Abstract
Fuelled by the obesity epidemic, there is considerable interest in the developmental origins of white adipose tissue (WAT) and the stem and progenitor cells from which it arises. Whereas increased visceral fat mass is associated with metabolic dysfunction, increased subcutaneous WAT is protective. There are six visceral fat depots: perirenal, gonadal, epicardial, retroperitoneal, omental and mesenteric, and it is a subject of much debate whether these have a common developmental origin and whether this differs from that for subcutaneous WAT. Here we show that all six visceral WAT depots receive a significant contribution from cells expressing Wt1 late in gestation. Conversely, no subcutaneous WAT or brown adipose tissue arises from Wt1-expressing cells. Postnatally, a subset of visceral WAT continues to arise from Wt1-expressing cells, consistent with the finding that Wt1 marks a proportion of cell populations enriched in WAT progenitors. We show that all visceral fat depots have a mesothelial layer like the visceral organs with which they are associated, and provide several lines of evidence that Wt1-expressing mesothelium can produce adipocytes. These results reveal a major ontogenetic difference between visceral and subcutaneous WAT, and pinpoint the lateral plate mesoderm as a major source of visceral WAT. They also support the notion that visceral WAT progenitors are heterogeneous, and suggest that mesothelium is a source of adipocytes.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Rodeheffer, M. S., Birsoy, K. & Friedman, J. M. Identification of white adipocyte progenitor cells in vivo. Cell 135, 240–249 (2008).
Tang, W. et al. White fat progenitor cells reside in the adipose vasculature. Science 322, 583–586 (2008).
Tran, K. V. et al. The vascular endothelium of the adipose tissue gives rise to both white and brown fat cells. Cell Metab. 15, 222–229 (2012).
Berry, R. & Rodeheffer, M. S. Characterization of the adipocyte cellular lineage in vivo. Nat. Cell Biol. 15, 302–308 (2013).
Berry, R. et al. Esrrg functions in early branch generation of the ureteric bud and is essential for normal development of the renal papilla. Hum. Mol. Genet. 20, 917–926 (2011).
Billon, N. & Dani, C. Developmental origins of the adipocyte lineage: new insights from genetics and genomics studies. Stem. Cell Rev. 8, 55–66 (2012).
Macotela, Y. et al. Intrinsic differences in adipocyte precursor cells from different white fat depots. Diabetes 61, 1691–1699 (2012).
Tchkonia, T. et al. Identification of depot-specific human fat cell progenitors through distinct expression profiles and developmental gene patterns. Am. J. Physiol. Endocrinol. Metab. 292, E298–E307 (2007).
Yamamoto, Y. et al. Adipose depots possess unique developmental gene signatures. Obesity 18, 872–878 (2010).
Martinez-Estrada, O. M. et al. Wt1 is required for cardiovascular progenitor cell formation through transcriptional control of Snail and E-cadherin. Nat. Genet. 42, 89–93 (2010).
Essafi, A. et al. A wt1-controlled chromatin switching mechanism underpins tissue-specific wnt4 activation and repression. Dev. Cell 21, 559–574 (2011).
Chau, Y. Y. et al. Acute multiple organ failure in adult mice deleted for the developmental regulator Wt1. PLoS Genet. 7, e1002404 (2011).
Muzumdar, M. D., Tasic, B., Miyamichi, K., Li, L. & Luo, L. A global double-fluorescent Cre reporter mouse. Genesis 45, 593–605 (2007).
Chau, Y. Y. & Hastie, N. D. The role of Wt1 in regulating mesenchyme in cancer, development, and tissue homeostasis. Trends Genet. 28, 515–524 (2012).
Wilm, B., Ipenberg, A., Hastie, N. D., Burch, J. B. & Bader, D. M. The serosal mesothelium is a major source of smooth muscle cells of the gut vasculature. Dev. 132, 5317–5328 (2005).
Rosito, G. A. et al. Pericardial fat, visceral abdominal fat, cardiovascular disease risk factors, and vascular calcification in a community-based sample: the Framingham Heart Study. Circulation 117, 605–613 (2008).
Ding, J. et al. The association of pericardial fat with incident coronary heart disease: The multi-ethnic study of atherosclerosis (MESA). Am. J. Clin. Nutr. 90, 499–504 (2009).
Asahina, K., Zhou, B., Pu, W. T. & Tsukamoto, H. Septum transversum-derived mesothelium gives rise to hepatic stellate cells and perivascular mesenchymal cells in developing mouse liver. Hepatology 53, 983–995 (2011).
Ijpenberg, A. et al. Wt1 and retinoic acid signaling are essential for stellate cell development and liver morphogenesis. Dev. Biol. 312, 157–170 (2007).
Rinkevich, Y. et al. Identification and prospective isolation of a mesothelial precursor lineage giving rise to smooth muscle cells and fibroblasts for mammalian internal organs, and their vasculature. Nat. Cell Biol. 14, 1251–1260 (2012).
Que, J. et al. Mesothelium contributes to vascular smooth muscle and mesenchyme during lung development. Proc. Natl Acad. Sci. USA 105, 16626–16630 (2008).
Carmona, R., Cano, E., Mattiotti, A., Gaztambide, J. & Munoz-Chapuli, R. Cells derived from the coelomic epithelium contribute to multiple gastrointestinal tissues in mouse embryos. PLoS One 8, e55890 (2013).
Cano, E., Carmona, R. & Munoz-Chapuli, R. Wt1-expressing progenitors contribute to multiple tissues in the developing lung. Am. J. Physiol. Lung Cell Mol. Physiol. 305, L322–L332 (2013).
Han, J. et al. The spatiotemporal development of adipose tissue. Development 138, 5027–5037 (2011).
Kanamori-Katayama, M. et al. LRRN4 and UPK3B are markers of primary mesothelial cells. PLoS One 6, e25391 (2011).
Sanchez-Gurmaches, J. et al. PTEN loss in the Myf5 lineage redistributes body fat and reveals subsets of white adipocytes that arise from Myf5 precursors. Cell Metab. 16, 348–362 (2012).
Hosen, N. et al. The Wilms’ tumor gene WT1-GFP knock-in mouse reveals the dynamic regulation of WT1 expression in normal and leukemic hematopoiesis. Leukemia 21, 1783–1791 (2007).
del Monte, G. et al. Differential Notch signaling in the epicardium is required for cardiac inflow development and coronary vessel morphogenesis. Circ. Res. 108, 824–836 (2011).
Wessels, A. et al. Epicardially derived fibroblasts preferentially contribute to the parietal leaflets of the atrioventricular valves in the murine heart. Dev. Biol. 366, 111–124 (2012).
Acknowledgements
We thank P. Perry and M. Pearson (MRC HGU, IGMM, Edinburgh) for assistance with image capturing and time-lapse videoing, C. Nicol and L. Lettice for assistance with graphic design and publication (MRC HGU, IGMM, Edinburgh), L. Ramage (University/BHF Centre for Cardiovascular Science, Edinburgh) for technical assistance with human adipose tissues, R. Carmona and E. Cano (University of Malaga, Malaga) for assistance with immunostaining, E. Freyer (MRC HGU, IGMM, Edinburgh) and M. Waterfall (School of Biological Sciences, Edinburgh University, Edinburgh) for assistance in running the FACS facility. Human sample collection was funded by the EU FP7 programme, the British Heart Foundation and the Medical Research Council. This work has been supported by a Medical Research Council core grant to the Human Genetics Unit (Edinburgh), grant BFU2011-25304 (MINECO, Spain), grant BFU2012-25304, and the Wellcome Trust (091374/z/10/z). A. Schedl was supported by grants from ARC (SL120120304626), ANR (ADSTEM) and FRM (DEQ20090515425).
Author information
Authors and Affiliations
Contributions
Conceived and designed the experiments: Y-Y.C., R. Bandiera, A. Serrels, O.M.M.E., R.M.C., A. Schedl and N.H. Performed the experiments: Y-Y.C., R. Bandiera, A. Schedl, O.M.M.E., W.Q., M.L., J.S., A.T., R. Berry, S.M., R.H.S. and R.M.C. Analysed the data: Y-Y.C., R. Bandiera, A. Serrels, O.M.M.E., R.M.C. and N.H. Contributed reagents/materials/analysis tools: R. Bandiera, A. Serrels, M.L., R.H.S., B.R.W. and R.M.C. Wrote the paper: Y-Y.C. and N.H.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
Supplementary Figure 1 Representative FACS analysis of Lipidtox staining from the floating layer from adipose tissue digestion.
(a) Single cells (P1) from the fl oating layers were stained with LipidTox (Deep Red) or without and analysed by FACS (n = 3). (b) Immunofl uoresence of bone marrow from Wt1-CreERT2.mTmG mice (induced at E14.5 and harvested at one-two month old). Adipocytes in the bone marrow were stained with perilipin antibody (indicated in red; cell nuclei were stained with DAPI in blue). No GFP+ cells were detected. (c) FACS analysis of bone marrow cells. No GFP+ cellswere detected. Bone marrow cells from Cre-negative mice were used for the GFP gating (n = 3).
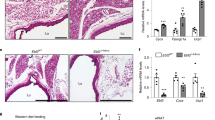
Supplementary Figure 2 Immunofluorescence images of adipose tissues from lineage tracing study.
One dose of tamoxifen was given to pregnant animals at E14.5 and mice were analysed at 1.2 years-old (n = 4). Sections from various fat pads were stained with GFP antibody(indicate in red), Wt1-antibody (indicate in green), and cell nuclei were stained with DAPI (blue). a, epicadial; b, omental; c, perirenal; d, retroperitoneal; e, mesenteric. (f) Subcutaneous section stained with anti-RFP antibody (indicated in green) and GFP (indicated in red).
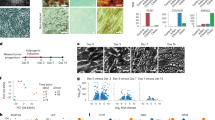
Supplementary Figure 3 Differentiation of SVFs into myotubes, osteoblasts, and adipocytes.
(a) Myotube differentiation assay. SVFs from Wt1CreERT2.mTmG mice were co-cultured with C2C12 myoblast and maintained in myotube differentiation medium for two weeks. Cells were stained with GFP (green) and desmin (red) or RFP (red) and desmin (green).Multinucleated myotubes expressing both GFP and desmin or RFP and desmin were observed (yellow). (b) adipocyte differentiation assay. SVFs were cultured in adipocyte differentiation medium for 10 days. GFP signal is indicated in green and RFP signal is indicated in red. Fluorescence images were merged with bright field image. Lipid droplets were seen in adipocytes. (c) Osteoblast differentiation assay. Sorted cells were cultured in osteoblast differentiation medium (DMEM, 10% FCS, P/S, ascrobate-2-phosphate, η-glycerophosphate and dexamethasome for 3 weeks. Alkaline phosphatase assay (blue) was used to detect osteoblasts. Osteoblast differentiation assay which was performed in SVFs sorted from different fat pads was shown in (d).
Supplementary Figure 4 Visceral fat pads are covered by mesothelia and short tamoxifen-pulsing labeled mesothelia.
(a) Visceral fat pads in adult mice are covered by mesothelia. Immunofluorescence staining of sections from epididymal, epicardial, mesenteric, and omental fat pads (from Wt1-GFP mice) with GFP (green) and cytokeratin (red, a marker for mesothelia) antibodies. DAPI is indicated in blue. (b)Short tamoxifen-pulsing labeled mesothelia in lineage tracing experiment. Immunohistochemistry using GFP (green) and cytokeratin (red) antibodies on samples from the region of GI tract in Wt1-CreERT2; mTmG embryos induced with tamoxifen at E14.5 and analysed at E16.5. Cell nuclei is stained with DAPI (blue).
Supplementary Figure 5 The epididymal appendage is covered by mesothelia and lipid-filled cells in the explant culture are adipocytes.
(a) Whole mount immunostaning of epididymal appendage from postnatal day 2 CD1 pups with a mesothelin (red) antibody. Immunoflurescence image of mesothelin is overlapped with the brightfield image. (b) Immunofluorescence staining of sections from E18.5 Wt1-GFP embryo showing the GFP+ layer (green) covering the surface of GI tract is mesothelin (red) positive. Cell nuclei are stained with DAPI (blue). (c) Lipid droplets-fi lled GFP+ cells (green) in the epididymal appendage explant culture (Wt1-Cre^ERT2; mTmG) express adipocyte marker (FABP4, red) and (d) perilipin (green). GFP in (d) is indicated in red.
Supplementary information
Supplementary Information
Supplementary Information (PDF 964 kb)
Supplementary Table 1
Supplementary Information (XLSX 9 kb)
Supplementary Table 2
Supplementary Information (XLSX 11 kb)
Supplementary Table 3
Supplementary Information (XLSX 9 kb)
Supplementary Table 4
Supplementary Information (XLSX 9 kb)
Supplementary Table 5
Supplementary Information (XLSX 23 kb)
Time-lapse video of adipocytes formation in a Cre-positive epididymal appendage ex vivo culture.
Time-lapse of the epididymal appendage in a Cre-positive Wt1-lineage tracing model (Wt1-CreERT2; mTmG) cultured in matrigel and adipocyte differentiating medium (including 1 μM 4-OH tamoxifen). Cells in the mesothelium expressed RFP at the beginning and then started expressing GFP. Adipocytes became visible in cells with lipid droplets. (AVI 24689 kb)
Time-lapse video of adipocytes formation in a Cre-negative epididymal appendage ex vivo culture.
Time-lapse of the epididymal appendage in a Cre-negatgive Wt1-lineage tracing model (Wt1-CreERT2; mTmG) cultured in matrigel and adipocyte differentiating medium (including 1 μM 4-OH tamoxifen). No GFP cells were detected. (AVI 33133 kb)
Rights and permissions
About this article
Cite this article
Chau, YY., Bandiera, R., Serrels, A. et al. Visceral and subcutaneous fat have different origins and evidence supports a mesothelial source. Nat Cell Biol 16, 367–375 (2014). https://doi.org/10.1038/ncb2922
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb2922
This article is cited by
-
Rab18 maintains homeostasis of subcutaneous adipose tissue to prevent obesity-induced metabolic disorders
Science China Life Sciences (2024)
-
Characteristic and fate determination of adipose precursors during adipose tissue remodeling
Cell Regeneration (2023)
-
Stage-specific nutritional management and developmental programming to optimize meat production
Journal of Animal Science and Biotechnology (2023)
-
Dynamic chromatin architecture of the porcine adipose tissues with weight gain and loss
Nature Communications (2023)
-
Genetic lineage tracing identifies cardiac mesenchymal-to-adipose transition in an arrhythmogenic cardiomyopathy model
Science China Life Sciences (2023)