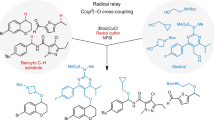
Abstract
The past decade has witnessed significant advances in C–H bond functionalizations with the discovery of new mechanisms. Non-precious transition-metal-catalysed radical oxidative coupling for C(sp3)–H bond transformations is an appealing strategy for C–C bond formations. The radical oxidative C(sp3)–H/C(sp3)–H cross-coupling reactions of α-C(sp3)–H bonds of amines with free radicals represent a conceptual and practical challenge. We herein develop the coordinating activation strategy to illustrate the nickel-catalysed radical oxidative cross-coupling between C(sp3)–H bonds and (hetero)arylmethyl free radicals. The protocol can tolerate a rich variety of α-amino acids and (hetero)arylmethanes as well as arylmethylenes and arylmethines, affording a large library of α-tertiary and α-quaternary β-aromatic α-amino acids. This process also features low-cost metal catalyst, readily handled and easily removable coordinating group, synthetic simplicity and gram-scale production, which would enable the potential for economical production at commercial scale in the future.
Similar content being viewed by others
Introduction
Transition-metal-catalysed functionalization of C(sp3)–H bonds is an appealing, yet challenging strategy for C(sp3)–C bond formations1,2,3,4,5,6,7,8,9,10. This attractive tactics in the rapid synthesis and late-stage diversification of organic scaffolds ranging from functional organic molecules to natural products and pharmaceutical candidates has drawn extensive attention11,12,13. Aromatic α-amino acids, especially β-arylalanine derivatives, are common structural units frequently found in natural products, pharmaceuticals, biologically active molecules, glycopeptides and proteins, and are also important synthetically useful compounds (Fig. 1a)14,15. Although a number of strategies have been used for the preparation of non-proteinogenic amino acids, traditional methodologies typically encounter highly hazardous reagents, tedious multiple synthesis and purification, rigorous reaction conditions such as very strong bases that may be intolerant of sensitive functionalities, and/or limited substrate scope16. Thus, there remains a great need for a general, diversity-orientated, economically attractive and environmentally benign protocol to prepare aromatic α-amino acids. In recent years, the chelation-directed palladium-catalysed remote methyl and methene C(sp3)–H arylation of amino-acid derivatives with aryl haildes and arylsilane reagents through C–H activation has illustrated the potential to synthesize diverse collections of non-proteinogenic β-aromatic α-amino acids (Fig. 1b, left)17,18,19,20,21,22,23,24,25,26. Despite significant progress, one of the main disadvantages of these strategies is the use of noble metal catalysts typically in combination with stoichiometric silver salts as the additive, which would diminish the appeal of practical application. Given that both α-amino acids and benzylic units27,28,29,30,31,32 are among the most widespread scaffolds, we surmised that the oxidative benzylation of α-C(sp3)–H bonds of α-amino acids with benzylic C(sp3)–H bonds would be a distinct, straightforward gateway to long-range unnatural β-arylalanine derivatives involving both α-tertiary and α-quaternary carbon centers (Fig. 1b, right).
(a) Selected medicinal and natural molecules containing β-aromatic α-amino-acid derivatives. (b), Transition-metal-catalysed two distinct gateways to β-aromatic α-amino-acid derivatives. Left, in previous work, the synthesis of β-arylalanine blocks through the palladium-catalysed chelation-directed β-C(sp3)–H activation strategy. Right, in the current work, the preparation of β-arylalanine blocks through oxidative C(sp3)–H/C(sp3)–H cross-coupling. (c), Coordinating activation strategies for oxidative C(sp3)–H/C(sp3)–H cross-coupling. Left, in our previous work, iron(III)-catalyzed oxidative C(sp3)–H/C(sp3)–H cross-coupling of α-tertiary α-amino acids with various nucleophiles to deliver α-quaternary α-amino acids derivatives. Right, in this report, the oxidative C(sp3)–H/C(sp3)–H cross-coupling of α-amino acids with a radical species through coordinating activation.
In recent years, the oxidative C(sp3)–H/C(sp3)–H cross-coupling reactions of α-C(sp3)–H bonds of amines with a variety of nucleophilic C(sp3)–H centers typically via aza-Henry-type or Mannich-type reactions have been developed as an appealing and powerful strategy for the synthesis of amine derivatives6,7,8,9. Despite substantial progress, these reactions often exhibit a significantly narrow substrate scope and are very sensitive to steric hindrance to the approach of the imine (or imine ion) intermediate, which puts constraints on the construction of the α-quaternary center. On the other hand, the scopes of nucleophiles are typically restricted to α-C(sp3)–H of electron-withdrawing groups, organometallic reagents and C(sp2)–H of electron-rich (hetero)aromatics, in which the functionalities are not commonly found in natural products and pharmaceutical agents. Thus, it is highly desirable to develop an innovative strategy to overcome these daunting restrictions to enrich structural diversity of coupled products, albeit it is thought as one of the most challenging tasks in the organic synthesis community. In principle, it should be possible to perform the cross-coupling between α-C(sp3)–H bonds of α-amino acids and arylmethyl free radicals, easily generated from arylmethyl C(sp3)–H bond by peroxide via single-electron transfer (SET), to produce β-arylalanine derivatives (Fig. 1c, right). However, although a variety of nucleophiles have been well explored in recent years, expanding this chemistry from nucleophiles to free radicals as the coupling partner represents a conceptual and practical challenge.
The development of conceptually new strategies for the formation of new C–C or C–X bonds has been a crucial topic in synthetic organic chemistry community33. Recently, we disclosed the iron(III)-catalysed oxidative C(sp3)–H/C(sp3)–H cross-coupling of α-tertiary α-amino acids with various nucleophiles to deliver α-quaternary α-amino-acid derivatives by tethering the coordinating 2-pyridinecarbonyl group at the nitrogen atom of α-tertiary α-amino acids, in which the coordination of a metal center towards the coordinating group could promote the formation of a ketimine intermediate and subsequential nucleophilic attack (Fig. 1c, left)34. Inspired by this work, we presumed that the coordination activation of a suitable coordinating group could enable α-C(sp3)–H bond of amine substrate for directly trapping a radical species35 and further stabilize the resulting radical intermediate for a SET (Fig. 1c, right)36. In this context, activated α-amino-acid substrate could catch the in situ generated benzyl free radical to produce β-arylalanine derivative. Here we would seek to address the discovery, development, and solution to the significant challenge. In this work, using the coordinating activation strategy, we demonstrate a practical gateway to β-aromatic α-amino-acid derivatives through the nickel-catalysed radical oxidative C(sp3)–H/C(sp3)–H cross-coupling between α-amino acids and (hetero)arylmethanes as well as arylmethylenes and arylmethines.
Results
Optimization of the reaction conditions
To illustrate the feasibility of the coordinating activation strategy, the coordinating 2-pyridinecarbonyl group was first assembled on phenylalanine ethyl ester. The cross-coupling reaction of the resulting ethyl 3-phenyl-2-(picolinamido)propanoate 1a with toluene 2a was then conducted as a model reaction (Table 1). In the absence of any metal catalyst, only a trace amount of ethyl 2-benzyl-3-phenyl-2-(picolinamido)propanoate 3a was detected (Table 1, entry 1). The use of commonly used iron, copper and ruthenium salts as the catalyst was also deeply disappointed (Table 1, entry 2). Fortunately, Ni(II) salts displayed the catalytic activity and 20 mol% of Ni(acac)2 gave the desired product 3a in 83% yield in the presence of DTBP (di-tert-butyl peroxide, 4.0 equiv) at 140 °C under N2 for 18 h (Table 1, entry 7, for detailed optimization see Supplementary Table 1). Subsequently, a series of N-protecting groups (PGs) on phenylalanine ethyl ester were examined under the optimized conditions (Table 2). It was found that 2-quinolinecarbonyl, 1-isoquinolinecarbonyl, 2-pyrimidinecarbonyl, and 2-imidazolecarbonyl groups were also effective auxiliaries to promote the coupling reaction, but resulted in diminished yields. Other N-protecting groups such as benzoyl, 2-benzo[d]thiazolecarbonyl, 3-pyridinecarbonyl, 4-pyridinecarbonyl, phenyl and acetyl were incapable of promoting the coupling.
Scope of benzylic substrates
With the optimal system in hand, we examined the scope of benzylic substrates with ethyl 3-phenyl-2-(picolinamido)propanoate 1a as illustrated in Table 3. To our delight, the current catalytic system was suitable for a wide range of arylmethanes (Table 3, 3a–3k). No matter whether the benzene ring of toluene is substituted with either electron-donating, electron-withdrawing or sterically hindered group, all of them delivered the desired products in synthetically useful yields. This protocol was tolerant of synthetically valuable functional groups on the phenyl moiety (for example, chloro, bromo, alkoxy and phenoxy groups), which could allow an opportunity for further transformations. The oxidative process also occurred well with a heteroarylmethane coupling partner, affording the targeted compound 3l in 61% yield. In addition, both 2-methylnaphthalene and 1-methylnaphthalene smoothly underwent the coupling process to yield the desired products 3m and 3n. In addition to (hetero)arylmethanes, we were also pleased to find that arylmethylene groups could also smoothly afford β,β-disubstituted β′-aryl α-amino-acid derivatives (Table 3, 3o–3t). For example, the oxdative cross-coupling reaction of diarylmethylenes with 1a afforded β,β′-diaryl α-amino acids 3o–3p. Ethylbenzene, butylbenzene and 1,2,3,4-tetrahydronaphthalene formed the coupled products in good yields with dr values of ∼1:1 (Table 3, 3q–3s). Gratifyingly, (methoxymethyl)benzene could also couple with 1a to afford β-methoxyl β,β′-diaryl α-amino-acid derivative 3t. Besides (hetero)arylmethanes and arylmethylenes, arylmethine groups could couple with 1a and exhibited good compatibility (Table 3, 3u–3v). α-Amino-acid amide could also smoothly react with toluene to afford the desired product (Table 3, 3w). More importantly, the synthesis of α-quaternary β,β′-diaryl α-amino acid 3a was conducted without problems on an ∼1.5-g scale in the presence of a lower catalyst loading (10 mol %; 86% yield on 1 mmol scale; 78% yield on 5-mmol scale; for details, see the Supplementary Information), which would enable the potential for economical production at commercial scale.
Scope of α-tertiary α-amino-acid substrates
We next turned our attention to the scope of α-amino-acid substrates (Table 4). To our surprise, the widespread natural and non-proteinogenic α-amino acids did smoothly couple with toluene to produce a greatly widened range of α-quaternary β-aromatic α-amino acids, which clearly exhibited the power of coordinating activation strategy. For example, the reactions of α-alkyl-substituted α-amino-acid substrates (for example, alanine, valine, tertiary leucine, leucine and isoleucine) with toluene afforded the coupled products in good yields (Table 4, 4b–4f). It is worth noting that a variety of α-amino-acid derivatives with other reactive functionalities (for example, aspartic acid, glutamic acid, methionine, benzoylglycine, tyrosine, tryptophane, lysine and serine derivatives) could also react with toluene in satisfactory yields (Table 4, 4g–4p). Moreover, the phenylalanine substrates with either an electron-donating or electron-withdrawing group on the phenyl ring gave the targeted compounds in synthetically useful yields (Table 4, 4l, and 4p–4r). Notably, the proline substrate failed to produce the desired product under the optimal reaction conditions (Table 4, 4s).
Synthesis of α-tertiary β-aromatic α-amino-acid derivatives
Finally, we examined the feasibility of using glycine as a substrate. This simplest amino acid could react with a variety of (hetero)arylmethanes at slightly elevated temperature. The coordinating activation strategy could offer a distinct gateway to obtain a wide range of natural and unnatural α-tertiary β-arylalanine derivatives (Table 5).
Deprotection of the coordinating group
The coordinating group could be easily removed in good yields by treatment with BF3·Et2O in enthanol at 140 °C for 32 h (Fig. 2). Thus, our protocol has provided an efficient, practical and reliable strategy for the synthesis of various natural and unnatural α-tertiary and α-quaternary β-aromatic α-amino acids.
Discussion
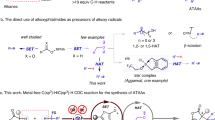
To gain insight into the possible mechanism of this oxidative cross-coupling process, the following competition experiments were performed. First, a radical trapping reagent (for example, 2,2,6,6-tetramethylpiperidine oxide, and 2,6-di-tert-butyl-4-methylphenol) could markedly suppress the coupling reaction of 1a with 2a (Fig. 3a), suggesting that a free radical intermediate was involved in this reaction. Second, kinetic isotope effects (KIE) were investigated with regard to the C(sp3)–H/D bonds for the toluene substrates. A clear KIE value of 5.0 was observed in a 1:1 mixture of toluene 2a and toluene-D8 ([D8]-2a) (Fig. 3b), indicating that the benzylic C(sp3)–H bond breaking might be the rate-limiting step37.
Considering that this type of oxidative C(sp3)–H/C(sp3)–H cross-coupling reactions may involve the α-ketimine ester intermediate, generated from the metal-coordinating amino-acid radical species via an intramolecular SET34, we attempted to synthesize α-ketimine ester 1w′, but all efforts to isolate this compound failed (Fig. 4a). Given that the coupling reaction of N-benzhydrylpicolinamide 1x with mesitylene 2e could afford the desired product 7 in 37% yield under the standard reaction conditions (Fig. 4b), we tried to attain N-(diphenylmethylene)picolinamide 1w, an alternative to α-ketimine ester. However, the reaction of 1w with 2e gave only a trace amount of 7 under the standard conditions (Fig. 4c). These results implied that the oxidative C(sp3)–H/C(sp3)–H cross-coupling reaction developed herein did not involve the direct formation of imine intermediate.
(a), Left, α-ketimine ester. Right, an alternative to α-ketimine ester. (b), The coupling reaction of N-benzhydrylpicolinamide 1x with mesitylene 2e under the standard reaction conditions. (c) The coupling reaction of N-(diphenylmethylene)picolinamide 1w with mesitylene 2e under the standard reaction conditions.
On the basis of the above observations, the plausible catalytic path is proposed in Fig. 5. First, a tert-butoxy radical (tBuO˙) is generated from DTBP with the aid of the low-valent Ni2+ species with concomitant formation of the high-valent Ni3+ species38,39. Subsequently, 2-picolinamido α-amino-acid ester coordinates with the high-valent nickel catalyst to yield the intermediate IM1. The benzylic radical, formed through the C(sp3)–H homolytic cleavage of arylmethane by a tert-butoxy radical, attacks α-carbon of IM1. The resulting radical cation intermediate IM2 (refs 40, 41) undergoes an intramolecular SET by the high valent Ni3+ to give the intermediate IM3. Finally, the release of the low-valent Ni2+ species delivers the targeted product and fulfills a catalytic cycle. The role of the coordination of metal towards the picolinamido group is assumed to involve the following aspects. First, the coordination of Ni3+ toward the picolinamido group can enable the activation of α-C(sp3)–H bond and makes it susceptible to an attack by the benzylic radical35. Second, the coordination enables to stabilize the resulting radical cation intermediate IM2 (ref. 36). Third, an intramolecular SET process is achieved more easily.
In summary, we have developed the coordinating activation strategy to allow the radical oxidative benzylation of an α-C(sp3)–H bond of α-amino acid with a simple benzylic C(sp3)–H bond by utilizing a low-cost and easily available nickel catalyst. In previous reports, the oxidative C(sp3)–H/C(sp3)–H cross-coupling reactions of α-C(sp3)–H bonds of amines with a variety of nucleophilic C(sp3)–H centers typically undergo aza-Henry-type or Mannich-type reactions through imine (or imine ion) intermediate. In the current work, the coordination-activated α-C(sp3)–H bonds of amine substrates directly trap a benzylic radical species rather than the formation of imine intermediate. Considering that the free radical acts as a real coupling partner, the transformation reported herein has further enriched the type of the oxidative cross-coupling reactions. The protocol can tolerate a broad range of α-amino acids and (hetero)arylmethanes as well as arylmethylenes and arylmethines, which would offer a great opportunity to assemble a large library of both α-tertiary and α-quaternary β-aromatic α-amino acids. More importantly, this reaction can proceed well on a larger scale, and the coordinating group can be handled readily and removed easily. We anticipate that this process would enable the potential for economical production at commercial scale in the future.
Methods
General methods
For 1H and 13C nuclear magnetic resonance (NMR) spectra of compounds in this manuscript and details of the synthetic procedures, see Supplementary Figs 2–100 and Supplementary Methods.
General procedure for the synthesis of β-arylalanines
Reaction conditions A. An oven-dried Schlenk tube with a magnetic stir bar was charged with amino-acid derivative 1 (0.25 mmol), arylmethane 2 (1.0 ml) and Ni(acac)2 (12.8 mg, 0.05 mmol) under N2 atmosphere. The reaction solution was stirred at room temperature for several minutes and DTBP (182.6 μl, 1.0 mmol) was then added. The tube was sealed with a teflon-coated cap and the mixture was stirred at 140 °C for 18 h. After being cooled to ambient temperature, the solution was diluted with 20 ml of CH2Cl2, filtered through a celite pad and washed with 10–20 ml of CH2Cl2. The combined organic phases were concentrated and the residue was purified by column chromatography on silica gel to provide the desired product.
Reaction conditions B. An oven-dried Schlenk tube with a magnetic stir bar was charged with amino-acid derivative 1 (0.25 mmol), arylmethane 2 (2.5 mmol), Ni(acac)2 (12.8 mg, 0.05 mmol) and benzene (0.5 ml) under an N2 atmosphere. The reaction solution was stirred at room temperature for several minutes and DTBP (182.6 μl, 1.0 mmol) was then added. The tube was sealed with a teflon-coated cap and the mixture was stirred at 140 °C for 18 h. After being cooled to ambient temperature, the solution was diluted with 20 ml of CH2Cl2, filtered through a celite pad, and washed with 10–20 ml of CH2Cl2. The combined organic phases were concentrated and the residue was purified by column chromatography on silica gel to provide the desired product.
Additional information
How to cite this article: Li, K. et al. Coordinating activation strategy for C(sp3)–H/C(sp3)–H cross-coupling to access β-aromatic α-amino acids. Nat. Commun. 6:8404 doi: 10.1038/ncomms9404 (2015).
References
Bellina, F. & Rossi, R. Transition metal-catalyzed direct arylation of substrates with activated sp3-hybridized C–H bonds and some of their synthetic equivalents with aryl halides and pseudohalides. Chem. Rev. 110, 1082–1146 (2010).
Jazzar, R., Hitce, J., Renaudat, A., Sofack-Kreutzer, J. & Baudoin, O. Functionalization of organic molecules by transition-metal-catalyzed C(sp3)–H activation. Chem. Eur. J. 16, 2654–2672 (2010).
Baudoin, O. Transition metal-catalyzed arylation of unactivated C(sp3)–H bonds. Chem. Soc. Rev. 40, 4902–4911 (2011).
Rouquet, G. & Chatani, N. Catalytic functionalization of C(sp2)–H and C(sp3)–H bonds by using bidentate directing groups. Angew. Chem. Int. Ed. 52, 11726–11743 (2013).
Franzoni, I. & Mazet, C. Recent trends in Pd-catalyzed remote functionalization of carbonyl compounds. Org. Biomol. Chem. 12, 233–241 (2014).
Li, Z., Bohle, D. S. & Li, C.-J. Cu-catalyzed cross-dehydrogenative coupling: A versatile strategy for C–C bond formations via the oxidative activation of sp3 C–H bonds. Proc. Natl Acad. Sci. USA 103, 8928–8933 (2006).
Yeung, C. S. & Dong, V. M. Catalytic dehydrogenative cross-coupling: forming carbon-carbon bonds by oxidizing two carbon-hydrogen bonds. Chem. Rev. 111, 1215–1292 (2011).
Zhang, C., Tang, C. & Jiao, N. Recent advances in copper-catalyzed dehydrogenative functionalization via a single electron transfer (SET) process. Chem. Soc. Rev. 41, 3464–3484 (2012).
Girard, S. A., Knauber, T. & Li, C.-J. The cross-dehydrogenative coupling of C(sp3)–H bonds: A versatile strategy for C–C bond formations. Angew. Chem. Int. Ed. 53, 74–100 (2014).
Noisier, A. F. M. & Brimble, M. A. C−H functionalization in the synthesis of amino acids and peptides. Chem. Rev. 114, 8775–8806 (2014).
McMurray, L., O’Hara, F. & Gaunt, M. J. Recent developments in natural product synthesis using metal-catalysed C–H bond functionalisation. Chem. Soc. Rev. 40, 1885–1898 (2011).
Gutekunst, W. R. & Baran, P. S. C–H functionalization logic in total synthesis. Chem. Soc. Rev. 40, 1976–1991 (2011).
Yamaguchi, J., Yamaguchi, A. D. & Itami, K. C–H bond functionalization: emerging synthetic tools for natural products and pharmaceuticals. Angew. Chem. Int. Ed. 51, 8960–9009 (2012).
Hughes A. B. (ed.). Amino Acids, Peptides and Proteins in Organic Chemistry: Origins and Synthesis of Amino Acid Wiley-VCH Verlag GmbH & Co. KGaA (2009).
Greenspan, P. D. et al. Identification of dipeptidyl nitriles as potent and selective inhibitors of Cathepsin B through structure-based drug design. J. Med. Chem. 44, 4524–4534 (2001).
Nájera, C. & Sansano, J. M. Catalytic asymmetric synthesis of α-amino acids. Chem. Rev. 107, 4584–4671 (2007).
Reddy, B. V. S., Reddy, L. R. & Corey, E. J. Novel acetoxylation and C−C coupling reactions at unactivated positions in α-amino acid derivatives. Org. Lett. 8, 3391–3394 (2006).
Tran, L. D. & Daugulis, O. Nonnatural amino acid synthesis by using carbon–hydrogen bond functionalization methodology. Angew. Chem. Int. Ed. 51, 5188–5191 (2012).
Zhang, Q. et al. Stereoselective synthesis of chiral α-amino-β-lactams through palladium(II)-catalyzed sequential monoarylation/amidation of C(sp3)−H bonds. Angew. Chem. Int. Ed. 52, 13588–13592 (2013).
Wang, B., Nack, W. A., He, G., Zhang, S.-Y. & Chen, G. Palladium-catalyzed trifluoroacetate-promoted mono-arylation of β-methyl group of alanine at room temperature: Synthesis of β-arylated α-amino acids through sequential C–H functionalization. Chem. Sci. 5, 3952–3957 (2014).
He, J. et al. Ligand-controlled C(sp3)–H arylation and olefination in synthesis of unnatural chiral α-amino acids. Science 343, 1216–1220 (2014).
Chen, K., Zhang, S.-Q., Xu, J.-W., Hu, F. & Shi, B.-F. A general and practical palladium-catalyzed monoarylation of β-methyl C(sp3)–H of alanine. Chem. Commun. 50, 13924–13927 (2014).
Gong, W., Zhang, G., Liu, T., Giri, R. & Yu, J.-Q. Site-selective C(sp3)–H functionalization of di-, tri- and tetrapeptides at the N-terminus. J. Am. Chem. Soc. 136, 16940–16946 (2014).
Chen, G. et al. Ligand-enabled β-C−H arylation of α-amino acids using a simple and practical auxiliary. J. Am. Chem. Soc. 137, 3338–3351 (2015).
He, J., Takise, R., Fu, H. & Yu, J.-Q. Ligand-enabled cross-coupling of C(sp3)−H bonds with arylsilanes. J. Am. Chem. Soc. 137, 4618–4621 (2015).
Aspin, S., Goutierre, A.-S., Larini, P., Jazzar, R. & Baudoin, O. Synthesis of aromatic α-amino esters: Palladium-catalyzed long-range arylation of primary C(sp3)–H bonds. Angew. Chem. Int. Ed. 51, 10808–10811 (2012).
Li, Z., Cao, L. & Li, C.-J. FeCl2-catalyzed selective C–C bond formation by oxidative activation of a benzylic C–H bond. Angew. Chem. Int. Ed. 46, 6505–6507 (2007).
Deng, G., Zhao, L. & Li, C.-J. Ruthenium-catalyzed oxidative cross-coupling of chelating arenes and cycloalkanes. Angew. Chem. Int. Ed. 47, 6278–6282 (2008).
Guin, S., Rout, S. K., Banerjee, A., Nandi, S. & Patel, B. K. Four tandem C–H activations: A sequential C–C and C–O bond making via a Pd-catalyzed cross dehydrogenative coupling (CDC) approach. Org. Lett. 14, 5294–5297 (2012).
Aihara, Y., Tobisu, M., Fukumoto, Y. & Chatani, N. Ni(II)-catalyzed oxidative coupling between C(sp2)−H in benzamides and C(sp3)−H in toluene derivatives. J. Am. Chem. Soc. 136, 15509–15512 (2014).
Kianmehr, E., Faghih, N. & Khan, K. M. Palladium-catalyzed regioselective benzylation-annulation of pyridine N-oxides with toluene derivatives via multiple C−H bond activations: Benzylation versus arylation. Org. Lett. 17, 414–417 (2015).
Ali, W., Behera, A., Guin, S. & Patel, B. K. Regiospecific benzoylation of electron-deficient N-heterocycles with methylbenzenes via a minisci-type reaction. J. Org. Chem. 80, 5625–5632 (2015).
Collins, K. D. & Glorius, F. A robustness screen for the rapid assessment of chemical reactions. Nat. Chem. 5, 597–601 (2013).
Li, K., Tan, G., Huang, J., Song, F. & You, J. Iron-catalyzed oxidative C–H/C–H cross-coupling: An efficient route to α-quaternary α-amino acid derivatives. Angew. Chem. Int. Ed. 52, 12942–12945 (2013).
Sonobe, T., Oisaki, K. & Kanai, M. Catalytic aerobic production of imines en route to mild, green, and concise derivatizations of amines. Chem. Sci. 3, 3249–3255 (2012).
Hennessy, E. T. & Betley, T. A. Complex N-heterocycle synthesis via iron-catalyzed, direct C–H bond amination. Science 340, 591–595 (2013).
Zhou, S.-L., Guo, L.-N., Wang, H. & Duan, X.-H. Copper-catalyzed oxidative benzylarylation of acrylamides by benzylic C–H bond functionalization for the synthesis of oxindoles. Chem. Eur. J. 19, 12970–12973 (2013).
Liu, D., Liu, C., Li, H. & Lei, A. Direct functionalization of tetrahydrofuran and 1,4-dioxane: Nickel-catalyzed oxidative C(sp3)–H arylation. Angew. Chem. Int. Ed. 52, 4453–4456 (2013).
Liu, Q., Jackstell, R. & Beller, M. Oxidative catalytic coupling reactions: Selective formation of C–C and C–X bonds using radical processes. Angew. Chem. Int. Ed. 52, 13871–13873 (2013).
Liu, C., Liu, D. & Lei, A. Recent advances of transition-metal catalyzed radical oxidative cross-couplings. Acc. Chem. Res. 47, 3459–3470 (2014).
Studer, A. & Curran, D. P. The electron is a catalyst. Nat. Chem. 6, 765–773 (2014).
Acknowledgements
We acknowledge support for this work from the National NSF of China (Nos 21432005, 21272160, and 21321061).
Author information
Authors and Affiliations
Contributions
K.L. and Q.W. performed the experiments and analysed the data. J.L. and J.Y. designed and directed the project and wrote the manuscript. All authors contributed to the discussions.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Figures 1-100, Supplementary Table 1, Supplementary Methods and Supplementary References. (PDF 14206 kb)
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Li, K., Wu, Q., Lan, J. et al. Coordinating activation strategy for C(sp3)–H/C(sp3)–H cross-coupling to access β-aromatic α-amino acids. Nat Commun 6, 8404 (2015). https://doi.org/10.1038/ncomms9404
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/ncomms9404
This article is cited by
-
α-Amino acid and peptide synthesis using catalytic cross-dehydrogenative coupling
Nature Synthesis (2022)
-
Multimetallic catalysed radical oxidative C(sp3)–H/C(sp)–H cross-coupling between unactivated alkanes and terminal alkynes
Nature Communications (2016)
-
Nickel-Catalyzed Aromatic C–H Functionalization
Topics in Current Chemistry (2016)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.