Abstract
In past decades, catalytic cross-coupling reactions between organic halides and organometallic reagents to construct carbon–carbon bond have achieved a tremendous progress. However, organolithium reagents have rarely been used in cross-coupling reactions, due mainly to their high reactivity. Another limitation of this transformation using organolithium reagents is how to control reactivity with excellent selectivity. Although palladium catalysis has been applied in this field recently, the development of an approach to replace catalytic systems of noble metals with nonprecious metals is currently in high demand. Herein, we report an efficient synthetic protocol involving iron-catalysed cross-coupling reactions employing organolithium compounds as key coupling partners to unite aryl, alkyl and benzyl fragments and also disclose an efficient iron-catalysed release-capture ethylene coupling with isopropyllithium.
Similar content being viewed by others
Introduction
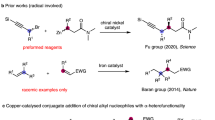
Transition metal-catalysed cross-coupling has emerged as a highly useful, selective and widely applicable method for synthesizing structurally diverse organic compounds via carbon–carbon bond formation1,2. Since the discoveries of cross-coupling reactions, palladium-catalysed cross-coupling with organic halides and organometallic reagents, has dominated this area as an exceptionally powerful approach to assemble C–C bond (Fig. 1a)3. Although Murahashi et al. disclosed a palladium-catalysed cross-coupling reaction of alkenyl halides with various organolithium compounds, direct use of organolithium reagents in cross-coupling reactions has been neglected for a long time, mainly due to the high reactivity and low stability of organolithium reagents4,5,6,7. Recently, Feringa and co-workers developed palladium-based catalytic systems to directly generate C–C bond using organolithium compounds as cross-coupling partners (Fig. 1b)8,9,10,11,12,13. Although palladium-based catalysts typically mediated such reactions, there are increasing concerns about their long-term sustainability in the synthetic community because of its high cost, low natural abundance, environmentally deleterious extraction, toxicity and competition for its use from the automotive and consumer electronics sectors14. Therefore, there is a growing interest in replacing palladium-based catalysts with those more Earth-abundant elements. With its low cost, high natural abundance and low toxicity, iron is indeed a particularly appealing alternative, and accordingly, the development of iron-catalysed cross-coupling is undergoing an explosive growth15,16,17,18,19,20. Herein, we develop an iron-catalysed cross-coupling strategy of organolithium reagents with organic halides to form C–C bonds, examples including C(sp2)-C(sp3) bonds, C(sp3)-C(sp3) bonds and a rare method to form a novel C(sp2)-C(sp3) bond via in-situ generation of ethylene from tetrahydrofuran (THF).
Results
Serendipity
Previously, we demonstrated that the rigid tetraphenylene (tetrabenzo[a,c,e,g]cyclooctatetraene) is a structurally and functionally exceptional molecule21,22. To improve the efficiency of the coupling step, we proposed to synthesize tetraphenylene derivatives through a one-pot iron-catalysed intramolecular cross-coupling protocol23. Although we obtained a trace amount of tetraphenylene, the serendipity is that 2-n-butylbiphenyl was observed (Fig. 2). Therefore, we recognized the potential use of alkyllithum reagents in iron-catalysed cross-coupling reactions.
Optimization
Encouraged by the reaction shown in Fig. 2, we attempted to couple 4-methoxybromobenzene (1a) with n-BuLi (2a) under the same condition. As expected, the target product p-methoxybutylbenzene (3a), together with a trace amount of the isomerized cross-coupling product (3a’), were detected by gas chromatography-mass spectrometry (GC–MS), although the homo-coupling product (4) is the major product, together with dehalogenated product (5; Table 1, entry 1). In the absence of triethylamine (Et3N) and at 22 °C, the ratio of the desired product was almost the same (Table 1, entries 2–3). Then, in the presence of FeCl2 (10 mol%), ligands (20 mol%) and 1a (0.2 mmol) in THF (1.0 ml), several traditional bidentate ligands with different bite angles (for expanded screening results, see Supplementary Table 1) and monodentate electron-rich phosphine ligands (Fig. 3) were examined through a slow addition of dilute organolithium reagent 2a (0.3 mmol, 0.35 M) within 1 h at 22 °C using a syringe pump (Table 1, entries 4–11). To our delight, a distinct improvement was displayed by GC–MS. When trimethyl phosphite (L5) was used as a ligand, the desired product was isolated in 68% yield (Table 1, entry 8). Further screening of iron salts revealed that iron(II) chloride gave a promising yield with trimethyl phosphite as the ligand (Table 1, entries 12–15). Recently, Fürstner pioneered the use of iron(III) acetylactonate-catalysed cross-coupling reactions with Grignard reagents24,25,26,27, however, this iron catalyst demonstrated lower reactivity with lithium reagents (Table 1, entry 16). Surprisingly, upon addition of tetramethylethylenediamine (TMEDA, L8) into the solution of iron(III) chloride in THF, the homo-coupling by-product (4) was suppressed dramatically (Table 1, entry 17). This catalytic system developed by Nakamura had been employed in cross-coupling reactions with Grignard reagents and arylzinc reagents28,29,30,31. Therefore, the complex of TMEDA with iron(III) chloride was prepared according to Nakamura’s procedure for use in our next stage of optimization (Table 1, entries 18–24). When the cross-coupling reaction was conducted at 0 °C, the generation of the dehalogenated by-product (5) was reduced and the expected product was isolated in 85% (0.2 mmol scale; Table 1, entry 19). To further improve this procedure, we also screened the reaction media. A comparison of results obtained in THF revealed that the ratios of desired product were decreased in toluene and diethyl ether (Table 1, entries 20–21). When the catalyst loading was reduced to 3 and 1 mol%, respectively, the overall efficiency was not reduced in an obvious manner (Table 1, entries 22–23).
Monodentate and bidentate ligands were screened, also see Supplementary Information.
C(sp2)-C(sp3) cross-coupling of aryl halides with alkyllithiums
To expand the scope of the iron-catalysed reactions, C(sp2)-C(sp3) cross-coupling of aryl halides with alkyllithium reagents was further investigated. Initially, we compared the reactivity of different aryl halides (Table 2, 3a). 4-Methoxychlorobenzene (5% conversion) was more inert than 4-methoxybromobenzene (1a). The target product was generated exclusively, when 4-methoxyiodobenzene was used as a starting substrate. Unexpectedly, 4-methoxyphenyltrifluoromethanesulfonyl triflate (an aryl triflate) decomposed to the corresponding phenol (see note in Table 2). In consideration of their commercial availability, we made use of aryl bromides for further investigation (Table 2, 3b-3r). It was uncovered that varying the position of the methoxy group on the benzene ring led to a pronounced effect on the reaction outcome, presumably due to chelation of oxygen with lithium (Table 2, 3b-3c). In the case of bromobenzene, the GC yield was given due to the volatility issue (Table 2, 3d). Electron-donating and bulky functional groups facilitated cross-coupling reaction without sacrificing the yield of the corresponding products (Table 2, 3e-3g). However, a strongly electron-withdrawing substituent was found to lead to halogen-metal exchange (Table 2, 3h). Remarkably, a series of alkyllithiums were freshly prepared and were found to be compatible with this protocol, being able to couple with 4-bromo-N,N-dimethylaniline (Table 2, 3i-3o). Polyaromatic compounds were found to undergo alkylation in moderate yields (Table 2, 3p-3q). In addition, a double alkylation product was obtained in 65% yield (Table 2, 3r).
Release-capture ethylene coupling with isopropyllithium
When isopropyllithium, a typical secondary organolithium, was utilized in the iron catalysis system with 4-methoxybromobenzene (1a), 1-isopentyl-4-methoxybenzene (3aTHF) was obtained together with a trace amount of cross-coupling product 1-isopropyl-4-methoxybenzene. After prolonging the reaction time to overnight at 22 °C, the yield of 3aTHF was optimized up to 71% (Table 3, 3aTHF). To our best knowledge, this is an unusual example of transition metal-catalysed cross-coupling reaction involving freshly prepared ethylene generated by decomposing THF with isopropyllithium. Several aryl bromides were then investigated to explore the substituent effect at various positions of the benzene ring. Possible chelation effect and steric effect were demonstrated when the benzene ortho-position was occupied by a methoxy group or a bulky group (Table 3, 3bTHF and 3cTHF). It is noted that when FeCl2 with P(OMe)3 was used as the catalyst in place of [(FeCl3)2(TMEDA)3], the yield of 3cTHF could be improved. Remote substituents could be tolerated, leading to the formation of the corresponding products in 37–77% yield (Table 3, 3dTHF-3fTHF). Moreover, a naphthyl compound was found to participate efficiently (Table 3, 3gTHF). On the basis of the previously reported reactions32, we would like to propose a plausible pathway for this release-capture ethylene process. Thus, as shown in Fig. 4, THF is deprotonated at its 2-position by isopropyllithium to form 2-lithioTHF (I). Then, a subsequent intramolecular reverse [3+2] cycloaddition of the anion would release ethylene and generate the lithium enolate (II). Finally, the resulting ethylene could be caught in situ to give the doubly homologated lithium product (III). Moreover, further evidence for our proposed pathway was obtained from a relevant deuterium-labelled crossover experiment utilizing deuterated tetrahydrofuran (THF-d8) as solvent. Thus, treatment of 4-methoxybromobenzene (1a) with isopropyllithium in THF-d8, led to the release of ethylene-d4. The expected deuterated product 3aTHF-d8 (Table 3) was obtained in 61% yield.
Cross-coupling of alkyl bromides with organolithiums
We next extended the iron catalysis strategy to alkyl bromides with organolithium reagents. Typically, commercially available 1-bromo-3-phenylpropane was assessed with n-BuLi to explore the possibility of C(sp3)-C(sp3) cross-coupling. Gratifyingly, the reaction proceeded smoothly and the desired product was isolated in 77% yield (Table 4, 3aa). Other organolithium reagents, such as cyclopropyllithium, 9H-fluoren-9-yllithium and (trimethylsilyl)methyllithium, were allowed to couple with 1-bromo-3-phenylpropane to provide the corresponding C(sp3)-C(sp3) cross-coupling products in good to excellent yields (Table 4, 3ab-3ad). Benzylic compounds, possessing a typical C(sp3)-Br bonds, were also used as coupling partners. As expected, the cross-coupling products with yields ranging from 11 to 71% were generated, when n-BuLi and (trimethylsilyl)methyllithium were used as coupling partners (Table 4, 3ae-3am). Under the same condition, 2-(3-bromopropyl)naphthalene was also alkylated (Table 4, 3an). Subsequently, bromocyclohexane successfully underwent a similar reaction to form the relevant coupling product in 44% yield (Table 4, 3ao).
In summary, we have disclosed iron-catalysed cross-coupling of organolithium compounds to form diverse carbon–carbon bonds efficiently. These results are expected to expand the scope of iron catalysis as well as the use of organolithium reagents. We trust that these reactions would provide milder, cheaper and more environmentally friendly approaches towards cross-coupling products. An extension of this catalytic system to broaden its scope, and to investigate its mechanistic nature is underway in our laboratory.
Discussion
To provide a support against the involvement of trace amounts of other metal species, such as Pd, Pt, Co and Ni in our iron catalysts that would catalyse C–C bond formation, inductively coupled plasma mass spectrometry was performed on samples of FeCl3 to detect the trace quantities of these metals (see Supplementary Information for details). Moreover, we conducted experiments to mimic the catalyst system to prove that relevant products were not isolated when the concentration of Co and Ni were as low as those present in the iron salts (see Supplementary Information for details). We also performed preliminary mechanistic analysis of this transformation utilizing several control experiments (see Supplementary Information for details). It was likely that the reaction involved radical species.
Noteworthy, the capability to procure useful product quantities for laboratory and industry usage through scalable routes is emerging as a very essential goal in catalytic reactions today. Therefore, we also confirmed the scalable feasibility of these iron-catalysed reactions, as shown in Fig. 5. As can be seen, several typical scale-up reactions in multi-gram scales provided relevant desired products in satisfied yields.
Methods
Iron-catalysed cross-coupling of 4-methoxybromobenzene (1a) and n-BuLi (2a)
To an oven-dried vial, equipped with a magnetic stirring bar, was charged with [(FeCl3)2(TMEDA)3] (3.96 mg, 0.006 mmol, 3 mol%) in a glove box, following by the subsequent addition of 4-methoxybromobenzene (1a; 0.2 mmol) and THF (1.0 ml). Then, after the sealed vial with a rubber stopper was taken out from the glove box, the reaction mixture was cooled to 0 °C, n-BuLi (2a; 0.30 mmol, 1.6 M or 2.4 M in hexane, diluted with THF to a final concentration of 0.35 M) was added to the mixture using a syringe pump in 1 h. After the addition was completed, the reaction mixture was stirred at 0 °C for 1 h. Then, after quenching with a saturated solution of aqueous NH4Cl, the reaction mixture was extracted with CH2Cl2 three times. The combined organic solvent was evaporated under reduced pressure to afford the crude product, which was then purified by column chromatography on silica gel or preparative thin-layer chromatography.
Additional information
How to cite this article: Jia, Z. et al. Iron-catalysed cross-coupling of organolithium compounds with organic halides. Nat. Commun. 7:10614 doi: 10.1038/ncomms10614 (2016).
Change history
14 June 2016
A correction has been published and is appended to both the HTML and PDF versions of this paper. The error has not been fixed in the paper.
References
de Meijere, A., Brase, S. & Oestreich, M. Metal-Catalyzed Cross-Coupling Reactions and More, Vol. 1, 2 and 3 Wiley-VCH (2014).
Negishi, E. Magical power of transition metals: past, present, and future. Angew. Chem. Int. Ed. 50, 6738–6764 (2011).
Nicolaou, K. C., Bulger, P. G. & Sarlah, D. Palladium-catalyzed cross-coupling reactions in total synthesis. Angew. Chem. Int. Ed. 44, 4442–4489 (2005).
Murahashi, S., Yamamura, M., Yanagisawa, K., Mita, N. & Kondo, K. Stereoselective synthesis of alkenes and alkenyl sulfides from alkenyl halides using palladium and ruthenium catalysts. J. Org. Chem. 44, 2408–2417 (1979).
Clayden, J. Organolithiums: Selectivity for Synthesis Oxford (2002).
Rappoport, Z. & Marek, I. The Chemistry of Organolithium Compounds (Wiley-VHC (2004).
Luisi, R. & Capriati, V. Lithium Compounds in Organic Synthesis: From Fundamentals to Applications Wiley-VCH (2014).
Giannerini, M., Fañanás-Mastral, M. & Feringa, B. L. Direct catalytic cross-coupling of organolithium compounds. Nat. Chem 5, 667–672 (2013).
Vila, C., Giannerini, M., Hornillos, V., Fañanás-Mastral, M. & Feringa, B. L. Palladium-catalysed direct cross-coupling of secondary alkyllithium reagents. Chem. Sci 5, 1361–1367 (2014).
Vila, C. et al. Palladium-catalysed direct cross-coupling of organolithium reagents with aryl and vinyl triflates. Chem.-Eur. J 20, 13078–13083 (2014).
Castello, L. M. et al. Palladium-catalyzed cross-coupling of aryllithium reagents with 2-alkoxy-substituted aryl chlorides: Mild and efficient synthesis of 3,3’-diaryl BINOLs. Org. Lett. 17, 62–65 (2015).
Hornillos, V., Giannerini, M., Vila, C., Fañanás-Mastral, M. & Feringa, B. L. Direct catalytic cross-coupling of alkenyllithium compounds. Chem. Sci 6, 1394–1398 (2015).
Heijnen, D., Hornillos, V., Corbet, B. P., Giannerini, M. & Feringa, B. L. Palladium-catalyzed C(sp3)–C(sp2) cross-coupling of (trimethylsilyl)methyllithium with (hetero)aryl halides. Org. Lett. 17, 2262–2265 (2015).
Nakamura, E. & Sato, K. Managing the scarcity of chemical elements. Nat. Mater. 10, 158–161 (2011).
Plietker, B. Iron Catalysis in Organic Chemistry Wiley-VCH (2008).
Bolm, C., Legros, J., Le Paih, J. & Zani, L. Iron-catalyzed reactions in organic synthesis. Chem. Rev. 104, 6217–6254 (2004).
Correa, A., Garcia Mancheno, O. & Bolm, C. Iron-catalysed carbon-heteroatom and heteroatom-heteroatom bond forming processes. Chem. Soc. Rev. 37, 1108–1117 (2008).
Sherry, B. D. & Fürstner, A. The promise and challenge of iron-catalyzed cross coupling. Acc. Chem. Res. 41, 1500–1511 (2008).
Bauer, I. & Knölker, H.-J. Iron catalysis in organic synthesis. Chem. Rev. 115, 3170–3387 (2015).
Bedford, R. B. How Low Does Iron Go? Chasing the active species in Fe-catalyzed cross-coupling reactions. Acc. Chem. Res. 48, 1485–1493 (2015).
Han, J.-W., Li, X. & Wong, H. N. C. Our expedition in eight-membered ring compounds: From planar dehydrocyclooctenes to tub-shaped chiral tetraphenylenes. Chem. Rec. 15, 107–131 (2015).
Han, J.-W., Chen, J.-X., Li, X., Peng, X.-S. & Wong, H. N. C. Recent developments and applications of chiral tetraphenylenes. Synlett. 24, 2188–2198 (2013).
Toummini, D., Ouazzani, F. & Taillefer, M. Iron-catalyzed homocoupling of aryl halides and derivatives in the presence of alkyllithiums. Org. Lett. 15, 4690–4693 (2013).
Fürstner, A. & Leitner, A. Iron-catalyzed cross-coupling reactions of alkyl-Grignard reagents with aryl chlorides, tosylates, and triflates. Angew. Chem. Int. Ed. 41, 609–612 (2002).
Fürstner, A., Leitner, A., Méndez, M. & Krause, H. Iron-catalyzed cross-coupling reactions. J. Am. Chem. Soc. 124, 13856–13863 (2002).
Fürstner, A. & Méndez, M. Iron-catalyzed cross-coupling reactions: Efficient synthesis of 2, 3-allenol derivatives. Angew. Chem. Int. Ed. 42, 5355–5357 (2003).
Scheiper, B., Glorius, F., Leitner, A. & Fürstner, A. Catalysis-based enantioselective total synthesis of the macrocyclic spermidine alkaloid isooncinotine. Proc. Natl Acad. Sci. USA 101, 11960–11965 (2004).
Nakamura, M., Matsuo, K., Ito, S. & Nakamura, E. Iron-catalyzed cross-coupling of primary and secondary alkyl halides with aryl Grignard reagents. J. Am. Chem. Soc. 126, 3686–3687 (2004).
Noda, D., Sunada, Y., Hatakeyama, T., Nakamura, M. & Nagashima, H. Effect of TMEDA on iron-catalyzed coupling reactions of ArMgX with Alkyl halides. J. Am. Chem. Soc. 131, 6078–6079 (2009).
Ito, S., Fujiwara, Y.-I., Nakamura, E. & Nakamura, M. Iron-catalyzed cross-coupling of alkyl sulfonates with arylzinc reagents. Org. Lett. 11, 4306–4309 (2009).
Bedford, R. B. et al. TMEDA in iron-catalyzed kumada coupling: Amine adduct versus homoleptic “ate” complex formation. Angew. Chem. Int. Ed. 53, 1804–1808 (2014).
Bartlett, P. D., Friedman, S. & Stiles, M. The reaction of isopropyllithium and t-butyllithium with simple olefins. J. Am. Chem. Soc. 75, 1771–1772 (1953).
Acknowledgements
This work was supported by a grant to the State Key Laboratory of Synthetic Chemistry from the Innovation and Technology Commission, the National Natural Science Foundation of China/Research Grants Council Joint Research Scheme (N_CUHK451/13), the Research Grants Council of the Hong Kong SAR, China (GRF Project 403012 and CRF projects), the Chinese Academy of Sciences-Croucher Foundation Funding Scheme for Joint Laboratories and the National Natural Science Foundation of China (NSFC no. 21272199). The Shenzhen Science and Technology Innovation Committee for the Municipal Key Laboratory Scheme (ZDSY20130401150914965) and the Shenzhen Basic Research Program (JCYJ20120619151721025, JCYJ20140425184428455) are also gratefully acknowledged.
Author information
Authors and Affiliations
Contributions
Z.J. performed the experiments and wrote the draft of the manuscript, Q.L. helped to perform experiments to assess scope of coupling-partners as well as to help preparing the manuscript. X.-S.P. and H.N.C.W. provided overall supervision. All authors discussed the results and commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Figures 1-72, Supplementary Tables 1-2, Supplementary Methods and Supplementary References (PDF 2585 kb)
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Jia, Z., Liu, Q., Peng, XS. et al. Iron-catalysed cross-coupling of organolithium compounds with organic halides. Nat Commun 7, 10614 (2016). https://doi.org/10.1038/ncomms10614
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/ncomms10614
This article is cited by
-
Organosodium compounds for catalytic cross-coupling
Nature Catalysis (2019)
-
Iron-Catalyzed C–H Functionalization Processes
Topics in Current Chemistry (2016)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.