Abstract
Metastatic cancer is a systemic disease, and metastasis determinants might elicit completely different effects in various target organs. Here we show that tumour-secreted DKK1 is a serological marker of breast cancer metastasis organotropism and inhibits lung metastasis. DKK1 suppresses PTGS2-induced macrophage and neutrophil recruitment in lung metastases by antagonizing cancer cell non-canonical WNT/PCP–RAC1–JNK signalling. In the lungs, DKK1 also inhibits WNT/Ca2+–CaMKII–NF-κB signalling and suppresses LTBP1-mediated TGF-β secretion of cancer cells. In contrast, DKK1 promotes breast-to-bone metastasis by regulating canonical WNT signalling of osteoblasts. Importantly, targeting canonical WNT may not be beneficial to treatment of metastatic cancer, while combinatory therapy against JNK and TGF-β signalling effectively prevents metastasis to both the lungs and bone. Thus, DKK1 represents a class of Janus-faced molecules with dichotomous roles in organotropic metastasis, and our data provide a rationale for new anti-metastasis approaches.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Minn, A. J. et al. Genes that mediate breast cancer metastasis to lung. Nature 436, 518–524 (2005).
Kang, Y. et al. A multigenic program mediating breast cancer metastasis to bone. Cancer Cell 3, 537–549 (2003).
Bos, P. D. et al. Genes that mediate breast cancer metastasis to the brain. Nature 459, 1005–1009 (2009).
Michigami, T. et al. The effect of the bisphosphonate ibandronate on breast cancer metastasis to visceral organs. Breast Cancer Res. Treat. 75, 249–258 (2002).
De, A. Wnt/Ca2 + signaling pathway: a brief overview. Acta Biochim. Biophys. Sin (Shanghai) 43, 745–756 (2011).
Gao, C. & Chen, Y. G. Dishevelled: the hub of Wnt signaling. Cell Signal. 22, 717–727 (2010).
Glinka, A. et al. Dickkopf-1 is a member of a new family of secreted proteins and functions in head induction. Nature 391, 357–362 (1998).
Caneparo, L. et al. Dickkopf-1 regulates gastrulation movements by coordinated modulation of Wnt/β catenin and Wnt/PCP activities, through interaction with the Dally-like homolog Knypek. Genes Dev. 21, 465–480 (2007).
Cha, S. W., Tadjuidje, E., Tao, Q., Wylie, C. & Heasman, J. Wnt5a and Wnt11 interact in a maternal Dkk1-regulated fashion to activate both canonical and non-canonical signaling in Xenopus axis formation. Development 135, 3719–3729 (2008).
Pukrop, T. et al. Wnt 5a signaling is critical for macrophage-induced invasion of breast cancer cell lines. Proc. Natl Acad. Sci. USA 103, 5454–5459 (2006).
Mikheev, A. M. et al. Dickkopf-1 mediated tumor suppression in human breast carcinoma cells. Breast Cancer Res. Treat. 112, 263–273 (2008).
Anastas, J. N. & Moon, R. T. WNT signalling pathways as therapeutic targets in cancer. Nat. Rev. Cancer 13, 11–26 (2013).
Krishnan, V., Bryant, H. U. & Macdougald, O. A. Regulation of bone mass by Wnt signaling. J. Clin. Invest. 116, 1202–1209 (2006).
Wang, F. S. et al. Knocking down dickkopf-1 alleviates estrogen deficiency induction of bone loss. A histomorphological study in ovariectomized rats. Bone 40, 485–492 (2007).
Mundy, G. R. Metastasis to bone: causes, consequences and therapeutic opportunities. Nat. Rev. Cancer 2, 584–593 (2002).
Mariz, K., Ingolf, J. B., Daniel, H., Teresa, N. J. & Erich-Franz, S. The Wnt inhibitor dickkopf-1: a link between breast cancer and bone metastases. Clin. Exp. Metastasis 32, 857–866 (2015).
Minn, A. J. et al. Lung metastasis genes couple breast tumor size and metastatic spread. Proc. Natl Acad. Sci. USA 104, 6740–6745 (2007).
Smid, M. et al. Subtypes of breast cancer show preferential site of relapse. Cancer Res. 68, 3108–3114 (2008).
Labelle, M., Begum, S. & Hynes, R. O. Platelets guide the formation of early metastatic niches. Proc. Natl Acad. Sci. USA 111, E3053–E3061 (2014).
Acharyya, S. et al. A CXCL1 paracrine network links cancer chemoresistance and metastasis. Cell 150, 165–178 (2012).
Lin, E. Y., Nguyen, A. V., Russell, R. G. & Pollard, J. W. Colony-stimulating factor 1 promotes progression of mammary tumors to malignancy. J. Exp. Med. 193, 727–740 (2001).
Qian, B.-Z. et al. CCL2 recruits inflammatory monocytes to facilitate breast-tumour metastasis. Nature 475, 222–225 (2011).
Malanchi, I. et al. Interactions between cancer stem cells and their niche govern metastatic colonization. Nature 481, 85–89 (2012).
Ghajar, C. M. et al. The perivascular niche regulates breast tumour dormancy. Nat. Cell Biol. 15, 807–817 (2013).
Karnoub, A. E. et al. Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature 449, 557–563 (2007).
Malladi, S. et al. Metastatic latency and immune evasion through autocrine inhibition of WNT. Cell 165, 45–60 (2016).
Coffelt, S. B., Wellenstein, M. D. & de Visser, K. E. Neutrophils in cancer: neutral no more. Nat. Rev. Cancer 16, 431–446 (2016).
Niida, A. et al. DKK1, a negative regulator of Wnt signaling, is a target of the β-catenin/TCF pathway. Oncogene 23, 8520–8526 (2004).
Shi, C. & Pamer, E. G. Monocyte recruitment during infection and inflammation. Nat. Rev. Immunol. 11, 762–774 (2011).
Talmadge, J. E. & Gabrilovich, D. I. History of myeloid-derived suppressor cells. Nat. Rev. Cancer 13, 739–752 (2013).
Kalinski, P. Regulation of immune responses by prostaglandin E2. J. Immunol. 188, 21–28 (2012).
Miyazono, K., Olofsson, A., Colosetti, P. & Heldin, C. H. A role of the latent TGF-β1-binding protein in the assembly and secretion of TGF-β 1. EMBO J. 10, 1091–1101 (1991).
Padua, D. et al. TGF-β primes breast tumors for lung metastasis seeding through angiopoietin-like 4. Cell 133, 66–77 (2008).
Gleizes, P. E. et al. TGF-β latency: biological significance and mechanisms of activation. Stem Cells 15, 190–197 (1997).
Lelekakis, M. et al. A novel orthotopic model of breast cancer metastasis to bone. Clin. Exp. Metastasis 17, 163–170 (1999).
Boyce, B. F. & Xing, L. Functions of RANKL/RANK/OPG in bone modeling and remodeling. Arch Biochem. Biophys. 473, 139–146 (2008).
Buijs, J. T., Stayrook, K. R. & Guise, T. A. The role of TGF-β in bone metastasis: novel therapeutic perspectives. BoneKEy Rep. 1, 96 (2012).
Bronte, V. et al. Recommendations for myeloid-derived suppressor cell nomenclature and characterization standards. Nat. Commun. 7, 12150 (2016).
Lu, D. et al. Dkk3 prevents familial dilated cardiomyopathy development through Wnt pathway. Lab Invest. 96, 239–248 (2016).
Hoang, B. H. et al. Dickkopf 3 inhibits invasion and motility of Saos-2 osteosarcoma cells by modulating the Wnt-β-catenin pathway. Cancer Res. 64, 2734–2739 (2004).
Krupnik, V. E. et al. Functional and structural diversity of the human Dickkopf gene family. Gene 238, 301–313 (1999).
Mao, B. & Niehrs, C. Kremen2 modulates Dickkopf2 activity during Wnt/LRP6 signaling. Gene 302, 179–183 (2003).
Li, L., Mao, J., Sun, L., Liu, W. & Wu, D. Second cysteine-rich domain of Dickkopf-2 activates canonical Wnt signaling pathway via LRP-6 independently of dishevelled. J. Biol. Chem. 277, 5977–5981 (2002).
Fatima, S. et al. Dickkopf 4 (DKK4) acts on Wnt/β-catenin pathway by influencing β-catenin in hepatocellular carcinoma. Oncogene 31, 4233–4244 (2012).
Niehrs, C. Function and biological roles of the Dickkopf family of Wnt modulators. Oncogene 25, 7469–7481 (2006).
Wang, Y. et al. DLC1-dependent parathyroid hormone-like hormone inhibition suppresses breast cancer bone metastasis. J. Clin. Invest. 124, 1646–1659 (2014).
Aslakson, C. J. & Miller, F. R. Selective events in the metastatic process defined by analysis of the sequential dissemination of subpopulations of a mouse mammary tumor. Cancer Res. 52, 1399–1405 (1992).
Santner, S. J. et al. Malignant MCF10CA1 cell lines derived from premalignant human breast epithelial MCF10AT cells. Breast Cancer Res. Treat. 65, 101–110 (2001).
Jin, L. et al. Differential secretome analysis reveals CST6 as a suppressor of breast cancer bone metastasis. Cell Res. 22, 1356–1373 (2012).
Weischenfeldt, J. & Porse, B. Bone marrow-derived macrophages (BMM): Isolation and applications. CSH Protoc. 2008 (2008) pdb prot5080.
Di Mitri, D. et al. Tumour-infiltrating Gr-1+ myeloid cells antagonize senescence in cancer. Nature 515, 134–137 (2014).
Liang, Y. et al. Epigenetic activation of TWIST1 by MTDH promotes cancer stem-like cell traits in breast cancer. Cancer Res. 75, 3672–3680 (2015).
Acknowledgements
We thank L. Li at SIBS for providing the TOP/FOPFLASH, ΔN-CTNNB1 and ΔC-LEF1 constructs; D. Xie at SIBS for providing the ΔN-TCF4 construct; Y. Xiao at SIBS for providing the RELA construct; M. Liu at SIBS for providing the PTGS2 construct; and X. Miao, H. Zhang, S Yan and P. Zhou at the Institute of Health Sciences core facilities for technical support. G.H. was funded by the National Natural Science Foundation of China (81430070, 81661148048, 31371409), the Chinese Academy of Sciences (QYZDB-SSW-SMC013, XDA12050101), the Ministry of Science and Technology of China (2013CB910904) and the Science and Technology Commission of Shanghai Municipality (14431900800).
Author information
Authors and Affiliations
Contributions
G.H. supervised the work. X.Zhuang and G.H. designed the experiments and drafted the manuscript. X.Zhuang, H.Z., M.C., F.P., J.Y. and X.Zhang performed the experiments. X.L. and Q.Y. contributed in clinical sample collection and analysis. Q.Y. helped design the experiments. All authors discussed the results and commented on the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
Supplementary Figure 1 Analyses of the roles of DKK1 in lung metastasis and intrinsic malignant properties of cancer cells.
(a) Serological DKK3 levels in Qilu breast cancer patients with different metastatic status. DKK2 and DKK4 were undetected in the majority (>96%) of serum samples. (b) mRNA analyses to validate DKK1 overexpression and knockdown (KD) in SCP28 cells (n = 3 biologically independent samples per group). (c) TOPFLASH luciferase activity in HeLa with or without recombinant WNT3A (rWNT3A) treatment to validate DKK1 overexpression (n = 6 biologically independent samples per group). (d) BLI of lung metastasis by SCP28 with or without DKK1 overexpression 7 days after intravenous injection (n = 10 mice per group). (e) Growth curve of lung metastasis by SCP28 from week 4 to 9 (n = 10 mice per group). (f) Validation of murine Dkk1 overexpression in 4T1 and the MMTV-PyMT-derived primary cancer cells (n = 3 biologically independent samples per group). (g–k) Analysis of in vitro cell growth (g, n = 4 biologically independent samples per group), tumor sphere formation (h, n = 3 biologically independent samples per group), cell apoptosis (i), wound-healing migration (j, n = 13 and 7 biologically independent samples per group for the left and right panel, respectively), and trans-endothelium invasion across lung-derived ST1.6R or bone-derived HBMEC-60 endothelial cells (k, n = 3 biologically independent samples per group) after DKK1 overexpression in SCP28. ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001 by repeated measures ANOVA (e) or Student’s t test (others). Unprocessed original scans of blots are shown in Supplementary Fig. 9. Box plots display values of minimum, first quartile, median, third quartile, and maximum. Bar graphs are shown as mean ± s.d.
Supplementary Figure 2 DKK1 suppresses macrophage and neutrophil recruitment in lung metastases.
(a) Workflow of flow cytometry for stromal content of lung metastases. Cancer cells were transfected with GFP and GFP− cells were gated as host cells. (b) Proportions of endothelial cells, fibroblasts, mesenchymal stem cells (MSCs) and natural killer (NK) cells in stroma of lung metastases with DKK1 overexpression or knockdown (n = 8 and n = 3 biologically independent samples in the left and right panel, respectively). (c) Analyses of CD11b+Gr-1+ myeloid cells and macrophages in spleen and bone marrow of mice injected with SCP28. (d) Upper, the CD11b+ cells in lung metastases were stained with antibodies against Ly6G (1A8) and Ly6C (HK1.4). Lower, the CD11b+Ly6Cm/hi cells in lung metastases were sequentially stained with antibodies against Ly6G (1A8) and Gr-1 (RB6). (e) CD11b+Ly6G−Ly6Chi cell contents in SCP28 lung metastases. (f) Expression of granulocytic and monocytic markers in different CD11b+ cell subsets. n = 4 biologically independent samples per group. (g) Giemsa staining of CD11b+Ly6G+Ly6Cm and CD11b+Ly6G−Ly6Chi cells in lung metastases. (h) Growth of THP-1 cultured with CM of control or DKK1-overexpressing SCP28 (n = 3 biologically independent samples). (i) DKK1 knockdown in MCF7. (j) Recruitment of bone marrow-derived macrophages by CM of control or Dkk1-overexpressing 4T1 (n = 3 biologically independent samples). The cell identity is validated by F4/80 immunofluorescent staining (left). Scale bar, 50 μm. (k,l) Validation of macrophage and neutrophil clearance in lung metastases by clodronate liposome and anti-Gr-1 (RB6-8C5) or anti-Ly6G (1A8). (m) Lung metastasis BLI quantitation of control and DKK1 knockdown SCP28 in mice treated with both clodronate liposome and RB6-8C5 (n = 5 mice per group). (n) In vitro growth of control and DKK1-overexpressing SCP28, when co-cultured with different amount of bone marrow-derived macrophages (n = 3 biologically independent samples). ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001; ns, not significant, by Student’s t test. Statistical source data for c are provided in Supplementary Table 6. Unprocessed original scans of blots are shown in Supplementary Fig. 9. Box plots display values of minimum, first quartile, median, third quartile, and maximum. Bar graphs are shown as mean ± s.d.
Supplementary Figure 3 The role of DKK1 in immunocyte suppression is mediated by regulating non-canonical WNT signaling pathway.
(a) ΔC-LEF1 (left), ΔN-TCF4 (upper right) and ΔN-CTNNB1 (lower right) overexpression in SCP28 with DKK1 knockdown or overexpression. (b–d) The regulatory function of ΔC-LEF1, ΔN-TCF4 and ΔN-CTNNB1 on canonical WNT signalling was validated by TOP/FOP reporter ratios at the basal status (b, n = 4 and n = 6 biologically independent samples for the left and right panel, respectively) or after LiCl induction (c, n = 6 biologically independent samples per group), or the changes in mRNA levels of WNT target genes LEF1 and AXIN2 in HeLa cells. (d, n = 3 biologically independent samples). (e) SCP28 Lung metastasis of intravenously injected SCP28 with DKK1 knockdown and ΔC-LEF1 (n = 9 mice per group). (f) THP-1 migration induced by CM of SCP28 with DKK1 knockdown and ΔC-LEF1 (n = 8 biologically independent samples per group). (g,h) Representative cell sorting results for macrophage and CD11b+Gr-1+ myeloid contents in lung metastases by SCP28 with DKK1 overexpression and/or ΔN-CTNNB1 (g), or SCP28 with DKK1 knockdown and ΔC-LEF1 or ΔN-TCF4 (h). (i) WNT5A overexpression in SCP28 and the upregulation of downstream non-canonical WNT signaling (p65 and JUN), and PTGS2 and LTBP1. (j) THP-1 migration recruited by CM of WNT5A-overexpressing SCP28 (n = 8 biologically independent samples per group). (k) The effects of DKK1 and WNT5A dual overexpression on PTGS2 and LTBP1 expression, JUN and p65 phosphorylation. (l,m) The effects of expressing other WNT inhibitory ligands, DKK2-4 and SFRP2, on PTGS2 and LTBP1 expression (l) and THP-1 migration (m, n = 7 biologically independent samples per group). ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.01, by ANOVA followed by Dunnett’s (b,c,e,f), or Student’s t test (others). Statistical source data for h are provided in Supplementary Table 6. Unprocessed original scans of blots are shown in Supplementary Fig. 9. Box plots display values of minimum, first quartile, median, third quartile, and maximum. Bar graphs are shown as mean ± s.d.
Supplementary Figure 4 DKK1 suppresses immunocyte recruitment by regulating the RAC1-JNK-PTGS2 axis.
(a) JUN and p65 phosphorylation and PTGS2 expression after DKK1 overexpression in 4T1.2 and MCF10CA1a. (b) RAC1 activation and JUN, JNK, CamKII and p65 phosphorylation after DKK1 knockdown in SCP28. (c,d) ERK1/2, AKT, PKC, p38 phosphorylation and Rho activation after DKK1 overexpression or knockdown in SCP28. (e) The effect of DKK1 on NFAT signaling in SCP28. Shown are the expression levels of various NFAT family members in SPC28 (left, n = 3 biologically independent samples per group), and the cytoplasmic and nuclear proportion of the mainly expressed NFATc3 after DKK1 overexpression. (f) THP-1 recruitment was not affected by direct administration of SP600125 into CM from control or DKK1-overexpressing SCP28 cells (n = 8 biologically independent samples per group). (g) Ptgs2 mRNA level after Dkk1 overexpression in 4T1.2 (n = 3 biologically independent samples per group). (h) PTGS2 expression was not affected by inhibitors of PKC (Gö6850) and CaMKII (KN-93) in SCP28 (n = 3 biologically independent samples per group). (i) Primary murine macrophage recruitment by 4T1 CM pre-treated with SP600125 or directedly administrated PGE2 (n = 3 biologically independent samples per group). (j) The expression of PTGER1-4 in various murine cell lines and bone marrow (BM)-derived cells (left), and in various human cell lines (right). n = 3 biologically independent samples per group. Mφ M0 and M2 refer to naïve macrophages derived from murine bone marrow, and those further differentiated by mIL13 treatment (25 ng ml−1, 72 h); THP-1 M0, M1 and M2 refer to naïve, M1 and M2 macrophages derived from THP-1, by treatments of PMA (30 ng ml−1, 72 h), PMA (72 h) + LPS (100 ng ml−1, 66 h, 6 h after PMA treatment) and PMA (6h) + IL13 (25 ng ml−1, 66 h, 6 h after PMA treatment). ∗∗P < 0.01, ∗P < 0.05 by Student’s t test. Unprocessed original scans of blots are shown in Supplementary Fig. 9. Box plots display values of minimum, first quartile, median, third quartile, and maximum. Bar graphs are shown as mean ± s.d.
Supplementary Figure 5 DKK1 regulates LTBP1 and TGF-β bioavailability by suppressing NF-κB.
(a) LTBP1 mRNA level in SCP28 after DKK1 overexpression (n = 3 biologically independent samples per group). (b) LTBP1 secretion level in 4T1.2 and MCF10CA1a after DKK1 overexpression (n = 3 biologically independent samples per group). (c) TGF-β1 mRNA level in SCP28 after DKK1 overexpression. n = 3 biologically independent samples per group. (d) SBE activity (n = 5 biologically independent samples per group) in HeLa cells treated by CM from DKK1-overexpressing SCP28, with or without heating activation of latent TGF-β1 in the CM. (e) The effects of PKC (Gö6850) and CaMKII (KN-93) inhibitors on p65 phosphorylation and LTBP1 expression in SCP28 (left), or SCP28 with DKK1 overexpression (middle) or knockdown (right). (f) The effects of KN-93 on p65 phosphorylation in MCF10CA1a with DKK1 overexpression. (g) The effects of SP600125 and BAY11-7082 on LTBP1 expression in Dkk1-overexpressing 4T1.2 (upper), and in SCP28 with DKK1 knockdown (lower). (h) The effect of RELA (p65) and IkBαM on LTBP1 expression in SCP28 with DKK1 overexpression. (i) NF-κB responsive reporter activity in HeLa cells transfected with DKK1 or IκBαM (n = 4 biologically independent samples per group). (j) p65 nuclear localization analyses in SCP28 after DKK1 overexpression by immunofluorescent staining (left) and subcellular fractionation (right). (k) Scheme of LTBP1 promoter reporter construction for NF-κB binding site analysis. The sequence of predicted NF-κB binding site was shown. (l) Luciferase activity of LTBP1 wild-type or truncated (ΔPLTBP1, without the NF-κB binding site) promoter in HeLa treated with NF-κB inhibitor BAY11-7082 or IkBαM (n = 4 biologically independent samples per group). ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.01, by ANOVA followed by Dunnett’s test (i) or Student’s t test (others). Unprocessed original scans of blots are shown in Supplementary Fig. 9. Bar graphs are shown as mean ± s.d.
Supplementary Figure 6 PTGS2 and LTBP1 mediate the role of DKK1 in lung metastasis.
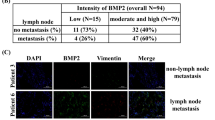
(a) SCP28 CM-induced THP-1 recruitment was not affected by LTBP1 knockdown (n = 8 biologically independent samples per group). (b) IHC analysis of p-SMAD3 in lung metastases by intravenously injected SCP28 with DKK1 KD3 and LTBP1 KD2 (n = 12 samples from 3 mice per group). Scale bar, 50 μm. (c) Expression of DKK1, PTGS2 and LTBP1 in breast cancer cells with varied lung (upper) and bone (lower) metastatic capabilities. n = 8 and 14 biologically independent samples for the upper and lower panel, respectively. (d) Serological DKK1 and PGE2 levels of the Qilu cohort patients (n = 60 patient serum samples). (e) Immunohistochemistry staining of DKK1, LTBP1 and PTGS2 in the Qilu cohort (n = 13 individual primary tumors per group). Shown on right are the representative images of DKK1, LTBP1 and PTGS2 IHC staining. Scale bars, 50 μm. ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001 by ANOVA followed by Dunnett’s test (b), Pearson correlation analysis (d) or Student’s t test (others). Box plots display values of minimum, first quartile, median, third quartile, and maximum. Bar graphs are shown as mean ± s.d.
Supplementary Figure 7 DKK1 promotes osteoclastogenesis by regulating osteoblast canonical WNT signaling.
(a) Intracardiac injection of SCP28 with DKK1 overexpression (n = 10 mice per group). Shown are bioluminescent imaging (BLI) analyses (left), representative BLI, X-ray, H/E, TRAP and Ki-67 staining of bone metastases (middle), and TRAP+ cell quantification along tumor-bone interface (right, n = 24 samples from 3 mice per group). middle, arrows point to overt bone lesions and TRAP+ cells; letters B and T denote bone and tumor areas. (b) Intracardiac injection of SCP28 cells with DKK1 knockdown (n = 10 mice per group). Shown are BLI and X-ray analyses (left), osteolytic area quantification (middle), and paralysis rates (right). (c) Primary tumor growth rate of orthotopically injected SCP28 cells with DKK1 overexpression in NOD/SCID mice (n = 10 mice per group). (d,e) Orthotopic injection of 4T1.2 cells with Dkk1 overexpression in BALB/c mice for bone and lung metastasis (n = 8 mice per group). Shown are validation of Dkk1 overexpression and 4T1.2 primary tumor growth (d), colony-formation analyses of cells in animal blood to quantitate the circulating cancer cells (e). (f) Osteoclastogenesis of primary bone marrow cultured in CM of SCP28 with DKK1 overexpression and knockdown (n = 4 biologically independent samples per group). Shown in the middle are representative images of TRAP staining. Arrow heads denote mature osteoclasts. (g) Osteoclastogenesis of primary bone marrow cultured in CM of MCF7 with DKK1 knockdown. n = 3 biologically independent samples for each group. (h) Rankl/Opg expression ratio of C2C12 and MC3T3 treated with recombinant human DKK1 and/or WNT3A proteins. n = 4 biologically independent samples for each group. Scale bars, 50 μm. ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001; ns, not significant, by repeated measures ANOVA (c,d), ANOVA followed by Dunnett’s (b,f right panel and g) or Holm’s test (h), or Student’s t test (others). Statistical source data for a are provided in Supplementary Table 6. Unprocessed original scans of blots are shown in Supplementary Fig. 9. Box plots display values of minimum, first quartile, median, third quartile, and maximum. Bar graphs are shown as mean ± s.d.
Supplementary Figure 8 The anti-metastasis effects of targeting canonical WNT or combinatory targeting of JNK and TGF-β.
(a–c) BALB/c mice with 4T1.2 orthotopic tumors were treated with the canonical WNT inhibitor XAV939 (n = 8 samples from 3 mice per group). Shown were CTNNB1 IHC staining of 4T1.2 primary tumors to validate the effect of XAV939 on WNT signaling (a, n = 8 biologically independent samples per group; Scale bar, 50 μm), primary tumor growth (b, n = 8 biologically independent animals per group), and quantitation of circulating tumor cells (c, n = 3 biologically independent animals per group). Circulating tumor cells were quantitated by qPCR of the Neomycin resistant marker gene, which was used to label the cancer cells. (d) Phosphorylated SMAD3 and JUN IHC in 4T1.2 primary tumors to validate the effects of the inhibitors on JNK-JUN and TGF-β signaling, in BALB/c mice treated with SP600125 (SP), SB431542 (SB) or both. (e) Osteoclastogenesis of primary bone marrow treated with SP600125 or SB431542 (n = 4 and n = 5 biologically independent samples per group for the left and right panel, respectively). ∗P < 0.05, ∗∗∗P < 0.001, ns, not significant, by repeated measures ANOVA (b), or Student’s t test (others). Bar graphs are shown as mean ± s.d.
Supplementary information
Supplementary Information
Supplementary Information (PDF 4114 kb)
Supplementary Table 1
Supplementary Information (XLS 15 kb)
Supplementary Table 2
Supplementary Information (XLS 15 kb)
Supplementary Table 3
Supplementary Information (XLS 15 kb)
Supplementary Table 4
Supplementary Information (XLSX 11 kb)
Supplementary Table 5
Supplementary Information (XLSX 16 kb)
Supplementary Table 6
Supplementary Information (XLSX 44 kb)
Rights and permissions
About this article
Cite this article
Zhuang, X., Zhang, H., Li, X. et al. Differential effects on lung and bone metastasis of breast cancer by Wnt signalling inhibitor DKK1. Nat Cell Biol 19, 1274–1285 (2017). https://doi.org/10.1038/ncb3613
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb3613
This article is cited by
-
The oncolytic bacteria-mediated delivery system of CCDC25 nucleic acid drug inhibits neutrophil extracellular traps induced tumor metastasis
Journal of Nanobiotechnology (2024)
-
LASS2 enhances chemosensitivity to cisplatin by inhibiting PP2A-mediated β-catenin dephosphorylation in a subset of stem-like bladder cancer cells
BMC Medicine (2024)
-
Fibroblasts inhibit osteogenesis by regulating nuclear-cytoplasmic shuttling of YAP in mesenchymal stem cells and secreting DKK1
Biological Research (2024)
-
LncRNA Malat1 suppresses pyroptosis and T cell-mediated killing of incipient metastatic cells
Nature Cancer (2024)
-
Ischemia-reperfusion injury: molecular mechanisms and therapeutic targets
Signal Transduction and Targeted Therapy (2024)