Abstract
Particle-stabilized Pickering emulsions have shown unusual behaviours such as the formation of non-spherical droplets and the sudden halt of coalescence between individual droplets. Here we report another unusual behaviour of Pickering emulsions—the simultaneous coalescence of multiple droplets in a single event. Using latex particles, silica particles and carbon nanotubes as model stabilizers, we show that multi-body coalescence can occur in both water-in-oil and oil-in-water emulsions. The number of droplets involved in the nth coalscence event equals four times the corresponding number of the tetrahedral sequence in close packing. Furthermore, coalescence is promoted by repulsive latex and silica particles but inhibited by attractive carbon nanotubes. The revelation of multi-body coalescence is expected to help better understand Pickering emulsions in natural systems and improve their designs in engineering applications.
Similar content being viewed by others
Introduction
Pickering emulsions are made of particle-stabilized droplets suspended in an immiscible continuous liquid phase1,2. They are important soft matter systems that form naturally in crude oils3 and food products4 and have been engineered for drug delivery5, water purification6 and material processing7,8,9,10. Compared with ordinary emulsions, Pickering emulsions are distinctively stable because the removal of interfacial particles requires a large amount of energy11. When individual Pickering droplets are forced to coalesce, the extraordinary stability brought about by interfacial particles can lead to the formation of non-spherical droplets12,13,14 and the arrest of droplet coalescence15,16. Little is known, however, about the coalescence of a collection of hundreds and thousands of Pickering droplets as in real emulsions. This is particularly important when particle stabilizers are used to produce near-monodispersed droplets17, during which the distribution of droplet size can be significantly broadened by coalescence under gravity, floatation and shear18,19.
Here we report for the first time that the presence of stabilizers at the oil–water interface can lead to multi-body coalescence in an ensemble of Pickering droplets—a phenomenon that has not been reported for either Pickering or ordinary emulsions. More interestingly, the number of droplets involved in coalescence equals four times the corresponding number of the tetrahedral sequence, indicating the inclusion of all closely packed nearest neighbours in a single coalescene event. As a result, a magic size distribition is produced with distinctive maxima related to each other through the cubic root of four times the tetrahedral numbers. Futhermore, interactions between stabilizers are found to affect the probability of coalescence by varying interfacial tension. Using model stabilizers including latex particles, silica particles and carbon nanotubes (CNTs), we show that coalescence is promoted by interparticle repulsion but inhibited by interparticle attraction.
Results
Selection of emulsion systems
To investigate coalescence of Pickering droplets in an ensemble, we select three representative emulsion systems, including latex particle-stabilized water droplets in dodecane, silica particle-stabilized 1,2-dichlorobenzene (DCB) droplets in water and CNT-stabilized water droplets in dodecane. Comparisons between the first two systems will show that multi-body coalescence occurs in both water-in-oil and oil-in-water emulsions. Subsequent comparisons with the CNT system will reveal differences between stabilizers lacking and having attractive interactions. Our results are organized in four sections as follows, including emulsion preparation and droplet size analysis, evolution of droplet size through multi-body coalescence, polydispersity and size evolution and coalescence probability and interparticle force.
Emulsion preparation and droplet size analysis
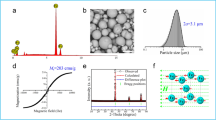
The three stabilizers and typical Pickering emulsions made from them are shown in Fig. 1. Latex and silica particles (Fig. 1a,b) are spheres with diameters of 0.8 μm and 1 μm, respectively. CNTs decorated with surface tension-tuning magnetite (Fe3O4) nanoparticles (Fig. 1c) are several micrometers long and have a diameter of ca. 15 nm (ref. 6). Pickering emulsions stabilized by latex particles, silica particles and CNTs are prepared following a conventional protocol20, involving two consecutive steps. First, water, oil and stabilizers are mixed and then shaken vigorously by hand for 10 min (Fig. 1d–f), forming Pickering emulsions containing stabilizer-wrapped droplets21. Then, emulsions are left standing undisturbed on top of a bench for 10 min, allowing droplets to precipitate (Fig. 1g–i), forming a closely packed ensemble (Fig. 1j–l) to induce coalescence. Pickering droplets prepared following this protocol have low uniformity indices between 0.2 and 0.4 (Supplementary Fig. 1)22,23,24, suggesting that the emulsions have only experienced limited coalescence21.
Results obtained with different analytical techniques are organized in columns: (a–c) transmission electron micrographs of stabilizers, (d–f) digital photographs of emulsions during mixing, (g–i) photographs taken at the end of standing and (j–l) optical micrographs of emulsions after standing. Results obtained with different stabilizers are organized in rows: (a,d,g,j) latex particle-stabilized water droplets in dodecane, (b,e,h,k) silica particle-stabilized 1,2-dichlorobenzene (DCB) droplets in water and (c,f,i,l) carbon nanotube-stabilized water droplets in dodecane. Mass ratio between droplets and the continuous phase: (d,g,j), 0.0667; (e,h,k), 0.0650; (f,i,l), 0.0667. Stabilizer-to-droplet mass ratio: (d,g,j), 0.02; (e,h,k), 0.03; (f,i,l), 0.02. Scale bars: a–c, 500 nm; inset in c, 5 nm and j–l, 250 μm.
After coalescence is complete, diameters of at least 500 droplets are measured using an optical microscope. Histograms, as shown in Fig. 2a–c, are constructed to facilitate detailed analyses of droplet size distribution. For all three types of emulsions, the diameter histogram can be readily deconvoluted into a series of Gaussian functions, indicating that each emulsion consists of several normally distributed populations of droplets. Of note, deconvolution is only possible when histograms are generated using at least 500 measurements. Similar histograms reported in the literature are usually made with significantly less measurements, often in the order of 50–100 (ref. 21). Under such conditions, the combination of multiple normal distributions degenerates to a single log-normal distribution25.
Results of different analyses are organized in columns: (a–c) diameter histograms showing distinctive maxima: dn's (n=0, 1, 2, 3), (d–f) inverse correlations of dn's with stabilizer-to-droplet mass ratio α and (g–i) linear correlations of dn's (n>0) with d0. Results for different stabilizers are organized in rows: (a,d,g) latex particle-stabilized water droplets in dodecane, (b,e,h) silica particle-stabilized 1,2-dichlorobenzene (DCB) droplets in water and (c,f,i) carbon nanotube-stabilized water droplets in dodecane. Curves in d–f are least-square regressions to equation (1). Lines in g–i are regressions to equation (2). Mass ratio between droplets and the continuous phase: (a,d,g), 0.0667; (b,e,h), 0.0650; (c,f,i), 0.0667. Stabilizer-to-droplet mass ratio: a, 0.02; b, 0.03 and c, 0.02. Error bars represent s.e.
The mean of each deconvoluted normal distribution, dn (n=0, 1, 2, 3), represents the mean diameter of the corresponding droplet population. We find that dn decreases with increasing stabilizer-to-droplet mass ratio α, as shown in Fig. 2d–f. The inverse dependence of dn on α can be readily explained by matching the total surface area of droplets and the total cross-section area of interfacial stabilizers25:

where ρ is the specific gravity of stabilizers with respect to the droplet phase, η is the porosity of interfacial packing and τn is packing thickness. Conformation of experimental data to equation (1) indicates that fulfiling the interfacial area requirement for accommodating all stabilizer particles is an important determinant of droplet size.
We further compare dn (n>0) with d0, revealing a linear relationship between them:

as shown in Fig. 2g–i. The scaling factor kn is estimated from the slope of linear regression. For n=1, 2 and 3, k1≈1.6, k2≈2.5 and k3≈3.4 for all three emulsion systems. The conservation of kn's among different emulsion systems suggests the presence of a univeral mechanism that controls the evolution of droplet size.
Evolution of droplet size through multi-body coalescence
To elucidate the mechanism of size evolution for Pickering droplets, we first focus on the mean diameter of deconvoluted droplet population without considering dispersion of the population. As shown in Fig. 3a (see Supplementary Table 1 for data), kn equals the cubic root of four times the corresponding tetrahedral number, Tn:
(a) Correlation of the scaling factor kn=dn/d0 (n=1, 2, 3) with the cubic root of four times the tetrahedral number, Tn. (b) Formation of dn droplets from the coalescence of one dn−1 droplet (no dn−1 droplet for n=1) and (Tn−Tn−1) d0 droplets in face-centred close packing. Note: droplets coloured in grey do not participate in coalescence. (c) Increase of interfacial particle thickness after coalescence. Extra-large symbols in a and c are used for clarity of presentation: squares, latex particle-stabilized water droplets in dodecane; diamonds, silica particle-stabilized 1,2-dichlorobenzene droplets in water; circles, carbon nanotube-stabilized water droplets in dodecane. Solid lines in a and c are least-square regressions (R2=0.99). Dashed lines are 95% confidence intervals. Error bars represent s.e.

suggesting that a dn droplet has the same volume as Tn d0 droplets and thus is formed by their coalescence. For n=1, 2 and 3, Tn=4, 16 and 40; therefore, the coalescence of these Pickering droplets is multi-body in nature.
Multi-body coalescence requires droplets to be closely packed, which is facilitated by the density difference between water and oil in our experimental systems (cf. Fig. 1g–i)26. As illustrated in Fig. 3b and Table 1, four nearest-neighbouring d0 droplets form a tetrahedron in a face-centred close (FCC) packed ensemble. When all four droplets coalesce simultaneously, the new droplet has a diameter of  , where T1=4. The d1 droplet has 12 nearest d0 neighbours in FCC, yielding a d2 droplet after coalescing with the d1 droplet:
, where T1=4. The d1 droplet has 12 nearest d0 neighbours in FCC, yielding a d2 droplet after coalescing with the d1 droplet:  , where T2=4+12=16. Similarly, a d3 droplet is formed by the coalescence of the d2 droplet with its 24 nearest neighbours:
, where T2=4+12=16. Similarly, a d3 droplet is formed by the coalescence of the d2 droplet with its 24 nearest neighbours:  , where T3=16+24=40.
, where T3=16+24=40.
We further examine multi-body coalescence by considering the material conservation of interfacial stabilizers before and after coalescence, which requires:

By combining this equation with equation (2), we obtain:

for constants ρ and η. Indeed, equation (5) holds for all three emulsion systems as illustrated in Fig. 3c (see Supplementary Table 2 for data).
Polydispersity and size evolution
In the analyses described above, we have assumed that each deconvoluted droplet population has a single diameter, dn (n=0, 1, 2, 3), equal to the mean of the Gaussian fit of experimental data. For the 0th droplet population, this assumption can be validated by considering that d0 droplets are formed under vigorous shaking—an independent and identical process with a finite variance27. However, will the coalescence of normally distributed d0 droplets produce normally distributed dn (n>0) droplets?
Coalescence progresses through the conservation of volume:

According to equation (6), we can prove that the probability density function of dn is the Tn-fold convolution power of the probability density function of  (see Supplementary Note 1 for derivation), which cannot be evaluated analytically. To obtain the distribution of the nth droplet population, we resort to the Monte Carlo method, which computes one million dn values from randomly selected d0's using equation (6).
(see Supplementary Note 1 for derivation), which cannot be evaluated analytically. To obtain the distribution of the nth droplet population, we resort to the Monte Carlo method, which computes one million dn values from randomly selected d0's using equation (6).
The histograms of simulated dn's (n>0) are shown in Fig. 4a, along with the normally distributed 0th population. The histograms can be well-approximated by normal distributions (similarity to normality >99.8%, as measured by Kolmogorov–Smirnov statistic)28,29, confirming that the normal distribution is conserved through coalescence. The means of simulated dn's are compared with those estimated from experimental data in Fig. 4b. The two data sets exhibit an excellent linear correlation with a near-unity slope of 1.01(±0.01) (R2=0.96), validating the use of Gaussian fits to estimate dn's.
(a) Evolution of the normal distribution of d0 with a mean of 25.3 μm and a s.d. of 7.5 μm (zeroth population in Fig. 2a) through multi-body coalescence (grey shades, simulated populations; coloured curves, Gaussian fits). (b) Comparison of mean diameters of dn's (n=1, 2, 3) obtained from fitting simulated data to Gaussian functions with those obtained from fitting experimental data to Gaussian functions. Symbols: squares, latex particle-stabilized water droplets in dodecane; diamonds, silica particle-stabilized 1,2-dichlorobenzene droplets in water; circles, carbon nanotube-stabilized water droplets in dodecane. Colours: red, n=1; green, n=2; purple, n=3. The solid line is obtained by linear regression (R2=0.96). Dashed curves bracket 95% confidence intervals.
Coalescence probability and interparticle force
Although Pickering droplets prepared with different stabilizers coalesce following the same tetrahedral sequence, the selection of stabilizer can, however, affect coalescence probability. This is revealed by examining the variation of relative abundance Nn/NT (n=0, 1, 2, 3) of each droplet population with α, as shown in Fig. 5. Here Nn is the number of dn droplets estimated by integrating the nth Gaussian fit and  . As α increases, Nn/NT (n>0) increases at the expense of N0/NT for latex and silica-stabilized droplets (Fig. 5a,b), indicating that the addition of stabilizers promotes coalescence. For CNT-stabilized droplets (Fig. 5c), the opposite is observed, revealing improved stability of d0 droplets and suppressed coalescence with the addition of CNTs.
. As α increases, Nn/NT (n>0) increases at the expense of N0/NT for latex and silica-stabilized droplets (Fig. 5a,b), indicating that the addition of stabilizers promotes coalescence. For CNT-stabilized droplets (Fig. 5c), the opposite is observed, revealing improved stability of d0 droplets and suppressed coalescence with the addition of CNTs.
(a) Latex particle-stabilized water droplets in dodecane (b) Silica particle-stabilized 1,2-dichlorobenzene (DCB) droplets in water. (c) Carbon nanotube (CNT)-stabilized water droplets in dodecane.  is the total number of droplets from all populations. Curves are regressions to exponential functions (R2>0.8). Error bars represent the standard deviation of the data.
is the total number of droplets from all populations. Curves are regressions to exponential functions (R2>0.8). Error bars represent the standard deviation of the data.
To understand why coalescence is promoted by latex and silica particles but suppressed by CNTs, we divide coalescence into two consecutive processes: packing and fusion, as illustrated in Fig. 6. Packing presses d0 droplets together and transforms them from spheres to rounded polyhedrons30. To pack droplets sufficiently close for coalescence, Laplace pressure p0 must be overcome by the external pressure provided by the droplets’ weight, P:

where γ is interfacial tension. Fusion between droplets then happens with the rupture of the separating liquid film, which requires the internal pressure of polyhedral droplets, pp, to exceed the disjoining pressure of the film, Π (a property of the continuous phase)31,32:

where C is a constant related to droplet packing fraction.
The interfacial tension includes contributions from both stabilizer-wrapped droplets, γd, and interactions between interfacial stabilizers, γs:

For stabilized droplets2,

where γow is the oil–water interfacial tension and θ is the contact angle formed by the continuous phase, the stabilizer surface and the droplet phase. According to equation (10), γd is constant for a given emulsion system; therefore, γ varies with γs.
γs can arise from the electrostatic repulsion between interfacial stabilizers. Latex particles, silica particles and CNTs are all negatively charged, as confirmed by their negative zeta potentials in water (latex, −18(±7) mV; silica, −21(±7) mV; CNTs, −13(±1) mV). Charge-induced repulsion, γcp, pushes stabilizer particles away from one another, reducing interfacial tension that pulls stabilizers together (that is, γs=−γcp<0):

An indication of interparticle repulsion is the random close packing33 patterns formed by latex and silica particles at the oil–water interface and revealed by confocal laser scanning microscopy, as shown in Fig. 7a–c and d–f, respectively. With low γ, equation (7) is readily fulfiled. The probability of coalescence is thus controlled by the difference between pp and Π according to equation (8). As α increases, d0 decreases according to equation (1). This leads to an increase of pp, improving the chances of overcoming Π to coalesce and produce more dn (n>0) droplets at greater α (cf. Fig. 5a,b).
Images obtained with different stabilizers are organized in rows: (a–c) latex particle-stabilized water droplets in dodecane, (d–f) silica particle-stabilized 1,2-dichlorobenzene droplets in water and (g–i) carbon nanotube-stabilized water droplets in dodecane. Different types of images are organized in columns: (a,d,g) three-dimensional reconstructions of spherical caps, (b,e,h) cross-sections at the tops of spheres and (c,f,i) cross-sections below the tops. Note: the diameter of individual carbon nanotubes is much smaller than the imaging resolution (ca. 300 nm); therefore, individual nanotubes and bundles of a few nanotubes are not visually resolved. Mass ratio between droplets and the continuous phase: (a–c), 0.0667; (d–f), 0.0650 and (g–i), 0.0667. Stabilizer-to-droplet mass ratio: (a–c), 0.02; (d–f), 0.03 and (g–i), 0.01. Scale bars, 10 μm.
Different from latex and silica particles, CNTs form an extended network at the oil–water interface, as revealed by the confocal micrographs shown in Fig. 7g–i. Formation of the network can be attributed to strong π–π attractions between individual nanotubes, which overtake electrostatic repulsions between them (that is, γs=γπ–π−γcp>0)6,34:

With high γ, equation (8) is readily fulfiled, leaving the control of coalescence probability to equation (7). As α increases, d0 decreases and p0 increases, resulting in a decrease of coalescence and minimal amounts of dn (n>0) droplets with large α (cf. Fig. 5c).
Discussion
We have shown that closely packed Pickering droplets can coalesce through a multi-body mechanism. We hypothesize that the determining factor of multi-body coalescence is the presence of stabilizers at the oil–water interface, which slows down coalescence. In ordinary emulsions where droplets are stabilized by surfactant molecules or ions, coalescence happens rapidly between two droplets35,36. Recent measurements have, however, shown that coalescence between two particle-stabilized droplets is orders of magnitude slower37. The extended transition time provides an opportunity for all of the nearest neighbours to be involved in a single coalescence event once coalescence is initiated between two droplets.
We formulate the multi-body coalescence theory in the FCC configuration. If Pickering droplets are packed in the hexagonal close packing (HCP) configuration, the number of droplets involved in the first coalescence event is the same as in FCC but decreases gradually for the second and third events, as illustrated in Table 2. The kn values for HCP are 1.6, 2.4 and 2.5 compared with 1.6, 2.5 and 3.4 for FCC. According to experimentally determined kn's, the packing of Pickering droplets is better represented by FCC. Nonetheless, multi-body coalescence requires only short-range ordering because significant coalescence occurs in the first few coordination shells surrounding an interstitial void. In the longer range, the lack of organization, such as that in random close packing33, should not affect the outcome of multi-body coalescence in Pickering emulsions.
Methods
Reagents
Reagent-grade chemicals were purchased from Sigma-Aldrich and Fisher Scientific except where otherwise stated. Deionized (DI) water (18.2 MΩ cm−1) used in solution making, washing and rinsing was generated using a Millipore system (Billerica, MA, USA) on site. We prepared Pickering emulsions by shaking and standing (see below)20.
Latex particle-stabilized water droplets in dodecane
Latex particles were obtained by drying an aqueous solution in vacuum overnight. The particles were then dispersed in dodecane (99%, TCI America) at various concentrations. To make Pickering emulsions, 50 μl DI water was added to 1 ml dodecane. The mixture was shaken by hand vigorously for 10 min and then left standing on bench for 10 min.
Silica particle-stabilized DCB droplets in water
Silica particles were first coated with (3-aminopropyl)trimethoxysilane (APTMS, 97%) to modify their surface wettability38. To do so, 0.1 ml particle solution (10 wt%) was dried in an oven at 120 °C overnight and was then mixed with 10 ml toluene (≥99.8%) and 100 μl APTMS. The mixture was shaken for 2 h. The particles were washed with toluene five times and with ethanol three times. The particles were then dried in an oven overnight to remove residual ethanol and immersed in water before use. To make a Pickering emulsion, 50 μl 1,2-DCB (99%, Alfa Aesar) was added to 1 ml DI water with different concentrations of silica particles. The mixture was shaken by hand vigorously and left standing undisturbed following the same procedure for making latex-stabilized emulsions.
CNT-stabilized water droplets in dodecane
Magnetite-decorated CNTs were prepared using multi-walled CNTs synthesized by chemical vapour deposition6. Catalysts were removed by washing with nitric acid. CNTs were then decorated with 10-nm magnetite nanoparticles using the polyol reduction method39. To make a CNT-stabilized Pickering emulsion, CNTs were dispersed in 10 ml water by sonication for 10 min, followed by an addition of 0.5 ml dodecane. The mixture was shaken and left standing quiescently following the same protocol for making latex and silica-stabilized emulsions.
Optical microscopy
Diameters of particle-stabilized droplets were measured using images taken by an optical microscope (Motic BA300POL). To do so, emulsions were poured on either glass (for silica-stabilized droplets) or plastic (for latex and CNT-stabilized droplets) Petri dishes. The emulsions were then diluted with the corresponding continuous phases to minimize droplet overlapping in the imaging field. For each sample, ~30 images were taken randomly with a × 10 objective lens (resolution: 1.25 μm per pixel). Diameters were measured using software ImageJ40. A few droplets stabilized by silica particles (<5%) were found at the arrested coalescence state with non-spherical shapes (Supplementary Fig. 2). They were excluded in subsequent diameter analyses.
Confocal laser scanning microscopy
Interfacial stabilizers were visualized using a confocal laser scanning microscope (Nikon A1R) equipped with a × 100 Plan Apo total internal reflection fluorescence objective lens. The oil phases were illuminated using oil-soluble Nile red. Water was illuminated using Alex Fluor 488. Concentrations of the fluorescent dyes were: latex particle-stabilized water droplets in dodecane, 0.001 mM Alex Fluor 488 in water and 0.1 mM Nile red in dodecane; silica particle-stabilized DCB droplets in water, 0.03 mM Nile red in DCB and 0.01 mM Alex Fluor 488 in water; CNT-stabilized water droplets in dodecane, 0.01 mM Alex Fluor 488 in water and 0.03 mM Nile red in dodecane. The oil-in-water emulsion stabilized by silica particles was imaged using a custom-made hydrophilic glass reservoir. To image water-in-oil emulsions stabilized by latex particles and CNTs, the reservoir was treated with a 1:100 octadecyltrichlorosilane toluene solution to create a hydrophobic coating before use.
Measurement of particle surface charge
Zeta potentials of latex particles, silica particles and CNTs were measured using a ZetaPlus analyzer (Brookhaven Instruments) in water at concentrations of 0.2 mg l−1, 0.2 mg l−1 and 0.12 mg l−1, respectively. Water pH was adjusted according to the conditions in corresponding emulsions. Latex particles and CNTs were dispersed in water in equilibrium with atmospheric carbon dioxide at pH 5.6. Silica particles were dispersed in dilute sodium hydroxide solution at pH 7.0. Before measurements, stabilizer suspensions were sonicated for 30 min. For each stabilizer, five measurements were made.
Additional information
How to cite this article: Wu, T. et al. Multi-body coalescence in Pickering emulsions. Nat. Commun. 6:5929 doi: 10.1038/ncomms6929 (2015).
References
Pickering, S. U. Emulsions. J. Chem. Soc. 91, 2001–2021 (1907).
Binks, B. P. Particles as surfactants-Similarities and differences. Curr. Opin. Colloid Interface Sci. 7, 21–41 (2002).
McLean, J. D. & Kilpatrick, P. K. Effects of asphaltene solvency on stability of water-in-crude-oil emulsions. J. Colloid Interface Sci. 189, 242–253 (1997).
Dickinson, E. Food emulsions and foams: stabilization by particles. Curr. Opin. Colloid Interface Sci. 15, 40–49 (2010).
Dinsmore, A. D. et al. Colloidosomes: selectively permeable capsules composed of colloidal particles. Science 298, 1006–1009 (2002).
Wang, H. et al. Removal of oil droplets from contaminated water using magnetic carbon nanotubes. Water Res. 47, 4198–4205 (2013).
Koos, E. & Willenbacher, N. Capillary forces in suspension rheology. Science 331, 897–900 (2011).
Stratford, K., Adhikari, R., Pagonabarraga, I., Desplat, J. C. & Cates, M. E. Colloidal jamming at interfaces: a route to fluid-bicontinuous gels. Science 309, 2198–2201 (2005).
Crossley, S., Faria, J., Shen, M. & Resasco, D. E. Solid nanoparticles that catalyze biofuel upgrade reactions at the water/oil interface. Science 327, 68–72 (2010).
Velev, O. D., Lenhoff, A. M. & Kaler, E. W. A class of microstructured particles through colloidal crystallization. Science 287, 2240–2243 (2000).
Aussillous, P. & Quere, D. Liquid marbles. Nature 411, 924–927 (2001).
Subramaniam, A. B., Abkarian, M., Mahadevan, L. & Stone, H. A. Non-spherical bubbles. Nature 438, 930–930 (2005).
Cui, M., Emrick, T. & Russell, T. P. Stabilizing liquid drops in nonequilibrium shapes by the interfacial jamming of nanoparticles. Science 342, 460–463 (2013).
Abkarian, M. et al. Dissolution arest and stability of particle-covered bubbles. Phys. Rev. Lett. 99, 188301 (2007).
Pawar, A. B., Caggioni, M., Ergun, R., Hartel, R. W. & Spicer, P. T. Arrested coalescence in Pickering emulsions. Soft Matter 7, 7710–7716 (2011).
Whitby, C. P. & Krebsz, M. Coalescence in concentrated Pickering emulsions under shear. Soft Matter 10, 4848–4854 (2014).
Nie, Z., Park, J. I., Li, W., Bon, S. A. F. & Kumacheva, E. An “inside-out” microfluidic approach to monodisperse emulsions stabilized by solid particles. J. Am. Chem. Soc. 130, 16508–16509 (2008).
Hasmy, A., Paredes, R., Sonneville-Aubrun, O., Cabane, B. & Botet, R. Dynamical transition in a model for dry foams. Phys. Rev. Lett. 82, 3368–3371 (1999).
Whitby, C. P., Fischer, F. E., Fornasiero, D. & Ralston, J. Shear-induced coalescence of oil-in-water Pickering emulsions. J. Colloid Interface Sci. 361, 170–177 (2011).
Wiley, R. M. Limited coalescence of oil droplets in coarse oil-in-water emulsions. J. Colloid Sci. 9, 427–437 (1954).
Arditty, S., Whitby, C. P., Binks, B. P., Schmitt, V. & Leal-Calderon, F. Some general features of limited coalescence in solid-stabilized emulsions. Eur. Phys. J. E Soft Matter 11, 273–281 (2003).
Binks, B. P. & Whitby, C. P. Silica particle-stabilized emulsions of silicone oil and water: aspects of emulsification. Langmuir 20, 1130–1137 (2004).
Romero, A., Cordobes, F., Puppo, M. C., Guerreroa, A. & Bengoechea, C. Rheology and droplet size distribution of emulsions stabilized by crayfish flour. Food Hydrocolloids 22, 1033–1043 (2008).
Schmitt, V., Cattelet, C. & Leal-Calderon, F. Coarsening of alkane-in-water emulsions stabilized by nonionic poly(oxyethylene) surfactants: The role of molecular permeation and coalescence. Langmuir 20, 46–52 (2004).
Wang, H. & Hobbie, E. K. Amphiphobic carbon nanotubes as macroemulsion surfactants. Langmuir 19, 3091–3093 (2003).
Whitby, C. P., Lotte, L. & Lang, C. Structure of concentrated oil-in-water Pickering emulsions. Soft Matter 8, 3784–3789 (2012).
Irani, R. R. Particle Size: Measurement, Interpretation, and Application John Wiley & Sons (1963).
Kolmogorov, A. N. Sulla determinazione empirica di una legge di distribuzione. G. Ist. Ital. Attuari 4, 83–91 (1933).
Smirnov, N. Table for estimating the goodness of fit of empirical distributions. Ann. Math. Stat. 19, 279–281 (1948).
Bibette, J., Morse, D. C., Witten, T. A. & Weitz, D. A. Stability criteria for emulsions. Phys. Rev. Lett. 69, 2439–2442 (1992).
Princen, H. M. Highly concentrated emulsions I. cylindrical systems. J. Colloid Interface Sci. 71, 55–66 (1979).
Princen, H. M., Aronson, M. P. & Moser, J. C. Highly concentrated emulsions II. Real systems. The effect of film thickness and contact angle on the volume fraction in creamed emulsions. J. Colloid Interface Sci. 75, 246–270 (1980).
Clusel, M., Corwin, E. I., Siemens, A. O. N. & Brujic, J. A. 'Granocentric' model for random packing of jammed emulsions. Nature 460, 611–615 (2009).
Feng, T., Hoagland, D. A. & Russell, T. P. Assembly of acid-functionalized single-walled carbon nanotubes at oil/water interfaces. Langmuir 30, 1072–1079 (2014).
von Smoluchowski, M. Experiments on a mathematical theory of kinetic coagulation of coloid solutions. Z. Phys. Chem. Stoechiom. Verwandtschafts. 92, 129–168 (1917).
Aldous, D. J. Deterministic and stochastic models for coalescence (aggregation and coagulation): a review of the mean-field theory for probabilists. Bernoulli 5, 3–48 (1999).
Chen, G. et al. Coalescence of Pickering emulsion droplets induced by an electric field. Phys. Rev. Lett. 110, 064502 (2013).
Krysztafkiewicz, A., Binkowski, S. & Wysocka, W. Pigments on amorphous silica carriers. Powder Technol. 132, 190–195 (2003).
Wang, H., Cao, L., Yan, S., Huang, N. & Xiao, Z. An efficient method for decoration of the multiwalled carbon nanotubes with nearly monodispersed magnetite nanoparticles. Mater. Sci. Eng. B 164, 191–194 (2009).
Schneider, C. A., Rasband, W. S. & Eliceiri, K. W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 9, 671–675 (2012).
Acknowledgements
C.N. thanks the Department of Energy Office of Nuclear Energy’s Nuclear Energy University Programmes, the National Science Foundation Environmental Engineering Programme and the Notre Dame Sustainable Energy Initiative for financial support. P.C.B.’s contribution was supported by the Energy Frontier Research Center Materials Science of Actinides. We thank Kun-Yi Lin for performing preliminary experiments on carbon nanotubes. We thank Antonio Simonetti, Diogo Bolster and Kapil Khandelwal for their inspiring comments and suggestions.
Author information
Authors and Affiliations
Contributions
C.N., H.W. and T.W. designed the study and analyzed data. T.W. and H.W. performed the experiments. B.J. contributed to the design of confocal measurements. F.L. performed Monte Carlo simulation and contributed to the statistical analysis of experimental data. P.C.B. contributed to the interpretation of droplet packing. All authors contributed to the writing of the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Figures 1-2, Supplementary Tables 1-2, Supplementary Note 1 and Supplementary References (PDF 458 kb)
Rights and permissions
About this article
Cite this article
Wu, T., Wang, H., Jing, B. et al. Multi-body coalescence in Pickering emulsions. Nat Commun 6, 5929 (2015). https://doi.org/10.1038/ncomms6929
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/ncomms6929
This article is cited by
-
General approach to surface-accessible plasmonic Pickering emulsions for SERS sensing and interfacial catalysis
Nature Communications (2023)
-
Enhanced coalescence of two oil droplets with clay particles
Korea-Australia Rheology Journal (2019)
-
Designer liquid-liquid interfaces made from transient double emulsions
Nature Communications (2018)
-
Higher-order assembly of crystalline cylindrical micelles into membrane-extendable colloidosomes
Nature Communications (2017)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.