Abstract
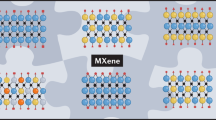
The family of 2D transition metal carbides, carbonitrides and nitrides (collectively referred to as MXenes) has expanded rapidly since the discovery of Ti3C2 in 2011. The materials reported so far always have surface terminations, such as hydroxyl, oxygen or fluorine, which impart hydrophilicity to their surfaces. About 20 different MXenes have been synthesized, and the structures and properties of dozens more have been theoretically predicted. The availability of solid solutions, the control of surface terminations and a recent discovery of multi-transition-metal layered MXenes offer the potential for synthesis of many new structures. The versatile chemistry of MXenes allows the tuning of properties for applications including energy storage, electromagnetic interference shielding, reinforcement for composites, water purification, gas- and biosensors, lubrication, and photo-, electro- and chemical catalysis. Attractive electronic, optical, plasmonic and thermoelectric properties have also been shown. In this Review, we present the synthesis, structure and properties of MXenes, as well as their energy storage and related applications, and an outlook for future research.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Nicolosi, V., Chhowalla, M., Kanatzidis, M. G., Strano, M. S. & Coleman, J. N. Liquid exfoliation of layered materials. Science 340, 1226419 (2013).
Fiori, G. et al. Electronics based on two-dimensional materials. Nat. Nanotechnol. 9, 768–779 (2014).
Xia, F., Wang, H., Xiao, D., Dubey, M. & Ramasubramaniam, A. Two-dimensional material nanophotonics. Nat. Photonics 8, 899–907 (2014).
Koppens, F. et al. Photodetectors based on graphene, other two-dimensional materials and hybrid systems. Nat. Nanotechnol. 9, 780–793 (2014).
Akinwande, D., Petrone, N. & Hone, J. Two-dimensional flexible nanoelectronics. Nat. Commun. 5, 5678 (2014).
Cepellotti, A. et al. Phonon hydrodynamics in two-dimensional materials. Nat. Commun. 6, 6400 (2015).
Geim, A. & Grigorieva, I. Van der Waals heterostructures. Nature 499, 419–425 (2013).
Lalmi, B. et al. Epitaxial growth of a silicene sheet. Appl. Phys. Lett. 97, 223109 (2010).
Cahangirov, S., Topsakal, M., Aktürk, E., S¸ahin, H. & Ciraci, S. Two-and one-dimensional honeycomb structures of silicon and germanium. Phys. Rev. Lett. 102, 236804 (2009).
Dávila, M., Xian, L., Cahangirov, S., Rubio, A. & Le Lay, G. Germanene: a novel two-dimensional germanium allotrope akin to graphene and silicene. New J. Phys. 16, 095002 (2014).
Liu, H. et al. Phosphorene: an unexplored 2D semiconductor with a high hole mobility. ACS Nano 8, 4033–4041 (2014).
Li, L. et al. Black phosphorus field-effect transistors. Nat. Nanotechnol. 9, 372–377 (2014).
Ataca, C., S¸ ahin, H. & Ciraci, S. Stable, single-layer MX2 transition-metal oxides and dichalcogenides in a honeycomb-like structure. J. Phys. Chem. C 116, 8983–8999 (2012).
Osada, M. & Sasaki, T. Two-dimensional dielectric nanosheets: novel nanoelectronics from nanocrystal building blocks. Adv. Mater. 24, 210–228 (2012).
Naguib, M. et al. Two-dimensional nanocrystals produced by exfoliation of Ti3AlC2 . Adv. Mater. 23, 4248–4253 (2011). This article reports the discovery of Ti3C2Tx MXene.
Naguib, M. et al. Two-dimensional transition metal carbides. ACS Nano 6, 1322–1331 (2012). This article reports the discovery of different MXenes, creating a family of 2D materials.
Naguib, M. et al. New two-dimensional niobium and vanadium carbides as promising materials for Li-ion batteries. J. Am. Chem. Soc. 135, 15966–15969 (2013).
Khazaei, M. et al. Novel electronic and magnetic properties of two-dimensional transition metal carbides and nitrides. Adv. Funct. Mater. 23, 2185–2192 (2013). The first computational study on electronic and magnetic properties of all the M2C MXenes.
Ghidiu, M. et al. Synthesis and characterization of two-dimensional Nb4C3 (MXene). Chem. Commun. 50, 9517–9520 (2014).
Anasori, B. et al. Two-dimensional, ordered, double transition metals carbides (MXenes). ACS Nano 9, 9507–9516 (2015). This study expanded the family MXenes by introducing ordered double transition metal MXenes.
Gogotsi, Y. Chemical vapour deposition: transition metal carbides go 2D. Nat. Mater. 14, 1079–1080 (2015).
Naguib, M., Mochalin, V. N., Barsoum, M. W. & Gogotsi, Y. MXenes: a new family of two-dimensional materials. Adv. Mater. 26, 992–1004 (2014).
Kurtoglu, M., Naguib, M., Gogotsi, Y. & Barsoum, M. W. First principles study of two-dimensional early transition metal carbides. MRS Commun. 2, 133–137 (2012).
Khazaei, M., Arai, M., Sasaki, T., Estili, M. & Sakka, Y. Two-dimensional molybdenum carbides: potential thermoelectric materials of the MXene family. Phys. Chem. Chem. Phys. 16, 7841–7849 (2014).
Urbankowski, P. et al. Synthesis of two-dimensional titanium nitride Ti4N3 (MXene). Nanoscale 8, 11385–11391 (2016). The first experimental report on the synthesis of a nitride MXene by etching in molten salts.
Ivanovskii, A. L. & Enyashin, A. N. Graphene-like transition-metal nanocarbides and nanonitrides. Russ. Chem. Rev. 82, 735–746 (2013).
Shein, I. R. & Ivanovskii, A. L. Graphene-like titanium carbides and nitrides Tin +1Cn, Tin + 1Nn (n = 1, 2, and 3) from de-intercalated MAX phases: first-principles probing of their structural, electronic properties and relative stability. Comput. Mater. Sci. 65, 104–114 (2012).
Xie, Y. & Kent, P. Hybrid density functional study of structural and electronic properties of functionalized Tin +1Xn (X = C, N) monolayers. Phys. Rev. B 87, 235441 (2013).
Gao, G. et al. Monolayer MXenes: promising half-metals and spin gapless semiconductors. Nanoscale 8, 8986–8994 (2016).
Khazaei, M. et al. Nearly free electron states in MXenes. Phys. Rev. B 93, 205125 (2016).
Barsoum, M. W. MAX Phases: Properties of Machinable Ternary Carbides and Nitrides (Wiley, 2013).
Barsoum, M. W. & Radovic, M. Elastic and mechanical properties of the MAX phases. Annu. Rev. Mater. Res. 41, 195–227 (2011).
Eklund, P., Beckers, M., Jansson, U., Högberg, H. & Hultman, L. The Mn +1AXn phases: materials science and thin-film processing. Thin Solid Films 518, 1851–1878 (2010).
Anasori, B. et al. Experimental and theoretical characterization of ordered MAX phases Mo2TiAlC2 and Mo2Ti2AlC3 . J. Appl. Phys. 118, 094304 (2015).
Ghidiu, M., Lukatskaya, M. R., Zhao, M.-Q., Gogotsi, Y. & Barsoum, M. W. Conductive two-dimensional titanium carbide ‘clay’ with high volumetric capacitance. Nature 516, 78–81 (2014). This study showed a new method for MXene synthesis and demonstrated clay-like behaviour of MXene produced by etching in HCl–LiF and its high volumetric capacitance.
Halim, J. et al. Transparent conductive two-dimensional titanium carbide epitaxial thin films. Chem. Mater. 26, 2374–2381 (2014).
Karlsson, L. H., Birch, J., Halim, J., Barsoum, M. W. & Persson, P. O. Atomically resolved structural and chemical investigation of single MXene sheets. Nano Lett. 15, 4955–4960 (2015).
Wang, L. et al. Synthesis and electrochemical performance of Ti3C2Tx with hydrothermal process. Electron. Mater. Lett. 12, 702–710 (2016).
Meshkian, R. et al. Synthesis of two-dimensional molybdenum carbide, Mo2C, from the gallium based atomic laminate Mo2Ga2C. Scripta Mater. 108, 147–150 (2015).
Halim, J. et al. Synthesis and characterization of 2D molybdenum carbide (MXene). Adv. Funct. Mater. 26, 3118–3127 (2016).
Zhou, J. et al. A two-dimensional zirconium carbide by selective etching of Al3C3 from nanolaminated Zr3Al3C5 . Angew. Chem. Int. Ed. 128, 5092–5097 (2016).
Lin, Z., He, L., Li, M., Wang, J. & Zhou, Y. Layered stacking characteristics of ternary zirconium aluminum carbides. J. Mater. Res. 22, 3058–3066 (2007).
Wang, J., Zhou, Y., Liao, T. & Lin, Z. Trend in crystal structure of layered ternary T-Al-C carbides (T = Sc, Ti, V, Cr, Zr, Nb, Mo, Hf, W, and Ta). J. Mater. Res. 22, 2685–2690 (2007).
Gesing, T. M. & Jeitschko, W. The crystal structures of Zr3Al3C5, ScAl3C3, and UAl3C3 and their relation to the structures of U2Al3C4 and Al4C3 . J. Solid State Chem. 140, 396–401 (1998).
Xie, J. et al. Atomically-thin molybdenum nitride nanosheets with exposed active surface sites for efficient hydrogen evolution. Chem. Sci. 5, 4615–4620 (2014).
Hoffman, E. N., Yushin, G., El-Raghy, T., Gogotsi, Y. & Barsoum, M. W. Micro and mesoporosity of carbon derived from ternary and binary metal carbides. Micropor. Mesopor. Mater. 112, 526–532 (2008).
Presser, V., Heon, M. & Gogotsi, Y. Carbide-derived carbons–from porous networks to nanotubes and graphene. Adv. Funct. Mater. 21, 810–833 (2011).
Barsoum, M. et al. The topotactic transformation of Ti3SiC2 into a partially ordered cubic Ti(C0.67Si0.06) phase by the diffusion of Si into molten cryolite. J. Electrochem. Soc. 146, 3919–3923 (1999).
El-Raghy, T., Barsoum, M. & Sika, M. Reaction of Al with Ti3SiC2 in the 800–1000ºC temperature range. Mater. Sci. Eng. A 298, 174–178 (2001).
Barsoum, M., Golczewski, J., Seifert, H. & Aldinger, F. Fabrication and electrical and thermal properties of Ti2InC, Hf2InC and (Ti, Hf)2InC. J. Alloys Compd. 340, 173–179 (2002).
Naguib, M. et al. On the topotactic transformation of Ti2AlC into a Ti–C–O–F cubic phase by heating in molten lithium fluoride in air. J. Am. Ceram. Soc. 94, 4556–4561 (2011).
Gusev, A. & Rempel, A. in Materials Science of Carbides, Nitrides and Borides (eds Gogotsi, Y. & Andrievski, R. A. ) 47–64 (Springer, 1999).
Xu, C. et al. Large-area high-quality 2D ultrathin Mo2C superconducting crystals. Nat. Mater. 14, 1135–1141 (2015).
Mashtalir, O., Naguib, M., Dyatkin, B., Gogotsi, Y. & Barsoum, M. W. Kinetics of aluminum extraction from Ti3AlC2 in hydrofluoric acid. Mater. Chem. Phys. 139, 147–152 (2013).
Cambaz, G. Z., Yushin, G. N., Gogotsi, Y. & Lutsenko, V. G. Anisotropic etching of SiC whiskers. Nano Lett. 6, 548–551 (2006).
Anasori, B. et al. Control of electronic properties of 2D carbides (MXenes) by manipulating their transition metal layers. Nanoscale Horiz. 1, 227–234 (2016).
Seh, Z. W. et al. Two-dimensional molybdenum carbide (MXene) as an efficient electrocatalyst for hydrogen evolution. ACS Energy Lett. 1, 589–594 (2016).
Ghidiu, M. et al. Ion-exchange and cation solvation reactions in Ti3C2 MXene. Chem. Mater. 28, 3507–3514 (2016).
Lipatov, A. et al. Effect of synthesis on quality, electronic properties and environmental stability of individual monolayer Ti3C2 MXene flakes. Adv. Electron. Mater. 2, 1600255 (2016).
Hu, T. et al. Interlayer coupling in two-dimensional titanium carbide MXenes. Phys. Chem. Chem. Phys. 18, 20256–20260 (2016).
Xu, J., Shim, J., Park, J.-H. & Lee, S. MXene electrode for the integration of WSe2 and MoS2 field effect transistors. Adv. Funct. Mater. 26, 5328–5334 (2016).
Lai, S. et al. Surface group modification and carrier transport property of layered transition metal carbides (Ti2CTx, T: –OH, –F and –O). Nanoscale 7, 19390–19396 (2015).
Mashtalir, O. et al. Intercalation and delamination of layered carbides and carbonitrides. Nat. Commun. 4, 1716 (2013). The first report on the intercalation of ions and polar organic molecules between MXene layers and the delamination of MXenes to make stable colloidal solutions.
Mashtalir, O., Lukatskaya, M. R., Zhao, M. Q., Barsoum, M. W. & Gogotsi, Y. Amine-assisted delamination of Nb2C MXene for Li-Ion energy storage devices. Adv. Mater. 27, 3501–3506 (2015).
Naguib, M., Unocic, R. R., Armstrong, B. L. & Nanda, J. Large-scale delamination of multi-layers transition metal carbides and carbonitrides “MXenes”. Dalton Trans. 44, 9353–9358 (2015).
Osti, N. C. et al. Effect of metal ion intercalation on the structure of MXene and water dynamics on its internal surfaces. ACS Appl. Mater. Interfaces 8, 8859–8863 (2016).
Sang, X. et al. Atomic defects in monolayer titanium carbide (Ti3C2Tx) MXene. ACS Nano 10, 9193–9200 (2016).
Shahzad, F. et al. Electromagnetic interference shielding with 2D transition metal carbides (MXenes) Science 353, 1137–1140 (2016).
Xie, X. et al. Porous heterostructured MXene/carbon nanotube composite paper with high volumetric capacity for sodium-based energy storage devices. Nano Energy 26, 513–523 (2016).
Shein, I. R. & Ivanovskii, A. L. Planar nano-block structures Tin +1Al0.5Cn and Tin +1Cn (n = 1, and 2) from MAX phases: structural, electronic properties and relative stability from first principles calculations. Superlattices Microstruct. 52, 147–157 (2012).
Xie, Y. et al. Role of surface structure on Li-ion energy storage capacity of two-dimensional transition-metal carbides. J. Am. Chem. Soc. 136, 6385–6394 (2014).
Yu, Y.-X. Prediction of mobility, enhanced storage capacity, and volume change during sodiation on interlayer-expanded functionalized Ti3C2 MXene anode materials for sodium-ion batteries. J. Phys. Chem. C 120, 5288–5296 (2016).
Ji, X. et al. Probing the electrochemical capacitance of MXene nanosheets for high-performance pseudocapacitors. Phys. Chem. Chem. Phys. 18, 4460–4467 (2016).
Zhang, X., Ma, Z., Zhao, X., Tang, Q. & Zhou, Z. Computational studies on structural and electronic properties of functionalized MXene monolayers and nanotubes. J. Mater. Chem. A 3, 4960–4966 (2015).
Wu, F. et al. Theoretical understanding of magnetic and electronic structures of Ti3C2 monolayer and its derivatives. Solid State Commun. 222, 9–13 (2015).
Tang, Q., Zhou, Z. & Shen, P. Are MXenes promising anode materials for Li ion batteries? Computational studies on electronic properties and Li storage capability of Ti3C2 and Ti3C2X2 (X = F, OH) monolayer. J. Am. Chem. Soc. 134, 16909–16916 (2012).
Li, X., Dai, Y., Ma, Y., Liu, Q. & Huang, B. Intriguing electronic properties of two-dimensional MoS2/TM2CO2(TM = Ti, Zr, or Hf) hetero-bilayers: type-II semiconductors with tunable band gaps. Nanotechnology 26, 135703 (2015).
Hu, J., Xu, B., Ouyang, C. Y., Zhang, Y. & Yang, S. Investigations on Nb2C monolayer as promising anode material for Li or non-Li ion batteries from first-principles calculations. RSC Adv. 6, 27467–27474 (2016).
Gandi, A. N., Alshareef, H. N. & Schwingenschlö gl, U. Thermoelectric performance of the MXenes M2CO2(M = Ti, Zr, or Hf). Chem. Mater. 28, 1647–1652 (2016).
Gan, L.-Y., Zhao, Y.-J., Huang, D. & Schwingenschlögl, U. First-principles analysis of MoS2/Ti2C and MoS2/Ti2CY2 (Y = F and OH) all-2D semiconductor/metal contacts. Phys. Rev. B 87, 245307 (2013).
Eames, C. & Islam, M. S. Ion intercalation into two-dimensional transition-metal carbides: global screening for new high-capacity battery materials. J. Am. Chem. Soc. 136, 16270–16276 (2014). A comprehensive computational study of MXenes for different cation battery applications.
Berdiyorov, G. R., Madjet, M. E. & Mahmoud, K. A. Ionic sieving through Ti3C2(OH)2 MXene: first-principles calculations. Appl. Phys. Lett. 108, 113110 (2016).
Ashton, M., Hennig, R. G. & Sinnott, S. B. Computational characterization of lightweight multilayer MXene Li-ion battery anodes. Appl. Phys. Lett. 108, 023901 (2016).
Ashton, M., Mathew, K., Hennig, R. G. & Sinnott, S. B. Predicted surface composition and thermodynamic stability of MXenes in solution. J. Phys. Chem. C 120, 3550–3556 (2016).
Yu, X.-f. et al. Monolayer Ti2CO2: a promising candidate for NH3 sensor or capturer with high sensitivity and selectivity. ACS Appl. Mater. Interfaces 7, 13707–13713 (2015).
Wang, X. et al. Atomic-scale recognition of surface structure and intercalation mechanism of Ti3C2X. J. Am. Chem. Soc. 137, 2715–2721 (2015).
Wang, H.-W., Naguib, M., Page, K., Wesolowski, D. J. & Gogotsi, Y. Resolving the structure of Ti3C2Tx MXenes through multi-level structural modeling of the atomic pair distribution function. Chem. Mater. 28, 349–359 (2015).
Halim, J. et al. X-Ray photoelectron spectroscopy of select multi-layered transition metal carbides (MXenes). Appl. Surf. Sci. 362, 406–417 (2016).
Enyashin, A. & Ivanovskii, A. Atomic structure, comparative stability and electronic properties of hydroxylated Ti2C and Ti3C2 nanotubes. Comp. Theor. Chem. 989, 27–32 (2012).
Magne, D., Mauchamp, V., Célérier, S., Chartier, P. & Cabioc'h, T. Spectroscopic evidence in the visible-ultraviolet energy range of surface functionalization sites in the multilayerTi3C2 MXene. Phys. Rev. B 91, 201409 (2015).
Li, L. Lattice dynamics and electronic structures of Ti3C2O2 and Mo2TiC2O2 (MXenes): the effect of Mo substitution. Comput. Mater. Sci. 124, 8–14 (2016).
Khazaei, M., Ranjbar, A., Arai, M. & Yunoki, S. Topological insulators in ordered double transition metals M′2M′′C2 (M′ = Mo, W; M′′ = Ti, Zr, Hf) MXenes. Phys. Rev. B 94, 125152 (2016).
Harris, K. J., Bugnet, M., Naguib, M., Barsoum, M. W. & Goward, G. R. Direct measurement of surface termination groups and their connectivity in the 2D MXene V2CTx using NMR spectroscopy. J. Phys. Chem. C 119, 13713–13720 (2015).
Hope, M. A. et al. NMR reveals the surface functionalisation of Ti3C2 MXene. Phys. Chem. Chem. Phys. 18, 5099–5102 (2016).
Mashtalir, O. et al. The effect of hydrazine intercalation on structure and capacitance of 2D titanium carbide (MXene). Nanoscale 8, 9128–9133 (2016).
Ying, Y. et al. Two-dimensional titanium carbide for efficiently reductive removal of highly toxic chromium (vi) from water. ACS Appl. Mater. Interfaces 7, 1795–1803 (2015).
Peng, Y.-Y. et al. All-MXene (2D titanium carbide) solid-state microsupercapacitors for on-chip energy storage. Energy Environ. Sci. 9, 2847–2854 (2016).
Mashtalir, O. et al. Dye adsorption and decomposition on two-dimensional titanium carbide in aqueous media. J. Mater. Chem. A 2, 14334–14338 (2014).
Wang, K. et al. Fabrication and thermal stability of two-dimensional carbide Ti3C2 nanosheets. Ceram. Int. 42, 8419–8424 (2016).
Naguib, M. et al. One-step synthesis of nanocrystalline transition metal oxides on thin sheets of disordered graphitic carbon by oxidation of MXenes. Chem. Commun. 50, 7420–7423 (2014).
Rakhi, R., Ahmed, B., Hedhili, M., Anjum, D. H. & Alshareef, H. Effect of post-etch annealing gas composition on the structural and electrochemical properties of Ti2CTx MXene electrodes for supercapacitor applications. Chem. Mater. 27, 5314–5323 (2015).
Wang, H. et al. Enhancement of the electrical properties of MXene Ti3C2 nanosheets by post-treatments of alkalization and calcination. Mater. Lett. 160, 537–540 (2015).
Ghassemi, H. et al. In situ environmental transmission electron microscopy study of oxidation of two-dimensional Ti3C2 and formation of carbon-supported TiO2 . J. Mater. Chem. A 2, 14339 (2014).
Naguib, M. MXenes: A New Family of Two-Dimensional Materials and its Application as Electrodes for Li-ion Batteries. Thesis, Drexel University (2014).
Lee, Y., Cho, S. B. & Chung, Y. C. Tunable indirect to direct band gap transition of monolayer Sc2CO2 by the strain effect. ACS Appl. Mater. Interfaces 6, 14724–14728 (2014).
Ma, Z. et al. Tunable band structures of heterostructured bilayers with transition-metal dichalcogenide and MXene monolayer. J. Phys. Chem. C 118, 5593–5599 (2014).
Zhao, S., Kang, W. & Xue, J. Manipulation of electronic and magnetic properties of M2C (M = Hf, Nb, Sc, Ta, Ti, V, Zr) monolayer by applying mechanical strains. Appl. Phys. Lett. 104, 133106 (2014).
Si, C., Zhou, J. & Sun, Z. Half-metallic ferromagnetism and surface functionalization-induced metal–insulator transition in graphene-like two-dimensional Cr2C crystals. ACS Appl. Mater. Interfaces 7, 17510–17515 (2015).
Weng, H. et al. Large-gap two-dimensional topological insulator in oxygen functionalized MXene. Phys. Rev. B 92, 075436 (2015).
Zhao, S., Kang, W. & Xue, J. MXene nanoribbons. J. Mater. Chem. C 3, 879–888 (2015).
Yang, J., Luo, X., Zhang, S. & Chen, L. Investigation of magnetic and electronic properties of transition metal doped Sc2CT2 (T = O, OH or F) using a first principles study. Phys. Chem. Chem. Phys. 18, 12914–12919 (2016).
Mauchamp, V. et al. Enhanced and tunable surface plasmons in two-dimensional Ti3C2 stacks: electronic structure versus boundary effects. Phys. Rev. B 89, 235428 (2014).
Borysiuk, V. N., Mochalin, V. N. & Gogotsi, Y. Molecular dynamic study of the mechanical properties of two-dimensional titanium carbides Tin +1Cn (MXenes). Nanotechnology 26, 265705 (2015).
Fu, Z. et al. Stabilization and strengthening effects of functional groups in two-dimensional titanium carbide. Phys. Rev. B 94, 104103 (2016).
Yorulmaz, U., Özden, A., Perkgöz, N. K., Ay, F. & Sevik, C. Vibrational and mechanical properties of single layer MXene structures: a first-principles investigation. Nanotechnology 27, 335702 (2016).
Zhang, H. et al. Computational studies on the structural, electronic and optical properties of graphene-like MXenes (M2CT2, M = Ti, Zr, Hf; T = O, F, OH) and their potential applications as visible-light driven photocatalysts. J. Mater. Chem. A 4, 12913–12920 (2016).
Ling, Z. et al. Flexible and conductive MXene films and nanocomposites with high capacitance. Proc. Natl Acad. Sci. USA 111, 16676–16681 (2014).
Boota, M. et al. Pseudocapacitive electrodes produced by oxidant-free polymerization of pyrrole between the layers of 2D titanium carbide (MXene). Adv. Mater. 28, 1517–1522 (2016).
Zhang, H. et al. Preparation, mechanical and anti-friction performance of MXene/polymer composites. Mater. Des. 92, 682–689 (2016).
Wu, X. et al. Polymer–Ti3C2Tx composite membranes to overcome the trade-off in solvent resistant nanofiltration for alcohol-based system. J. Membr. Sci. 515, 175–188 (2016).
Zhao, M.-Q. et al. Flexible MXene/carbon nanotube composite paper with high volumetric capacitance. Adv. Mater. 27, 339–345 (2015).
Liu, Y., Wang, W., Ying, Y., Wang, Y. & Peng, X. Binder-free layered Ti3C2/CNTs nanocomposite anodes with enhanced capacity and long-cycle life for lithium-ion batteries. Dalton Trans. 44, 7123–7126 (2015).
Dall'Agnese, Y., Rozier, P., Taberna, P.-L., Gogotsi, Y. & Simon, P. Capacitance of two-dimensional titanium carbide (MXene) and MXene/carbon nanotube composites in organic electrolytes. J. Power Sources 306, 510–515 (2016).
Dillon, A. D. et al. Highly conductive optical quality solution-processed films of 2D titanium carbide. Adv. Funct. Mater. 26, 4162–4168 (2016).
Hantanasirisakul, K. et al. Fabrication of Ti3C2Tx MXene transparent thin films with tunable optoelectronic properties. Adv. Electron. Mater. 2, 1600050 (2016).
Zha, X.-H. et al. Role of the surface effect on the structural, electronic and mechanical properties of the carbide MXenes. Europhys. Lett. 111, 26007 (2015).
Fashandi, H. et al. Dirac points with giant spin–orbit splitting in the electronic structure of two-dimensional transition-metal carbides. Phys. Rev. B 92, 155142 (2015).
Miranda, A., Halim, J., Barsoum, M. & Lorke, A. Electronic properties of freestanding Ti3C2Tx MXene monolayers. Appl. Phys. Lett. 108, 033102 (2016).
Paton, K. R. et al. Scalable production of large quantities of defect-free few-layer graphene by shear exfoliation in liquids. Nat. Mater. 13, 624–630 (2014).
Parvez, K. et al. Electrochemically exfoliated graphene as solution-processable, highly conductive electrodes for organic electronics. ACS Nano 7, 3598–3606 (2013).
Lane, N. J., Barsoum, M. W. & Rondinelli, J. M. Correlation effects and spin–orbit interactions in two-dimensional hexagonal 5d transition metal carbides, Tan +1Cn (n = 1,2,3). Europhys. Lett. 101, 57004 (2013).
Wang, G. A. Theoretical prediction of the intrinsic half-metallicity in surface-oxygen-passivated Cr2N MXene. J. Phys. Chem. C 120, 18850–18857 (2016).
Bonaccorso, F. et al. Graphene, related two-dimensional crystals, and hybrid systems for energy conversion and storage. Science 347, 1246501 (2015).
Feng, F., Wu, J., Wu, C. & Xie, Y. Regulating the electrical behaviors of 2D inorganic nanomaterials for energy applications. Small 11, 654–666 (2015).
Naguib, M. et al. MXene: a promising transision metal carbide anode for lithium-ion batteries. Electrochem. Commun. 16, 61–64 (2012).
Xie, Y. et al. Prediction and characterization of MXene nanosheet anodes for non-lithium-ion batteries. ACS Nano 8, 9606–9615 (2014).
Sun, D. et al. Structural transformation of MXene (V2C, Cr2C, and Ta2C) with O groups during lithiation: a first-principles investigation. ACS Appl. Mater. Interfaces 8, 74–81 (2015).
Ren, C. E. et al. Porous two-dimensional transition metal carbide (MXene) flakes for high-performance Li-ion storage. ChemElectroChem 3, 689–693 (2016).
Wang, X. et al. Pseudocapacitance of MXene nanosheets for high-power sodium-ion hybrid capacitors. Nat. Commun. 6, 6544 (2015). The first report on MXene application in Na-ion hybrid capacitors.
Dall'Agnese, Y., Taberna, P. L., Gogotsi, Y. & Simon, P. Two-dimensional vanadium carbide (MXene) as positive electrode for sodium-ion capacitors. J. Phys. Chem. Lett. 6, 2305–2309 (2015).
Yu, X.-f. et al. Mg intercalation into Ti2C building block. Chem. Phys. Lett. 629, 36–39 (2015).
Liang, X., Garsuch, A. & Nazar, L. F. Sulfur cathodes based on conductive MXene nanosheets for high-performance lithium–sulfur batteries. Angew. Chem. Int. Ed. 54, 3907–3911 (2015). The first report on the use of MXenes in Li–S batteries.
Zhao, X. et al. Fabrication of layered Ti3C2 with an accordion-like structure as a potential cathode material for high performance lithium–sulfur batteries. J. Mater. Chem. A 3, 7870–7876 (2015).
Luo, J. et al. Sn4+ ion decorated highly conductive Ti3C2 MXene: promising lithium-ion anodes with enhanced volumetric capacity and cyclic performance. ACS Nano 10, 2491–2499 (2016).
Lukatskaya, M. R. et al. Cation intercalation and high volumetric capacitance of two-dimensional titanium carbide. Science 341, 1502–1505 (2013). This is the first demonstration of MXenes being able to host a range of cations, such as Na+, K+, NH4+, Mg2+ and Al3+, enabling their use in supercapacitors.
Come, J. et al. Controlling the actuation properties of MXene paper electrodes upon cation intercalation. Nano Energy 17, 27–35 (2015).
Levi, M. D. et al. Solving the capacitive paradox of 2D MXene using electrochemical quartz-crystal admittance and in situ electronic conductance measurements. Adv. Energy Mater. 5, 1400815 (2015).
Tao, Y. et al. Towards ultrahigh volumetric capacitance: graphene derived highly dense but porous carbons for supercapacitors. Sci. Rep. 3, 2975 (2013).
Shen, B.-S. et al. All-solid-state flexible microsupercapacitor based on two-dimensional titanium carbide. Chin. Chem. Lett. 27, 1586–1591 (2016).
Lukatskaya, M. R. et al. Probing the mechanism of high capacitance in 2D titanium carbide using in situ X-ray absorption spectroscopy. Adv. Energy Mater. 5, 1500589 (2015).
Dall'Agnese, Y. et al. High capacitance of surface-modified 2D titanium carbide in acidic electrolyte. Electrochem. Commun. 48, 118–122 (2014).
Lin, Z. et al. Capacitance of Ti3C2Tx MXene in ionic liquid electrolyte. J. Power Sources 326, 575–579 (2016).
Peng, Q. et al. Unique lead adsorption behavior of activated hydroxyl group in two-dimensional titanium carbide. J. Am. Chem. Soc. 136, 4113–4116 (2014).
Xie, X. et al. Surface Al leached Ti3AlC2 substituting carbon for catalyst support served in a harsh corrosive electrochemical system. Nanoscale 6, 11035–11040 (2014).
Liu, H. et al. A novel nitrite biosensor based on the direct electrochemistry of hemoglobin immobilized on MXene-Ti3C2 . Sens. Actuators B 218, 60–66 (2015).
Zhang, X. et al. Preparation and tribological properties of Ti3C2(OH)2 nanosheets as additives in base oil. RSC Adv. 5, 2762–2767 (2015).
Xuan, J. et al. Organic-base-driven intercalation and delamination for the production of functionalized titanium carbide nanosheets with superior photothermal therapeutic performance. Angew. Chem. Int. Ed. 55, 14569–14574 (2016).
Qing, Y., Zhou, W., Luo, F. & Zhu, D. Titanium carbide (MXene) nanosheets as promising microwave absorbers. Ceram. Int. 42, 16412–16416 (2016).
Han, M. et al. Ti3C2 MXenes with modified surface for high-performance electromagnetic absorption and shielding in the X-Band. ACS Appl. Mater. Interfaces 8, 21011–21019 (2016).
Zou, G. et al. Synthesis of urchin-like rutile titania carbon nanocomposites by iron-facilitated phase transformation of MXene for environmental remediation. J. Mater. Chem. A 4, 489–499 (2016).
Guo, J., Peng, Q., Fu, H., Zou, G. & Zhang, Q. Heavy-metal adsorption behavior of two-dimensional alkalization-intercalated MXene by first-principles calculations. J. Phys. Chem. C 119, 20923–20930 (2015).
Zhang, Q. et al. Efficient phosphate sequestration for water purification by unique sandwich-like MXene/Magnetic iron oxide nanocomposites. Nanoscale 8, 7085–7093 (2016).
Ren, C. E. et al. Charge- and size-selective ion sieving through Ti3C2Tx MXene membranes. J. Phys. Chem. Lett. 6, 4026–4031 (2015).
Zhang, Y.-J. et al. Adsorption of uranyl species on hydroxylated titanium carbide nanosheet: a first-principles study. J. Hazard. Mater. 308, 402–410 (2016).
Wang, L. et al. Loading actinides in multi-layered structures for nuclear waste treatment: the first case study of uranium capture with vanadium carbide MXene. ACS Appl. Mater. Interfaces 8, 16396–16403 (2016).
Chen, J. et al. CO2 and temperature dual responsive “Smart” MXene phases. Chem. Commun. 51, 314–317 (2015).
Xiao, B., Li, Y.-c., Yu, X.-f. & Cheng, J.-b. MXenes: reusable materials for NH3 sensor or capturer by controlling the charge injection. Sens. Actuators B 235, 103–109 (2016).
Zhang, X. et al. Ti-anchored Ti2CO2 monolayer (MXene) as a single-atom catalyst for CO oxidation. J. Mater. Chem. A 4, 4871–4876 (2016).
Ma, T. Y., Cao, J. L., Jaroniec, M. & Qiao, S. Z. Interacting carbon nitride and titanium carbide nanosheets for high-performance oxygen evolution. Angew. Chem. Int. Ed. 55, 1138–1142 (2015).
Li, X., Zeng, C. & Fan, G. Ultrafast hydrogen generation from the hydrolysis of ammonia borane catalyzed by highly efficient bimetallic RuNi nanoparticles stabilized on Ti3C2X2 (X = OH and/or F). Int. J. Hydrogen Energy 40, 3883–3891 (2015).
Gao, Y. et al. Preparation of MXene–Cu2O nanocomposite and effect on thermal decomposition of ammonium perchlorate. Solid State Sci. 35, 62–65 (2014).
Peng, C. et al. Hybrids of two-dimensional Ti3C2 and TiO2 exposing {001} facets toward enhanced photocatalytic activity. ACS Appl. Mater. Interfaces 8, 6051–6060 (2016).
Azofra, L. M., Li, N., MacFarlane, D. R. & Sun, C. Promising prospects for 2D d2–d4 M3C2 transition metal carbides (MXenes) in N2 capture and conversion into ammonia. Energy Environ. Sci. 9, 2545–2549 (2016).
Xu, B. et al. Ultrathin MXene-micropattern-based field-effect transistor for probing neural activity. Adv. Mater. 28, 3333–3339 (2016).
Rasool, K. et al. Antibacterial activity of Ti3C2Tx MXene. ACS Nano 10, 3674–3684 (2016).
Yin, H. et al. Effect of MXene (nano-Ti3C2) on early-age hydration of cement paste. J. Nanomater. 16, 147 (2015).
Yang, J., Chen, B., Song, H., Tang, H. & Li, C. Synthesis, characterization, and tribological properties of two-dimensional Ti3C2 . Cryst. Res. Technol. 49, 926–932 (2014).
Acknowledgements
The authors worked with M. W. Barsoum (Drexel University) and P. Simon (Paul Sabatier University) on MXene synthesis and energy storage, respectively. Y.G. thanks numerous graduate students and post-docs, as well as collaborators at Drexel and elsewhere, who helped in the exploration of MXenes. Research on MXenes was supported by the Fluid Interface Reactions, Structures and Transport (FIRST) Center, an Energy Frontier Research Center funded by the US Department of Energy, Office of Science and Office of Basic Energy Sciences. B.A. was supported by King Abdullah University of Science and Technology under the KAUST-Drexel University Competitive Research Grant.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Supplementary information
Supplementary information S1 (figure)
Schematics of M2AX, M3AX2, M4AX3 crystal structures. (PDF 404 kb)
Supplementary information S2 (table)
Known M2AX, M3AX2, M4AX3 MAX phases to date1,2. (PDF 71 kb)
Supplementary information S3 (table)
MXene synthesis conditions (PDF 71 kb)
Supplementary information S4 (Box)
Delamination via intercalation (PDF 151 kb)
Supplementary information S5 (figure)
MXene crystal structures showing atomic ordering of M, X and T elements. (PDF 151 kb)
Supplementary information S6 (figure)
Effect of etching conditions on MXenes. (PDF 111 kb)
Supplementary information S7 (Box)
XRD patterns during MXene synthesis (PDF 129 kb)
Rights and permissions
About this article
Cite this article
Anasori, B., Lukatskaya, M. & Gogotsi, Y. 2D metal carbides and nitrides (MXenes) for energy storage. Nat Rev Mater 2, 16098 (2017). https://doi.org/10.1038/natrevmats.2016.98
Published:
DOI: https://doi.org/10.1038/natrevmats.2016.98
This article is cited by
-
Two-dimensional nanomaterials induced nano-bio interfacial effects and biomedical applications in cancer treatment
Journal of Nanobiotechnology (2024)
-
Fabrication of angstrom-scale two-dimensional channels for mass transport
Nature Protocols (2024)
-
An acetate electrolyte for enhanced pseudocapacitve capacity in aqueous ammonium ion batteries
Nature Communications (2024)
-
Functionalized MXene ink enables environmentally stable printed electronics
Nature Communications (2024)
-
Down-selection of biomolecules to assemble “reverse micelle” with perovskites
Nature Communications (2024)