Abstract
Arising from: R. N. Kaplan et al. Nature 438, 820–827 (2005)10.1038/nature04186; Kaplan et al. reply
Molecules such as vascular endothelial growth factor (VEGF) or placental growth factor—critical regulators of tumour angiogenesis—are also thought to mobilize into blood circulation bone marrow-derived cells (BMDCs)1, which may subsequently be recruited to tumours and facilitate tumour growth and metastasis2,3. A study4 has suggested that BMDCs form ‘metastatic niches’ in lungs before arrival of cancer cells, and showed that pharmacological inhibition of VEGF receptor 1 (VEGFR1, also known as Flt1)—cognate receptor for VEGF and placental growth factor—prevented BMDC infiltration in lungs and ‘metastatic niche’ formation. Here we report that blockade of VEGFR1 activity does not affect the rate of spontaneous metastasis formation in a clinically relevant and widely used preclinical model5,6,7,8. Therefore, alternative pathways probably mediate the priming of tissues for metastasis.
Similar content being viewed by others
Main
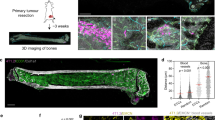
We assessed the role of VEGFR1 activity in spontaneous formation of macroscopic metastasis after pharmacological inhibition of VEGFR1 (with MF1-blocking antibody). We generated chimaeric C57BL mice in which BMDCs express green fluorescent protein (GFP) after lethal irradiation and restorative bone marrow transplantation (BMT) with cells from Actb-GFP/C57BL mice (BMT-Actb-GFP/C57BL mice). After bone marrow reconstitution, we subcutaneously implanted Lewis lung carcinoma (LLC1/LL2, a BMDC-rich tumour) or B16 melanoma (a tumour with significantly fewer BMDCs). Continuous treatment with MF1 did not delay primary tumour growth compared to non-specific IgG. Moreover, MF1 treatment did not reduce BMDC infiltration in LLC1 or B16 tumours (Fig. 1). We resected these tumours when they had grown to 1 cm in diameter (after 15–17 days) and metastatic foci had already formed in the lungs5,6. As no macroscopic metastases were detectable at the time of primary tumour removal, we measured the number of BMDCs in the lungs of BMT-Actb-GFP/C57BL mice before and after the formation of macroscopic metastases. BMDC infiltration at the time of resection (day 0) and at day 10 was relatively small but readily detectable and comparable with overall BMDC accumulation in tumour-free BMT-Actb-GFP/C57BL mice in both tumour models (Fig. 1).
The number of BMDCs was calculated as ratio of GFP-surface area to DAPI-surface area (±s.e.m.). DAPI was used to stain the nuclei of all cells (n = 6–8 mice per group). *P < 0.05.
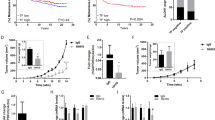
This suggests a key role for activated pulmonary alveolar macrophages—BMDCs that reside in the normal lung in comparable numbers in tumour-free non-BMT C57BL mice—as opposed to de novo BMDC recruitment before spontaneous metastasis. When most mice spontaneously developed macroscopic metastases (day 14), we detected a significant increase in de novo BMDC accumulation inside the LLC1 metastatic nodules and in the peri-tumour lung tissue, but not in B16 metastases, akin to BMDC incorporation in the primary tumours in these models (Fig. 1). VEGFR1 blockade with MF1 significantly reduced BMDC accumulation in LLC1 metastases. Surprisingly, VEGFR1 blockade did not decrease the occurrence, number, size or overall burden of spontaneous lung metastases on day 14 after LLC1 or B16 resection compared to control-treated mice (Table 1).
Previous studies in mice genetically deficient in VEGFR1-tyrosine kinase domain (flt1TK–/– mice) have shown that MMP-9 is induced by VEGFR1 signalling in lung stromal cells, which facilitates metastatic tumour growth in experimental metastasis models (that is, after intravenous infusion of cancer cells)9. When tested in flt1TK–/–/C57BL mice, LLC1 growth was similar and B16 growth was slightly delayed compared to C57BL mice. However, when evaluated at day 14, the number of mice with spontaneous macroscopic lung metastases or the number of metastatic nodules was not significantly different (Table 1). CD11b (Mac1) immunostaining in normal and metastatic lungs of these mice showed comparable myeloid BMDC infiltration in flt1TK–/–/C57BL and C57BL mice. Finally, since the extracellular domain of VEGFR1 is present on cells in flt1TK–/–/C57BL mice, we measured by flow cytometry the number of VEGFR1+ cells in circulating blood and in the tumour tissue. We detected no significant difference in circulating VEGFR1+ cells in LLC1- or B16-burdened mice, or in tissue-resident VEGFR1+ cells in normal lung or LLC1 tumours, and only marginal differences in B16 tumours10.
In conclusion, VEGFR1 modulates BMDC infiltration and primary/metastatic tumour growth in some models, consistent with previous reports11. However, pharmacological blockade or genetic deficiency in intracellular VEGFR1-TK domain neither eradicated nor significantly altered pre-metastatic BMDC infiltration or early spontaneous metastasis formation in lungs in these models.
Methods
Flt1TK–/–/C57BL (re-derived from flt1TK–/– mice10,12, kindly provided by M. Shibuya, Univ. Tokyo) and Actb-GFP/C57BL mice (Jackson Labs), C57BL mouse-syngeneic LLC1 lung carcinoma (CRL-1642) and B16 melanoma cell lines (CRL-6323)(both ATCC), the BMT and spontaneous metastasis formation models, and confocal microscopy and flow cytometric analyses have been previously described5,10,12. We used Matlab software for quantification. Rat anti-VEGFR1 monoclonal antibody (MF1, a gift from ImClone Systems) or IgG (Jackson Labs) was administered intraperitoneally (20 or 40 mg kg-1) to tumour-bearing mice three times per week13. We used Alexa-Fluor-647 (Molecular Probes)-labelled MF1 for flow cytometry. All other antibodies were purchased from BD-Pharmingen.
References
Rafii, S., Lyden, D., Benezra, R., Hattori, K. & Heissig, B. Vascular and haematopoietic stem cells: novel targets for anti-angiogenesis therapy? Nature Rev. Cancer 2, 826–835 (2002)
de Visser, K. E., Eichten, A. & Coussens, L. M. Paradoxical roles of the immune system during cancer development. Nature Rev. Cancer 6, 24–37 (2006)
Pollard, J. W. Nature Rev. Cancer Tumour-educated macrophages promote tumour progression and metastasis. 4, 71–78 (2004)
Kaplan, R. N. et al. VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature 438, 820–827 (2005)
Duda, D. G. et al. Evidence for incorporation of bone marrow-derived endothelial cells into perfused blood vessels in tumors. Blood 107, 2774–2776 (2006)
Gao, D. et al. Endothelial progenitor cells control the angiogenic switch in mouse lung metastasis. Science 319, 195–198 (2008)
Ohtaki, T. et al. Metastasis suppressor gene KiSS-1 encodes peptide ligand of a G-protein-coupled receptor. Nature 411, 613–617 (2001)
Padua, D. et al. TGFbeta primes breast tumors for lung metastasis seeding through angiopoietin-like 4. Cell 133, 66–77 (2008)
Hiratsuka, S. et al. MMP9 induction by vascular endothelial growth factor receptor-1 is involved in lung-specific metastasis. Cancer Cell 2, 289–300 (2002)
Dawson, M. R., Duda, D. G., Chae, S.-S., Fukumura, D. & Jain, R. K. VEGFR1 activity modulates myeloid cell infiltration in growing lung metastases but is not required for spontaneous metastasis formation. PLoS One 10.1371/journal.pone.0006525 (2009)
Fischer, C. et al. Anti-PlGF inhibits growth of VEGF(R)-inhibitor-resistant tumors without affecting healthy vessels. Cell 131, 463–475 (2007)
Hiratsuka, S., Minowa, O., Kuno, J., Noda, T. & Shibuya, M. Flt-1 lacking the tyrosine kinase domain is sufficient for normal development and angiogenesis in mice. Proc. Natl Acad. Sci. USA 95, 9349–9354 (1998)
Wu, Y. et al. Anti-vascular endothelial growth factor receptor-1 antagonist antibody as a therapeutic agent for cancer. Clin. Cancer Res. 12, 6573–6584 (2006)
Author information
Authors and Affiliations
Ethics declarations
Competing interests
Competing interests: R.K.J. is a consultant and receives research funding from AstraZeneca Pharmaceuticals and Dyax Corporation.
PowerPoint slides
Rights and permissions
About this article
Cite this article
Dawson, M., Duda, D., Fukumura, D. et al. VEGFR1-activity-independent metastasis formation. Nature 461, E4 (2009). https://doi.org/10.1038/nature08254
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nature08254
This article is cited by
-
A history of exploring cancer in context
Nature Reviews Cancer (2018)
-
Anti-angiogenesis for cancer revisited: Is there a role for combinations with immunotherapy?
Angiogenesis (2017)
-
Circulating tumour cells—a bona fide cause of metastatic cancer
Cancer and Metastasis Reviews (2014)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.