Abstract
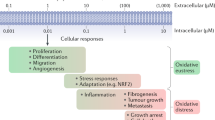
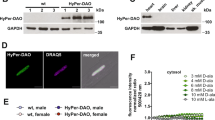
An emerging aspect of redox signaling is the pathway mediated by electrophilic byproducts, such as nitrated cyclic nucleotide (for example, 8-nitroguanosine 3′,5′-cyclic monophosphate (8-nitro-cGMP)) and nitro or keto derivatives of unsaturated fatty acids, generated via reactions of inflammation-related enzymes, reactive oxygen species, nitric oxide and secondary products. Here we report that enzymatically generated hydrogen sulfide anion (HS−) regulates the metabolism and signaling actions of various electrophiles. HS− reacts with electrophiles, best represented by 8-nitro-cGMP, via direct sulfhydration and modulates cellular redox signaling. The relevance of this reaction is reinforced by the significant 8-nitro-cGMP formation in mouse cardiac tissue after myocardial infarction that is modulated by alterations in HS− biosynthesis. Cardiac HS−, in turn, suppresses electrophile-mediated H-Ras activation and cardiac cell senescence, contributing to the beneficial effects of HS− on myocardial infarction–associated heart failure. Thus, this study reveals HS−-induced electrophile sulfhydration as a unique mechanism for regulating electrophile-mediated redox signaling.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Kajimura, M., Fukuda, R., Bateman, R.M., Yamamoto, T. & Suematsu, M. Interactions of multiple gas-transducing systems: hallmarks and uncertainties of CO, NO, and H2S gas biology. Antioxid. Redox Signal. 13, 157–192 (2010).
Li, L., Rose, P. & Moore, P.K. Hydrogen sulfide and cell signaling. Annu. Rev. Pharmacol. Toxicol. 51, 169–187 (2011).
Fridovich, I. The biology of oxygen radicals. Science 201, 875–880 (1978).
Sawa, T. et al. Protein S-guanylation by the biological signal 8-nitroguanosine 3′,5′-cyclic monophosphate. Nat. Chem. Biol. 3, 727–735 (2007).
Rhee, S.G. Cell signaling. H2O2, a necessary evil for cell signaling. Science 312, 1882–1883 (2006).
D′Autréaux, B. & Toledano, M.B. ROS as signalling molecules: mechanisms that generate specificity in ROS homeostasis. Nat. Rev. Mol. Cell Biol. 8, 813–824 (2007).
Akaike, T., Fujii, S., Sawa, T. & Ihara, H. Cell signaling mediated by nitrated cyclic guanine nucleotide. Nitric Oxide 23, 166–174 (2010).
Rudolph, T.K. & Freeman, B.A. Transduction of redox signaling by electrophile-protein reactions. Sci. Signal. 2, re7 (2009).
Forman, H.J., Fukuto, J.M. & Torres, M. Redox signaling: thiol chemistry defines which reactive oxygen and nitrogen species can act as second messengers. Am. J. Physiol. Cell Physiol. 287, C246–C256 (2004).
Zaki, M.H. et al. Cytoprotective function of heme oxygenase 1 induced by a nitrated cyclic nucleotide formed during murine salmonellosis. J. Immunol. 182, 3746–3756 (2009).
Uchida, K. & Shibata, T. 15-Deoxy-Δ12,14-prostaglandin J2: an electrophilic trigger of cellular responses. Chem. Res. Toxicol. 21, 138–144 (2008).
Fujii, S. et al. The critical role of nitric oxide signaling, via protein S-guanylation and nitrated cyclic GMP, in the antioxidant adaptive response. J. Biol. Chem. 285, 23970–23984 (2010).
Hughes, M.N., Centelles, M.N. & Moore, K.P. Making and working with hydrogen sulfide: the chemistry and generation of hydrogen sulfide in vitro and its measurement in vivo: a review. Free Radic. Biol. Med. 47, 1346–1353 (2009).
Tiranti, V. et al. Loss of ETHE1, a mitochondrial dioxygenase, causes fatal sulfide toxicity in ethylmalonic encephalopathy. Nat. Med. 15, 200–205 (2009).
Wintner, E.A. et al. A monobromobimane-based assay to measure the pharmacokinetic profile of reactive sulphide species in blood. Br. J. Pharmacol. 160, 941–957 (2010).
Winterbourn, C.C. & Metodiewa, D. Reactivity of biologically important thiol compounds with superoxide and hydrogen peroxide. Free Radic. Biol. Med. 27, 322–328 (1999).
Glushchenko, A.V. & Jocobsen, D.W. Molecular targeting of proteins by L-homocysteine: mechanistic implications for vascular disease. Antioxid. Redox Signal. 9, 1883–1898 (2007).
LoPachin, R.M., Gavin, T., Geohagen, B.C. & Das, S. Neurotoxic mechanisms of electrophilic type-2 alkenes: soft-soft interactions described by quantum mechanical parameters. Toxicol. Sci. 98, 561–570 (2007).
Ishii, I. et al. Murine cystathionine γ-lyase: complete cDNA and genomic sequences, promoter activity, tissue distribution and developmental expression. Biochem. J. 381, 113–123 (2004).
Lander, H.M. et al. Redox regulation of cell signalling. Nature 381, 380–381 (1996).
Oliva, J.L. et al. The cyclopentenone 15-deoxy-Δ12,14-prostaglandin J2 binds to and activates H-Ras. Proc. Natl. Acad. Sci. USA 100, 4772–4777 (2003).
Serrano, M., Lin, A.W., McCurrach, M.E., Beach, D. & Lowe, S.W. Oncogenic ras provokes premature cell senescence associated with accumulation of p53 and p16INK4a. Cell 88, 593–602 (1997).
Wu, C., Miloslavskaya, I., Demontis, S., Maestro, R. & Galaktionov, K. Regulation of cellular response to oncogenic and oxidative stress by Seladin-1. Nature 432, 640–645 (2004).
Shih, H., Lee, B., Lee, R.J. & Boyle, A.J. The aging heart and post-infarction left ventricular remodeling. J. Am. Coll. Cardiol. 57, 9–17 (2011).
Liu, Y.H. et al. Role of inducible nitric oxide synthase in cardiac function and remodeling in mice with heart failure due to myocardial infarction. Am. J. Physiol. Heart Circ. Physiol. 289, H2616–H2623 (2005).
Zhang, P. et al. Inducible nitric oxide synthase deficiency protects the heart from systolic overload-induced ventricular hypertrophy and congestive heart failure. Circ. Res. 100, 1089–1098 (2007).
Gelb, B.D. & Tartaglia, M. Ras signaling pathway mutations and hypertrophic cardiomyopathy: getting into and out of the thick of it. J. Clin. Invest. 121, 844–847 (2011).
Sano, M. et al. p53-induced inhibition of Hif-1 causes cardiac dysfunction during pressure overload. Nature 446, 444–448 (2007).
Hunter, J.J., Tanaka, N., Rockman, H.A., Ross, J. Jr. & Chien, K.R. Ventricular expression of a MLC-2v-ras fusion gene induces cardiac hypertrophy and selective diastolic dysfunction in transgenic mice. J. Biol. Chem. 270, 23173–23178 (1995).
Asano, K. et al. Constitutive and inducible nitric oxide synthase gene expression, regulation, and activity in human lung epithelial cells. Proc. Natl. Acad. Sci. USA 91, 10089–10093 (1994).
Pechkovsky, D.V. et al. Pattern of NOS2 and NOS3 mRNA expression in human A549 cells and primary cultured AEC II. Am. J. Physiol. Lung Cell Mol. Physiol. 282, L684–L692 (2002).
Nishida, M. et al. Heterologous down-regulation of angiotensin type 1 receptors by purinergic P2Y2 receptor stimulation through S-nitrosylation of NF-κB. Proc. Natl. Acad. Sci. USA 108, 6662–6667 (2011).
Hancock, J.F. Ras proteins: different signals from different locations. Nat. Rev. Mol. Cell Biol. 4, 373–384 (2003).
Prior, I.A. et al. GTP-dependent segregation of H-Ras from lipid rafts is required for biological activity. Nat. Cell Biol. 3, 368–375 (2001).
Roy, S. et al. Individual palmitoyl residues serve distinct roles in H-Ras trafficking, microlocalization, and signaling. Mol. Cell. Biol. 25, 6722–6733 (2005).
Carballal, S. et al. Reactivity of hydrogen sulfide with peroxynitrite and other oxidants of biological interest. Free Radic. Biol. Med. 50, 196–205 (2011).
Zhao, W., Zhang, J., Lu, Y. & Wang, R. The vasorelaxant effect of H2S as a novel endogenous gaseous KATP channel opener. EMBO J. 20, 6008–6016 (2001).
Bucci, M. et al. Hydrogen sulfide is an endogenous inhibitor of phosphodiesterase activity. Arterioscler. Thromb. Vasc. Biol. 30, 1998–2004 (2010).
Sun, P. et al. PRAK is essential for ras-induced senescence and tumor suppression. Cell 128, 295–308 (2007).
Yoshitake, J. et al. Nitric oxide as an endogenous mutagen for Sendai virus without antiviral activity. J. Virol. 78, 8709–8719 (2004).
Ohshima, H., Sawa, T. & Akaike, T. 8-Nitroguanine, a product of nitrative DNA damage caused by reactive nitrogen species: formation, occurrence, and implications in inflammation and carcinogenesis. Antioxid. Redox Signal. 8, 1033–1045 (2006).
Acknowledgements
We thank J.B. Gandy for her editing of the manuscript. Thanks are also due to T. Okamoto, S. Fujii, Md. M. Rahaman, S. Khan, K.A. Ahmed, J. Yoshitake, T. Matsunaga, M.H.A. Rahman, J. Sakamoto, J. Minkyung, K. Hara, M. Goto, F. Sohma, K. Taguchi, T. Miura, T. Toyama, Y. Shinkai, I. Ishii and M. Toyataka for technical assistance; S. Kasamatsu, T. Ida and K. Kunieda for 8-nitro-cGMP preparation; Y. Kawai for technical assistance with LC/MS/MS experiments; H. Arimoto for helpful discussion; and J. Wu for reading our paper to evaluate its concepts and interdisciplinary accessibility. This work was supported in part by Grants-in-Aid for Scientific Research and Grants-in-Aid for Scientific Research on Innovative Areas (Research in a Proposed Area) from the Ministry of Education, Sciences, Sports and Technology, Japan; a grant from the Japan Science and Technology Agency PRESTO program; grants from the Ministry of Health, Labor and Welfare of Japan; and grants from the US National Institutes of Health.
Author information
Authors and Affiliations
Contributions
M.N., T.A. and T. Sawa designed the experiment, performed analysis and wrote the paper; N.K., H. Inoue and T. Shibata performed MS analysis, cell biology and mouse and rat studies; K.O., H. Ihara, H.M., H.K., M.S. and M.Y. performed data analyses; B.A.F., A.v.d.V., K.U. and Y.K. designed the experiment and edited the paper.
Corresponding author
Ethics declarations
Competing interests
B.A.F. acknowledges financial interests in Complexa, Inc.
Supplementary information
Supplementary Text and Figures
Supplementary Methods and Supplementary Results (PDF 9048 kb)
Rights and permissions
About this article
Cite this article
Nishida, M., Sawa, T., Kitajima, N. et al. Hydrogen sulfide anion regulates redox signaling via electrophile sulfhydration. Nat Chem Biol 8, 714–724 (2012). https://doi.org/10.1038/nchembio.1018
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchembio.1018
This article is cited by
-
Sulfide regulation of cardiovascular function in health and disease
Nature Reviews Cardiology (2023)
-
Nanostructured Ce/CeO2-rGO: Highly Sensitive and Selective Electrochemical Hydrogen Sulfide (H2S) Sensor
Electrocatalysis (2023)
-
Supersulphides provide airway protection in viral and chronic lung diseases
Nature Communications (2023)
-
Progress on the reaction-based methods for detection of endogenous hydrogen sulfide
Analytical and Bioanalytical Chemistry (2022)
-
Spectroscopic study of cyclen-based 19F NMR probe for detection of hydrogen sulfide
Analytical Sciences (2022)