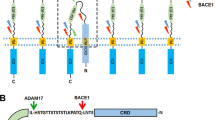
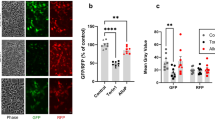
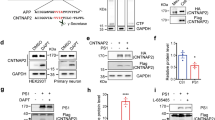
Abstract
Activity-dependent neuroprotective protein (ADNP), essential for brain formation, is a frequent autism spectrum disorder (ASD)-mutated gene. ADNP associates with microtubule end-binding proteins (EBs) through its SxIP motif, to regulate dendritic spine formation and brain plasticity. Here, we reveal SKIP, a novel four-amino-acid peptide representing an EB-binding site, as a replacement therapy in an outbred Adnp-deficient mouse model. We discovered, for the first time, axonal transport deficits in Adnp+/− mice (measured by manganese-enhanced magnetic resonance imaging), with significant male–female differences. RNA sequencing evaluations showed major age, sex and genotype differences. Function enrichment and focus on major gene expression changes further implicated channel/transporter function and the cytoskeleton. In particular, a significant maturation change (1 month-five months) was observed in beta1 tubulin (Tubb1) mRNA, only in Adnp+/+ males, and sex-dependent increase in calcium channel mRNA (Cacna1e) in Adnp+/+ males compared with females. At the protein level, the Adnp+/− mice exhibited impaired hippocampal expression of the calcium channel (voltage-dependent calcium channel, Cacnb1) as well as other key ASD-linked genes including the serotonin transporter (Slc6a4), and the autophagy regulator, BECN1 (Beclin1), in a sex-dependent manner. Intranasal SKIP treatment normalized social memory in 8- to 9-month-old Adnp+/−-treated mice to placebo-control levels, while protecting axonal transport and ameliorating changes in ASD-like gene expression. The control, all d-amino analog D-SKIP, did not mimic SKIP activity. SKIP presents a novel prototype for potential ASD drug development, a prevalent unmet medical need.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Accession codes
References
Gillberg C, Wing L . Autism: not an extremely rare disorder. Acta Psychiatr Scand 1999; 99: 399–406.
Abrahams BS, Geschwind DH . Advances in autism genetics: on the threshold of a new neurobiology. Nat Rev Genet 2008; 9: 341–355.
Helsmoortel C, Vulto-van Silfhout AT, Coe BP, Vandeweyer G, Rooms L, van den Ende J et al. A SWI/SNF-related autism syndrome caused by de novo mutations in ADNP. Nat Genet 2014; 46: 380–384.
Zamostiano R, Pinhasov A, Gelber E, Steingart RA, Seroussi E, Giladi E et al. Cloning and characterization of the human activity-dependent neuroprotective protein. J Biol Chem 2001; 276: 708–714.
Borozdin W, Graham JM Jr, Bohm D, Bamshad MJ, Spranger S, Burke L et al. Multigene deletions on chromosome 20q13.13-q13.2 including SALL4 result in an expanded phenotype of Okihiro syndrome plus developmental delay. Hum Mutat 2007; 28: 830.
Bassan M, Zamostiano R, Davidson A, Pinhasov A, Giladi E, Perl O et al. Complete sequence of a novel protein containing a femtomolar-activity-dependent neuroprotective peptide. J Neurochem 1999; 72: 1283–1293.
Pinhasov A, Mandel S, Torchinsky A, Giladi E, Pittel Z, Goldsweig AM et al. Activity-dependent neuroprotective protein: a novel gene essential for brain formation. Brain Res Dev Brain Res 2003; 144: 83–90.
Mandel S, Rechavi G, Gozes I . Activity-dependent neuroprotective protein (ADNP) differentially interacts with chromatin to regulate genes essential for embryogenesis. Dev Biol 2007; 303: 814–824.
Mandel S, Gozes I . Activity-dependent neuroprotective protein constitutes a novel element in the SWI/SNF chromatin remodeling complex. J Biol Chem 2007; 282: 34448–34456.
Dresner E, Malishkevich A, Arviv C, Leibman Barak S, Alon S, Ofir R et al. Novel evolutionary-conserved role for the activity-dependent neuroprotective protein (ADNP) family that is important for erythropoiesis. J Biol Chem 2012; 287: 40173–40185.
Schirer Y, Malishkevich A, Ophir Y, Lewis J, Giladi E, Gozes I . Novel marker for the onset of frontotemporal dementia: early increase in activity-dependent neuroprotective protein (ADNP) in the face of Tau mutation. PLoS One 2014; 9: e87383.
Ewers M, Walsh C, Trojanowski JQ, Shaw LM, Petersen RC, Jack CR Jr et al. Prediction of conversion from mild cognitive impairment to Alzheimer's disease dementia based upon biomarkers and neuropsychological test performance. Neurobiol Aging 2010; 33: 1203–1214.
Luo M, Shen D, Zhou X, Chen X, Wang W . MicroRNA-497 is a potential prognostic marker in human cervical cancer and functions as a tumor suppressor by targeting the insulin-like growth factor 1 receptor. Surgery 2013; 153: 836–847.
Gozes I, Yeheskel A, Pasmanik-Chor M . Activity-dependent neuroprotective protein (ADNP): a case study for highly conserved chordata-specific genes shaping the brain and mutated in cancer. J Alzheimers Dis 2014; 45: 57–73.
Malishkevich A, Amram N, Hacohen-Kleiman G, Magen I, Giladi E, Gozes I . Activity-dependent neuroprotective protein (ADNP) exhibits striking sexual dichotomy impacting on autistic and Alzheimer's pathologies. Transl Psychiatry 2015; 5: e501.
Gkogkas CG, Khoutorsky A, Ran I, Rampakakis E, Nevarko T, Weatherill DB et al. Autism-related deficits via dysregulated eIF4E-dependent translational control. Nature 2013; 493: 371–377.
Merenlender-Wagner A, Malishkevich A, Shemer Z, Udawela M, Gibbons A, Scarr E et al. Autophagy has a key role in the pathophysiology of schizophrenia. Mol Psychiatry 2015; 20: 126–132.
Dere E, Dahm L, Lu D, Hammerschmidt K, Ju A, Tantra M et al. Heterozygous ambra1 deficiency in mice: a genetic trait with autism-like behavior restricted to the female gender. Front Behav Neurosci 2014; 8: 181.
Oz S, Kapitansky O, Ivashco-Pachima Y, Malishkevich A, Giladi E, Skalka N et al. The NAP motif of activity-dependent neuroprotective protein (ADNP) regulates dendritic spines through microtubule end binding proteins. Mol Psychiatry 2014; 19: 1115–1124.
Vulih-Shultzman I, Pinhasov A, Mandel S, Grigoriadis N, Touloumi O, Pittel Z et al. Activity-dependent neuroprotective protein snippet NAP reduces tau hyperphosphorylation and enhances learning in a novel transgenic mouse model. J Pharmacol Exp Ther 2007; 323: 438–449.
Gozes I, Morimoto BH, Tiong J, Fox A, Sutherland K, Dangoor D et al. NAP: research and development of a peptide derived from activity-dependent neuroprotective protein (ADNP). CNS Drug Rev 2005; 11: 353–368.
Gozes I . Tau pathology: predictive diagnostics, targeted preventive and personalized medicine and application of advanced research in medical practice. EPMA J 2010; 1: 305–316.
Morimoto BH, Schmechel D, Hirman J, Blackwell A, Keith J, Gold M . A double-blind, placebo-controlled, ascending-dose, randomized study to evaluate the safety, tolerability and effects on cognition of AL-108 after 12 weeks of intranasal administration in subjects with mild cognitive impairment. Dement Geriatr Cogn Disord 2013; 35: 325–336.
Magen I, Gozes I . Microtubule-stabilizing peptides and small molecules protecting axonal transport and brain function: focus on davunetide (NAP). Neuropeptides 2013; 47: 489–495.
Jarskog LF, Dong Z, Kangarlu A, Colibazzi T, Girgis RR, Kegeles LS et al. Effects of davunetide on N-acetylaspartate and choline in dorsolateral prefrontal cortex in patients with schizophrenia. Neuropsychopharmacology 2013; 38: 1245–1252.
Javitt DC, Buchanan RW, Keefe RS, Kern R, McMahon RP, Green MF et al. Effect of the neuroprotective peptide davunetide (AL-108) on cognition and functional capacity in schizophrenia. Schizophr Res 2012; 136: 25–31.
Simmons A, Westman E, Muehlboeck S, Mecocci P, Vellas B, Tsolaki M et al. The AddNeuroMed framework for multi-centre MRI assessment of Alzheimer's disease: experience from the first 24 months. Int J Geriatr Psychiatry 2011; 26: 75–82.
Divinski I, Mittelman L, Gozes I . A femtomolar acting octapeptide interacts with tubulin and protects astrocytes against zinc intoxication. J Biol Chem 2004; 279: 28531–28538.
Ewers M, Cheng X, Nural HF, Walsh C, Meindl T, Teipel SJ et al. Increased CSF- BACE1 activity associated with decreased hippocampus volume in Alzheimer's disease. J Alzheimers Dis 2010; 25: 373–381.
Brenneman DE, Hauser J, Neale E, Rubinraut S, Fridkin M, Davidson A et al. Activity-dependent neurotrophic factor: structure-activity relationships of femtomolar-acting peptides. J Pharmacol Exp Ther 1998; 285: 619–627.
Wilkemeyer MF, Chen SY, Menkari CE, Brenneman DE, Sulik KK, Charness ME . Differential effects of ethanol antagonism and neuroprotection in peptide fragment NAPVSIPQ prevention of ethanol-induced developmental toxicity. Proc Natl Acad Sci USA 2003; 100: 8543–8548.
Sun H . A universal molecular descriptor system for prediction of logP, logS, logBB, and absorption. J Chem Inf Comput Sci 2004; 44: 748–757.
Honnappa S, Gouveia SM, Weisbrich A, Damberger FF, Bhavesh NS, Jawhari H et al. An EB1-binding motif acts as a microtubule tip localization signal. Cell 2009; 138: 366–376.
Bjelic S, De Groot CO, Scharer MA, Jaussi R, Bargsten K, Salzmann M et al. Interaction of mammalian end binding proteins with CAP-Gly domains of CLIP-170 and p150(glued). J Struct Biol 2012; 177: 160–167.
Raveh B, London N, Schueler-Furman O . Sub-angstrom modeling of complexes between flexible peptides and globular proteins. Proteins 2010; 78: 2029–2040.
Dangoor D, Biondi B, Gobbo M, Vachutinski Y, Fridkin M, Gozes I et al. Novel glycosylated VIP analogs: synthesis, biological activity, and metabolic stability. J Pept Sci 2008; 14: 321–328.
Alcalay RN, Giladi E, Pick CG, Gozes I . Intranasal administration of NAP, a neuroprotective peptide, decreases anxiety-like behavior in aging mice in the elevated plus maze. Neurosci Lett 2004; 361: 128–131.
Batool F, Hasnat A, Haleem MA, Haleem DJ . Dose-related effects of clozapine and risperidone on the pattern of brain regional serotonin and dopamine metabolism and on tests related to extrapyramidal functions in rats. Acta Pharm 2010; 60: 129–140.
McFarlane HG, Kusek GK, Yang M, Phoenix JL, Bolivar VJ, Crawley JN . Autism-like behavioral phenotypes in BTBR T+tf/J mice. Genes Brain Behav 2008; 7: 152–163.
El-Kordi A, Winkler D, Hammerschmidt K, Kastner A, Krueger D, Ronnenberg A et al. Development of an autism severity score for mice using Nlgn4 null mutants as a construct-valid model of heritable monogenic autism. Behav Brain Res 2013; 25.1: 41–49.
Matsuoka Y, Jouroukhin Y, Gray AJ, Ma L, Hirata-Fukae C, Li HF et al. A neuronal microtubule-interacting agent, NAPVSIPQ, reduces tau pathology and enhances cognitive function in a mouse model of Alzheimer's disease. J Pharmacol Exp Ther 2008; 325: 146–153.
Gregg B, Thiessen DD . A simple method of olfactory discrimination of urines for the Mongolian gerbil, Meriones unguiculatus. Physiol Behav 1981; 26: 1133–1136.
Heckemann RA, Keihaninejad S, Aljabar P, Gray KR, Nielsen C, Rueckert D et al. Automatic morphometry in Alzheimer's disease and mild cognitive impairment. Neuroimage 2011; 56: 2024–2037.
Vaisburd S, Shemer Z, Yeheskel A, Giladi E, Gozes I . Risperidone and NAP protect cognition and normalize gene expression in a schizophrenia mouse model. Sci Rep 2015; 5: 16300.
Gozes I, Helsmoortel C, Vandeweyer G, Van der Aa N, Kooy F, Sermone SB . The compassionate side of neuroscience: Tony Sermone's Undiagnosed genetic journey—ADNP mutation. J Mol Neurosci 2015; 56: 751–757.
Vandeweyer G, Helsmoortel C, Van Dijck A, Vulto-van Silfhout AT, Coe BP, Bernier R et al. The transcriptional regulator ADNP links the BAF (SWI/SNF) complexes with autism. Am J Med Genet C Semin Med Genet 2014; 166C: 315–326.
Hakim F, Wang Y, Carreras A, Hirotsu C, Zhang J, Peris E et al. Chronic sleep fragmentation during the sleep period induces hypothalamic endoplasmic reticulum stress and PTP1b-mediated leptin resistance in male mice. Sleep 2015; 38: 31–40.
Murphy DL, Fox MA, Timpano KR, Moya PR, Ren-Patterson R, Andrews AM et al. How the serotonin story is being rewritten by new gene-based discoveries principally related to SLC6A4, the serotonin transporter gene, which functions to influence all cellular serotonin systems. Neuropharmacology 2008; 55: 932–960.
Quraishe S, Cowan CM, Mudher A . NAP (davunetide) rescues neuronal dysfunction in a Drosophila model of tauopathy. Mol Psychiatry 2013; 18: 834–842.
Gozes I, Littauer UZ . Tubulin microheterogeneity increases with rat brain maturation. Nature 1978; 276: 411–413.
Gozes I, Sweadner KJ . Multiple tubulin forms are expressed by a single neurone. Nature 1981; 294: 477–480.
Nakamachi T, Ohtaki H, Yofu S, Dohi K, Watanabe J, Hayashi D et al. Pituitary adenylate cyclase-activating polypeptide (PACAP) type 1 receptor (PAC1R) co-localizes with activity-dependent neuroprotective protein (ADNP) in the mouse brains. Regul Pept 2008; 145: 88–95.
Li W, Moallem I, Paller KA, Gottfried JA . Subliminal smells can guide social preferences. Psychol Sci 2007; 18: 1044–1049.
Chih B, Afridi SK, Clark L, Scheiffele P . Disorder-associated mutations lead to functional inactivation of neuroligins. Hum Mol Genet 2004; 13: 1471–1477.
Krey JF, Dolmetsch RE . Molecular mechanisms of autism: a possible role for Ca2+ signaling. Curr Opin Neurobiol 2007; 17: 112–119.
Laurence JA, Fatemi SH . Glial fibrillary acidic protein is elevated in superior frontal, parietal and cerebellar cortices of autistic subjects. Cerebellum 2005; 4: 206–210.
Mulder EJ, Anderson GM, Kema IP, de Bildt A, van Lang ND, den Boer JA et al. Platelet serotonin levels in pervasive developmental disorders and mental retardation: diagnostic group differences, within-group distribution, and behavioral correlates. J Am Acad Child Adolesc Psychiatry 2004; 43: 491–499.
Whitaker-Azmitia PM . Serotonin and brain development: role in human developmental diseases. Brain Res Bull 2001; 56: 479–485.
De Rubeis S, He X, Goldberg AP, Poultney CS, Samocha K, Cicek AE et al. Synaptic, transcriptional and chromatin genes disrupted in autism. Nature 2014; 515: 209–215.
Ellegood J, Anagnostou E, Babineau BA, Crawley JN, Lin L, Genestine M et al. Clustering autism: using neuroanatomical differences in 26 mouse models to gain insight into the heterogeneity. Mol Psychiatry 2015; 20: 118–125.
Pagan C, Delorme R, Callebert J, Goubran-Botros H, Amsellem F, Drouot X et al. The serotonin-N-acetylserotonin-melatonin pathway as a biomarker for autism spectrum disorders. Transl Psychiatry 2014; 4: e479.
Jazin E, Cahill L . Sex differences in molecular neuroscience: from fruit flies to humans. Nat Rev Neurosci 2010; 11: 9–17.
Luo S, Garcia-Arencibia M, Zhao R, Puri C, Toh PP, Sadiq O et al. Bim inhibits autophagy by recruiting Beclin 1 to microtubules. Mol Cell 2012; 47: 359–370.
Pescosolido MF, Schwede M, Johnson Harrison A, Schmidt M, Gamsiz ED, Chen WS et al. Expansion of the clinical phenotype associated with mutations in activity-dependent neuroprotective protein. J Med Genet 2014; 51: 587–589.
Ponting CP, Goodstadt L . Separating derived from ancestral features of mouse and human genomes. Biochem Soc Trans 2009; 37: 734–739.
Cahill L . A half-truth is a whole lie: on the necessity of investigating sex influences on the brain. Endocrinology 2012; 153: 2541–2543.
Nissen KB, Andersen JJ, Haugaard-Kedstrom LM, Bach A, Stromgaard K . Design, synthesis, and characterization of fatty acid derivatives of a dimeric peptide-based postsynaptic density-95 (PSD-95) inhibitor. J Med Chem 2015; 58: 1575–1580.
Acknowledgements
Illana Gozes laboratory is supported by the AMN Foundation, the Israel Ministry of Science, Technology and Space, Israel Science Foundation, CFTAU Montreal Circle of Friends and the Adams family, Adams Super Center for Brain Studies, the Edersheim Levie-Gitter Institute for Functional Brain Imaging, the Diana and Zelman Elton (Elbaum) Laboratory for Molecular Neuroendocrinology and the Lily and Avraham Gildor Chair for the Investigation of Growth Factors at Tel Aviv University. Illana Gozes is a Humboldt Award Recipient and was a fellow at the Hanse-Wissenschftenkolleg, Germany. This study is in partial fulfillment graduate studies requirements for Noy Amram, Gal Hacohen Kleiman, Anna Malishkevich, Jeny Katz and Shlomo Sragovich at the Miriam and Sheldon G. Adelson Graduate School of Medicine, Sackler Faculty of Medicine, Tel Aviv University. We thank Orly Yaron, Genome Center Laboratory, Limor Frish, NMR Laboratory, and Yael Piontkewitz, Alfredo Federico Strauss Center for Computational Neuroimaging at Tel Aviv University and the Technion's Genomic Center for their input and excellent work. We thank Oxana Kapitansky for her help.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
SKIP is under patent protection and under term sheet agreement to Coronis Partners (IG conflict of interest).
Additional information
Supplementary Information accompanies the paper on the Molecular Psychiatry website
Supplementary information
Rights and permissions
About this article
Cite this article
Amram, N., Hacohen-Kleiman, G., Sragovich, S. et al. Sexual divergence in microtubule function: the novel intranasal microtubule targeting SKIP normalizes axonal transport and enhances memory. Mol Psychiatry 21, 1467–1476 (2016). https://doi.org/10.1038/mp.2015.208
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/mp.2015.208
This article is cited by
-
The cytoplasmic localization of ADNP through 14-3-3 promotes sex-dependent neuronal morphogenesis, cortical connectivity, and calcium signaling
Molecular Psychiatry (2023)
-
Adnp-mutant mice with cognitive inflexibility, CaMKIIα hyperactivity, and synaptic plasticity deficits
Molecular Psychiatry (2023)
-
Proteomic phenotype of cerebral organoids derived from autism spectrum disorder patients reveal disrupted energy metabolism, cellular components, and biological processes
Molecular Psychiatry (2022)
-
Autophagy deficiency in neurodevelopmental disorders
Cell & Bioscience (2021)
-
Discovery of autism/intellectual disability somatic mutations in Alzheimer's brains: mutated ADNP cytoskeletal impairments and repair as a case study
Molecular Psychiatry (2021)