Abstract
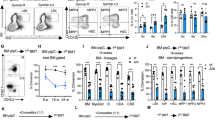
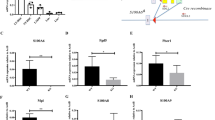
Coordinating the balance between haematopoietic stem cell (HSC) quiescence and self-renewal is crucial for maintaining haematopoiesis lifelong. Equally important for haematopoietic function is modulating HSC localization within the bone marrow niches, as maintenance of HSC function is tightly controlled by a complex network of intrinsic molecular mechanisms and extrinsic signalling interactions with their surrounding microenvironment1. In this study we demonstrate that nuclear factor erythroid 2-related factor 2 (Nfe2l2, or Nrf2), well established as a global regulator of the oxidative stress response, plays a regulatory role in several aspects of HSC homeostasis. Nrf2 deficiency results in an expansion of the haematopoietic stem and progenitor cell compartment due to cell-intrinsic hyperproliferation, which was accomplished at the expense of HSC quiescence and self-renewal. We further show that Nrf2 modulates both migration and retention of HSCs in their niche. Moreover, we identify a previously unrecognized link between Nrf2 and CXCR4, contributing, at least partially, to the maintenance of HSC function.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Kiel, M. J. & Morrison, S. J. Uncertainty in the niches that maintain haematopoietic stem cells. Nat. Rev. Immunol. 8, 290–301 (2008).
Zhang, J. & Li, L. Stem cell niche: microenvironment and beyond. J. Biol. Chem. 283, 9499–9503 (2008).
Schofield, R. The relationship between the spleen colony-forming cell and the haemopoietic stem cell. Blood Cells 4, 7–25 (1978).
Yun, Z., Maecker, H. L., Johnson, R. S. & Giaccia, A. J. Inhibition of PPAR γ2 gene expression by the HIF-1-regulated gene DEC1/Stra13: a mechanism for regulation of adipogenesis by hypoxia. Dev. Cell 2, 331–341 (2002).
Yun, Z., Lin, Q. & Giaccia, A. J. Adaptive myogenesis under hypoxia. Mol. Cell. Biol. 25, 3040–3055 (2005).
Ezashi, T., Das, P. & Roberts, R. M. Low O2 tensions and the prevention of differentiation of hES cells. Proc. Natl Acad. Sci. USA 102, 4783–4788 (2005).
Mazumdar, J. et al. O2 regulates stem cells through Wnt/β-catenin signalling. Nat. Cell Biol. 12, 1007–1013 (2010).
Takubo, K. et al. Regulation of the HIF- 1α level is essential for hematopoietic stem cells. Cell Stem Cell 7, 391–402 (2010).
Owusu-Ansah, E. & Banerjee, U. Reactive oxygen species prime Drosophila haematopoietic progenitors for differentiation. Nature 461, 537–541 (2009).
Tothova, Z. et al. FoxOs are critical mediators of hematopoietic stem cell resistance to physiologic oxidative stress. Cell 128, 325–339 (2007).
Ito, K. et al. Reactive oxygen species act through p38 MAPK to limit the lifespan of hematopoietic stem cells. Nat. Med. 12, 446–451 (2006).
Ito, K. et al. Regulation of oxidative stress by ATM is required for self-renewal of haematopoietic stem cells. Nature 431, 997–1002 (2004).
Moi, P., Chan, K., Asunis, I., Cao, A. & Kan, Y. W. Isolation of NF-E2-related factor 2 (Nrf2), a NF-E2-like basic leucine zipper transcriptional activator that binds to the tandem NF-E2/AP1 repeat of the β-globin locus control region. Proc. Natl Acad. Sci. USA 91, 9926–9930 (1994).
Li, W. & Kong, A. N. Molecular mechanisms of Nrf2-mediated antioxidant response. Mol. Carcinog. 48, 91–104 (2009).
Nguyen, T., Nioi, P. & Pickett, C. B. The Nrf2-antioxidant response element signaling pathway and its activation by oxidative stress. J. Biol. Chem. 284, 13291–13295 (2009).
Itoh, K. et al. Nrf2/small Maf heterodimer mediates the induction of phase II detoxifying enzyme genes through antioxidant response elements. Biochem. Biophys. Res. Commun. 236, 313–322 (1997).
Ishii, T. et al. Transcription factor Nrf2 coordinately regulates a group of oxidative stress-inducible genes in macrophages. J. Biol. Chem. 275, 16023–16029 (2000).
Chan, K., Lu, R., Chang, J. C. & Kan, Y. W. NRF2, a member of the NFE2 family of transcription factors, is not essential for murine erythropoiesis, growth, and development. Proc. Natl Acad. Sci. USA 93, 13943–13948 (1996).
Hochmuth, C. E., Biteau, B., Bohmann, D. & Jasper, H. Redox regulation by Keap1 and Nrf2 controls intestinal stem cell proliferation in Drosophila. Cell Stem Cell 8, 188–199 (2011).
Zuniga-Pflucker, J. C. T-cell development made simple. Nat. Rev. Immunol. 4, 67–72 (2004).
Tzeng, Y. S. et al. Loss of Cxcl12/Sdf-1 in adult mice decreases the quiescent state of hematopoietic stem/progenitor cells and alters the pattern of hematopoietic regeneration after myelosuppression. Blood 117, 429–439 (2011).
Sugiyama, T., Kohara, H., Noda, M. & Nagasawa, T. Maintenance of the hematopoietic stem cell pool by CXCL12-CXCR4 chemokine signaling in bone marrow stromal cell niches. Immunity 25, 977–988 (2006).
Nie, Y., Han, Y. C. & Zou, Y. R. CXCR4 is required for the quiescence of primitive hematopoietic cells. J. Exp. Med. 205, 777–783 (2008).
Motohashi, H. et al. NF-E2 domination over Nrf2 promotes ROS accumulation and megakaryocytic maturation. Blood 115, 677–686 (2010).
Kuroha, T. et al. Ablation of Nrf2 function does not increase the erythroid or megakaryocytic cell lineage dysfunction caused by p45 NF-E2 gene disruption. J. Biochem. 123, 376–379 (1998).
Juntilla, M. M. et al. AKT1 and AKT2 maintain hematopoietic stem cell function by regulating reactive oxygen species. Blood 115, 4030–4038 (2010).
Banning, A., Deubel, S., Kluth, D., Zhou, Z. & Brigelius-Flohe, R. The GI-GPx gene is a target for Nrf2. Mol. Cell. Biol. 25, 4914–4923 (2005).
Tullet, J. M. et al. Direct inhibition of the longevity-promoting factor SKN-1 by insulin-like signaling in C. elegans. Cell 132, 1025–1038 (2008).
Papaconstantinou, J. Insulin/IGF-1 and ROS signaling pathway cross-talk in aging and longevity determination. Mol. Cell. Endocrinol. 299, 89–100 (2009).
Geiger, H. & Rudolph, K. L. Aging in the lympho-hematopoietic stem cell compartment. Trends Immunol. 30, 360–365 (2009).
Zakrzewski, J. L. et al. Adoptive transfer of T-cell precursors enhances T-cell reconstitution after allogeneic hematopoietic stem cell transplantation. Nat. Med. 12, 1039–1047 (2006).
Hayashi, H. & Kume, T. Forkhead transcription factors regulate expression of the chemokine receptor CXCR4 in endothelial cells and CXCL12-induced cell migration. Biochem. Biophys. Res. Commun. 367, 584–589 (2008).
Na, I. K. et al. Concurrent visualization of trafficking, expansion, and activation of T lymphocytes and T-cell precursors in vivo. Blood 116, e18–e25 (2010).
Acknowledgements
We thank J. Chan (University of California, Irvine, USA) for providing the Nrf2−/− mice; the staff of the Memorial Sloan-Kettering Cancer Center Research Animal Resources Center for excellent animal care; and the staff of the Flow Cytometry Core Facility and M. S. Jiao of the Comparative Pathology Laboratories for assistance with sample preparation. This research was supported by National Institutes of Health award numbers RO1-HL069929 (M.R.M.v.d.B.), R01-AI100288 (M.R.M.v.d.B.), R01-AI080455 (M.R.M.v.d.B.), R01-AI101406 (M.R.M.v.d.B.) and P01-CA023766 (ROR). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Support was also received from the Radiation Effects Research Foundation (RERF-NIAID; M.R.M.v.d.B.), The Experimental Therapeutics Center of Memorial Sloan-Kettering Cancer Center funded by W. H. Goodwin and A. Goodwin, The Lymphoma Foundation, Alex’s Lemonade Stand, The Geoffrey Beene Cancer Research Center at Memorial Sloan-Kettering Cancer Center, and The Peter Solomon Fund. J.A.D. was supported by an Australian National Health and Medical Research Council Biomedical Training Fellowship and a Research Fellowship from the Leukemia and Lymphoma Society. E.V. was supported by a fellowship from the Italian Foundation for Cancer Research.
Author information
Authors and Affiliations
Contributions
J.J.T. designed and performed the experiments. J.A.D., K.T., J.S. and E.V. assisted with experiments and provided substantial intellectual inputs. N.V.S., M.L.W., O.M.S. and L.F.Y. assisted with experiments. A. M. Holland, A. M. Hanash, A.G. and H.H.T. provided intellectual inputs. M.A.S.M. and M.R.M.v.d.B. guided the research. J.J.T., J.A.D., K.T., A. M. Holland, Y.S. and M.R.M.v.d.B. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Information (PDF 338 kb)
Rights and permissions
About this article
Cite this article
Tsai, J., Dudakov, J., Takahashi, K. et al. Nrf2 regulates haematopoietic stem cell function. Nat Cell Biol 15, 309–316 (2013). https://doi.org/10.1038/ncb2699
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb2699
This article is cited by
-
Effects of fine particulate matter on bone marrow-conserved hematopoietic and mesenchymal stem cells: a systematic review
Experimental & Molecular Medicine (2024)
-
Cell-intrinsic factors governing quiescence vis-à-vis activation of adult hematopoietic stem cells
Molecular and Cellular Biochemistry (2023)
-
Andrographolide protects bone marrow mesenchymal stem cells against glucose and serum deprivation under hypoxia via the NRF2 signaling pathway
Stem Cell Research & Therapy (2022)
-
Thinking outside the box: non-canonical targets in multiple sclerosis
Nature Reviews Drug Discovery (2022)
-
Early and late stage MPN patients show distinct gene expression profiles in CD34+ cells
Annals of Hematology (2021)