Abstract
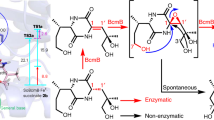
The [4+2] cycloaddition remains one of the most intriguing transformations in synthetic and natural products chemistry. In nature, however, there are remarkably few enzymes known to have this activity. We herein report an unprecedented enzymatic [4+2] cyclization cascade that has a central role in the biosynthesis of pyrroindomycins, which are pentacyclic spirotetramate natural products. Beginning with a linear intermediate that contains two pairs of 1,3-diene and alkene groups, the dedicated cyclases PyrE3 and PyrI4 act in tandem to catalyze the formation of two cyclohexene rings in the dialkyldecalin system and the tetramate spiro-conjugate of the molecules. The two cyclizations are completely enzyme dependent and proceed in a regio- and stereoselective manner to establish the enantiomerically pure pentacyclic core. Analysis of a related spirotetronate pathway confirms that homologs are functionally exchangeable, establishing the generality of these findings and explaining how nature creates diverse active molecules with similar rigid scaffolds.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Accession codes
References
Ding, W. et al. Pyrroindomycins, novel antibiotics produced by Streptomyces rugosporus sp. LL-42D005. I. Isolation and structure determination. J. Antibiot. (Tokyo) 47, 1250–1257 (1994).
Singh, M.P. et al. Pyrroindomycins, novel antibiotics produced by Streptomyces rugosporus LL-42D005. II. Biological activities. J. Antibiot. (Tokyo) 47, 1258–1265 (1994).
Vieweg, L., Reichau, S., Schobert, R., Leadlay, P.F. & Sussmuth, R.D. Recent advances in the field of bioactive tetronates. Nat. Prod. Rep. 31, 1554–1584 (2014).
Oikawa, H. & Tokiwano, T. Enzymatic catalysis of the Diels-Alder reaction in the biosynthesis of natural products. Nat. Prod. Rep. 21, 321–352 (2004).
Kim, H.J., Ruszczycky, M.W. & Liu, H.W. Current developments and challenges in the search for a naturally selected Diels-Alderase. Curr. Opin. Chem. Biol. 16, 124–131 (2012).
Kelly, W.L. Intramolecular cyclizations of polyketide biosynthesis: mining for a “Diels-Alderase”? Org. Biomol. Chem. 6, 4483–4493 (2008).
Townsend, C.A.A. “Diels-Alderase” at last. ChemBioChem 12, 2267–2269 (2011).
Kelly, W.L. Biochemistry: life imitates art. Nature 473, 35–36 (2011).
Wu, Q., Wu, Z., Qu, X. & Liu, W. Insights into pyrroindomycin biosynthesis reveal a uniform paradigm for tetramate/tetronate formation. J. Am. Chem. Soc. 134, 17342–17345 (2012).
Jia, X.Y. et al. Genetic characterization of the chlorothricin gene cluster as a model for spirotetronate antibiotic biosynthesis. Chem. Biol. 13, 575–585 (2006).
Fang, J. et al. Cloning and characterization of the tetrocarcin A gene cluster from Micromonospora chalcea NRRL 11289 reveals a highly conserved strategy for tetronate biosynthesis in spirotetronate antibiotics. J. Bacteriol. 190, 6014–6025 (2008).
Zhang, H. et al. Elucidation of the kijanimicin gene cluster: insights into the biosynthesis of spirotetronate antibiotics and nitrosugars. J. Am. Chem. Soc. 129, 14670–14683 (2007).
Li, S. et al. Dissecting glycosylation steps in lobophorin biosynthesis implies an iterative glycosyltransferase. Org. Lett. 15, 1374–1377 (2013).
Staunton, J. & Weissman, K.J. Polyketide biosynthesis: a millennium review. Nat. Prod. Rep. 18, 380–416 (2001).
Walsh, C.T. Polyketide and nonribosomal peptide antibiotics: modularity and versatility. Science 303, 1805–1810 (2004).
Weissman, K.J. & Leadlay, P.F. Combinatorial biosynthesis of reduced polyketides. Nat. Rev. Microbiol. 3, 925–936 (2005).
Sun, Y. et al. In vitro reconstruction of tetronate RK-682 biosynthesis. Nat. Chem. Biol. 6, 99–101 (2010).
Kanchanabanca, C. et al. Unusual acetylation-elimination in the formation of tetronate antibiotics. Angew. Chem. Int. Ed. Engl. 52, 5785–5788 (2013).
Koskiniemi, H. et al. Crystal structures of two aromatic hydroxylases involved in the early tailoring steps of angucycline biosynthesis. J. Mol. Biol. 372, 633–648 (2007).
Kallio, P. et al. Tracing the evolution of angucyclinone monooxygenases: structural determinants for C-12b hydroxylation and substrate inhibition in PgaE. Biochemistry 52, 4507–4516 (2013).
Wang, P. et al. Uncovering the enzymes that catalyze the final steps in oxytetracycline biosynthesis. J. Am. Chem. Soc. 135, 7138–7141 (2013).
Gottardi, E.M. et al. Abyssomicin biosynthesis: formation of an unusual polyketide, antibiotic-feeding studies and genetic analysis. ChemBioChem 12, 1401–1410 (2011).
He, H.Y. et al. Quartromicin biosynthesis: two alternative polyketide chains produced by one polyketide synthase assembly line. Chem. Biol. 19, 1313–1323 (2012).
Bisang, C. et al. A chain initiation factor common to both modular and aromatic polyketide synthases. Nature 401, 502–505 (1999).
Reeves, C.D. et al. Alteration of the substrate specificity of a modular polyketide synthase acyltransferase domain through site-specific mutations. Biochemistry 40, 15464–15470 (2001).
Keatinge-Clay, A.T. A tylosin ketoreductase reveals how chirality is determined in polyketides. Chem. Biol. 14, 898–908 (2007).
Wu, J., Zaleski, T.J., Valenzano, C., Khosla, C. & Cane, D.E. Polyketide double bond biosynthesis. Mechanistic analysis of the dehydratase-containing module 2 of the picromycin/methylmycin synthase. J. Am. Chem. Soc. 127, 17393–17404 (2005).
Kwan, D.H. et al. Prediction and manipulation of the stereochemistry of enoylreduction in modular polyketide synthases. Chem. Biol. 15, 1231–1240 (2008).
Bornemann, S. Flavoenzymes that catalyse reactions with no net redox change. Nat. Prod. Rep. 19, 761–772 (2002).
Hashimoto, T. et al. Biosynthesis of versipelostatin: identification of an enzyme-catalyzed [4+2]-cycloaddition required for macrocyclization of spirotetronate-containing polyketides. J. Am. Chem. Soc. 137, 572–575 (2015).
Ose, T. et al. Insight into a natural Diels-Alder reaction from the structure of macrophomate synthase. Nature 422, 185–189 (2003).
Oikawa, H., Katayama, K., Suzuki, Y. & Ichihara, A. Enzymatic activity catalysing exo-selective Diels-Alder reaction in solanapyrone biosynthesis. J. Chem. Soc. Chem. Commun.##1321–1322 (1995).
Auclair, K. et al. Lovastatin nonaketide synthase catalyzes an intramolecular Diels-Alder reaction of a substrate analogue. J. Am. Chem. Soc. 122, 11519–11520 (2000).
Ma, S.M. et al. Complete reconstitution of a highly reducing iterative polyketide synthase. Science 326, 589–592 (2009).
Kim, R.R. et al. Mechanistic insights on riboflavin synthase inspired by selective binding of the 6,7-dimethyl-8-ribityllumazine exomethylene anion. J. Am. Chem. Soc. 132, 2983–2990 (2010).
Kim, H.J., Ruszczycky, M.W., Choi, S.H., Liu, Y.N. & Liu, H.W. Enzyme-catalysed [4+2] cycloaddition is a key step in the biosynthesis of spinosyn A. Nature 473, 109–112 (2011).
Carey, F.A. in Organic Chemistry 4th edn. 365–397 (Mcgraw-Hill Higher Education, New York, 2000).
Roush, W.R., Reilly, M.L., Koyama, K. & Brown, B.B. A formal total synthesis of (+)-tetronolide, the aglycon of the tetrocarcins: enantio-and diastereoselective syntheses of the octahydronaphthalene (bottom-half) and spirotetronate (top-half) fragments. J. Org. Chem. 62, 8708–8721 (1997).
Roush, W.R. & Sciotti, R.J. Enantioselective total synthesis of (−)-chlorothricolide via the tandem inter- and intramolecular Diels−Alder reaction of a hexaenoate intermediate. J. Am. Chem. Soc. 120, 7411–7419 (1998).
Takahashi, S. et al. Reveromycin A biosynthesis uses RevG and RevJ for stereospecific spiroacetal formation. Nat. Chem. Biol. 7, 461–468 (2011).
Sun, P. et al. Spiroketal formation and modification in avermectin biosynthesis involves a dual activity of AveC. J. Am. Chem. Soc. 135, 1540–1548 (2013).
Green, M.R. & Sambrook, J. Molecular Cloning: a Laboratory Manual 4th edn. (Cold Spring Harbor Laboratory Press, 2012).
Keiser, T., Bibb, M.J., Buttner, M.J., Chater, K.F. & Hopwood, D.A. Practical Streptomyces Genetics (John Innes Foundation, 2000).
Frisch, M.J. et al. Gaussian 09, Revision B.01 (Gaussian, Inc., Wallingford CT, 2010).
Stephens, P.J. & Harada, N. ECD Cotton effect approximated by the Gaussian curve and other methods. Chirality 22, 229–233 (2010).
Varetto, U. MOLEKEL 5.4. (Swiss National Supercomputing Centre: Manno, Switzerland, 2009).
Roy, A., Kucukural, A. & Zhang, Y. I-TASSER: a unified platform for automated protein structure and function prediction. Nat. Protoc. 5, 725–738 (2010).
Acknowledgements
This work was supported in part by grants from the National Natural Science Foundation of China (91213303, 81202453, 31430005 and 91413101), STCSM (14JC1407700 and 13XD1404500), the Ministry of Science and Technology of China (2012AA02A706) and the National Research Foundation (OTKA K105871) of Hungary.
Author information
Authors and Affiliations
Contributions
Z.T., Z.W., H.Z., X.J. and D.C. performed the in vivo investigations. Z.T., Y.Y., S.Z., F.Y. and Q.Z. conducted the in vitro enzymatic investigations. P.S., Y.Y. and Z.W. characterized the chemical compounds. A.M. and T.K. conducted the DFT conformational analysis and TDDFT-ECD calculation. Z.T. performed the sequence analysis. Z.T., P.S. and W.L. analyzed the data and wrote the manuscript. W.L. directed the research.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Notes 1–3, Supplementary Figures 1–18 and Supplementary Tables 1–9. (PDF 6902 kb)
Supplementary Data Set
Crystallographic data for 3 (TXT 1823 kb)
Rights and permissions
About this article
Cite this article
Tian, Z., Sun, P., Yan, Y. et al. An enzymatic [4+2] cyclization cascade creates the pentacyclic core of pyrroindomycins. Nat Chem Biol 11, 259–265 (2015). https://doi.org/10.1038/nchembio.1769
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchembio.1769
This article is cited by
-
Tandem intermolecular [4 + 2] cycloadditions are catalysed by glycosylated enzymes for natural product biosynthesis
Nature Chemistry (2023)
-
A cyclase that catalyses competing 2 + 2 and 4 + 2 cycloadditions
Nature Chemistry (2023)
-
Selective cycloadditions
Nature Chemistry (2023)
-
An NmrA-like enzyme-catalysed redox-mediated Diels–Alder cycloaddition with anti-selectivity
Nature Chemistry (2023)
-
Discovery and characterization of a terpene biosynthetic pathway featuring a norbornene-forming Diels-Alderase
Nature Communications (2022)