703 (1998) .
Yi, C. S. & Yun, S. Y. Scope and mechanistic study of the ruthenium-catalyzed ortho-C-H bond activation and cyclization reactions of arylamines with terminal alkynes. J. Am. Chem. Soc. 127, 17000–17006 (2005) .
Morimoto, K. et al. Synthesis of fluorene derivatives through rhodium-catalyzed dehydrogenative cyclization. Angew. Chem. Int. Ed. 51, 5359–5362 (2012) .
He, H., Liu, W.-B., Dai, L.-X. & You, S.-L. Ir-Catalyzed cross-coupling of styrene derivatives with allylic carbonates: Free amine assisted vinyl C-H bond activation. J. Am. Chem. Soc. 131, 8346–8347 (2009) .
Liang, Z., Feng, R., Yin, H. & Zhang, Y. Free-amine directed arylation of biaryl-2-amines with aryl iodides by palladium catalysis. Org. Lett. 15, 4544–4547 (2013) .
Liang, D., Hu, Z., Peng, J., Huang, J. & Zhu, Q. Synthesis of phenanthridinones via palladium-catalyzed C(sp2)-H aminocarbonylation of unprotected o-arylanilines. Chem. Commun. 49, 173–175 (2013) .
McNally, A., Haffemayer, B., Collins, B. S. L. & Gaunt, M. J. Palladium-catalysed C-H activation of aliphatic amines to give strained nitrogen heterocycles. Nature 510, 129–133 (2014) .
Shi, Z. et al. Indoles from simple anilines and alkynes: Palladium-catalyzed C-H activation using dioxygen as the oxidant. Angew. Chem. Int. Ed. 48, 4572–4576 (2009) .
Zhang, G., Yu, H., Qin, G. & Huang, H. Rh-catalyzed oxidative C-H activation/annulation: converting anilines to indoles using molecular oxygen as the sole oxidant. Chem. Commun. 50, 4331–4334 (2014) .
Louillat, M.-L., Biafora, A., Legros, F. & Patureau, F. W. Ruthenium-catalyzed cross-dehydrogenative ortho-N-carbazolation of diarylamines: Versatile access to unsymmetrical diamines. Angew. Chem. Int. Ed. 53, 3505–3509 (2014) .
Sharma, U., Kancherla, R., Naveen, T., Agasti, S. & Maiti, D. Palladium-catalyzed annulation of diarylamines with olefins through C-H activation: Direct access to N-arylindoles. Angew. Chem. Int. Ed. 53, 11895–11899 (2014) .
Seoane, A., Casanova, N., Quiñones, N., Mascareñas, J. L. & Gulías, M. Straightforward assembly of benzoxepines by means of a rhodium(III)-catalyzed C-H functionalization of o-vinylphenols. J. Am. Chem. Soc. 136, 834–837 (2013) .
Amii, H., Kishikawa, Y. & Uneyama, K. Rh(I)-catalyzed coupling cyclization of N-Aryl trifluoroacetimidoyl chlorides with alkynes: One-pot synthesis of fluorinated quinolines. Org. Lett. 3, 1109–1112 (2001) .
Wu, L., Liu, Q., Jackstell, R. & Beller, M. Carbonylations of alkenes with CO surrogates. Angew. Chem. Int. Ed. 53, 6310–6320 (2014) .
Sam, B., Breit, B. & Krische, M. J. Paraformaldehyde and methanol as C1 feedstocks in metal-catalyzed C-C couplings of π-unsaturated reactants: Beyond hydroformylation. Angew. Chem. Int. Ed. 54, 3267–3274 (2015) .
Morimoto, T. & Kakiuchi, K. Evolution of carbonylation catalysis: no need for carbon monoxide. Angew. Chem. Int. Ed. 43, 5580–5588 (2004) .
Dixneuf, P. H. & Cadierno, V. Metal-Catalyzed Reactions in Water Wiley-VCH Verlag GmbH & Co. KGaA (2013) .
Kulkarni, A. & Török, B. Microwave-assisted multicomponent domino cyclization-aromatization: an efficient approach for the synthesis of substituted quinolines. Green Chem. 12, 875–878 (2010) .
Prajapati, S. M., Patel, K. D., Vekariya, R. H., Panchala, S. N. & Patel, H. D. Recent advances in the synthesis of quinolines: a review. RSC Adv. 4, 24463–24476 (2014) .
Cao, H. et al. Development, scope and mechanisms of multicomponent reactions of asymmetric electron-deficient alkynes with amines and formaldehyde. Chem. Euro. J. 14, 11623–11633 (2008) .
Burke, T. R. Jr. et al. Bicyclic compounds as ring-constrained inhibitors of protein-tyrosine kinase p56lck. J. Med. Chem. 36, 425–432 (1993) .
Liu, Q. et al. Regioselective Pd-catalyzed methoxycarbonylation of alkenes using both paraformaldehyde and methanol as CO surrogates. Angew. Chem. Int. Ed. 54, 4493–4497 (2015) .
Ferguson, J., Zeng, F., Alwis, N. & Alper, H. Synthesis of 2(1H)-quinolinones via Pd-catalyzed oxidative cyclocarbonylation of 2-vinylanilines. Org. Lett. 15, 1998–2001 (2013) .
Newsome, D. S. The water-gas shift reaction. Catal. Rev. Sci. Eng. 21, 275–318 (1980) .
Acknowledgements
This research was supported by the SERB (SB/FT/CS-065/2013) and CSIR-NCL (MLP028726). S.P.M., M.K.S. and V.G.L. thank to CSIR for fellowship. E.B. thanks to Drs. C. S. Gopinath, C. V. Ramana, B. L. V. Prasad, H. V. Thulasiram, Amitava Das, C. P. Vinod, Nitin Patil and M. Chakraborty for their constant support. We also thank Dr S. P. Borikar for GC–MS analysis (Organic Chemistry Division, NCL). Central NMR facility, NCL is greatly acknowledged.
Author information
Authors and Affiliations
Contributions
S.P.M. contributed to catalytic experiments, starting material synthesis, mechanistic studies and manuscript writing. M.K.S. and V.G.L. contributed to starting material synthesis. P.R.R. contributed to NMR studies. E.B. contributed to design and direction of the project and manuscript writing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Figures 1-98, Supplementary Tables 1-6, Supplementary Methods and Supplementary References (PDF 3952 kb)
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Midya, S., Sahoo, M., Landge, V. et al. Reversed reactivity of anilines with alkynes in the rhodium-catalysed C–H activation/carbonylation tandem. Nat Commun 6, 8591 (2015). https://doi.org/10.1038/ncomms9591
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/ncomms9591
This article is cited by
-
Nickel-catalyzed direct synthesis of dialkoxymethane ethers
Journal of Chemical Sciences (2017)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.