Abstract
Distinction between multiple primary cancers and intrapulmonary metastases in patients with synchronous multifocal lung cancer can be challenging. Histological and genotypic assessment of multifocal lung tumors have been suggested to influence the staging. The aim of this study was to determine the role of morphology and genotype in staging of surgically treated multifocal non-small cell lung carcinoma. Synchronous lung cancers from 60 patients (42 with adenocarcinoma and 18 with squamous cell carcinoma), clinically considered to represent intrapulmonary metastases, were histologically subtyped according to the 2015 World Health Organization classification of lung tumors and subjected to genotypic analysis (KRAS, EGFR, BRAF, PIK3CA, ALK, MET and ROS1 in adenocarcinoma and PIK3CA and p16 in squamous cell carcinoma). Concordance between clinical criteria and histological subtyping was identified in about 50% of cases (P<0.0001). Genotypically, 44% of adenocarcinomas and 60% of squamous cell carcinomas with identified molecular alterations were considered to be intrapulmonary metastases. Concordance between histological and molecular staging was observed in 89% of adenocarcinomas and 56% of squamous cell carcinomas. Univariate survival analyses failed to demonstrate significant differences in overall or cancer-specific survival in patients with adenocarcinoma and squamous cell carcinomas restaged according to histology and/or molecular profile. Lymph node metastases (N1/N2 vs N0) (P=0.03) and age >65 years (P=0.05) were associated with shorter overall survival. In addition, squamous cell carcinomas with p16 deletion showed shorter overall survival when compared with squamous cell carcinomas without p16 deletion (P=0.05). No correlation between other molecular alterations, clinico-pathological characteristics and prognosis was found. Our study demonstrates that a comprehensive genotypic and morphological assessment of surgically treated multifocal lung cancers is feasible but not sufficient to establish their clonal relationship and prognosis.
Similar content being viewed by others
Main
The reported incidence of multiple synchronous tumors of the lung in recent series is up to 20%.1, 2 Staging of such tumors as independent primary tumors or intrapulmonary metastases is often challenging. Morphological criteria, especially those proposed by Martini and Melamed,3 have been used as the main tool with the idea that morphology of metastases should match the primary tumor, while different morphology supports classification of tumors as unrelated separate primaries. However, clinico-pathological distinction between the two possibilities is not always possible and may not be prognostic.
Over the past decade, multiple studies using different molecular approaches to analysis of synchronous lung tumor nodules have emerged, including DNA microsatellite analysis, CGH/aCHG and most recently next-generation sequencing.2, 4, 5, 6, 7, 8, 9, 10 The data from published reports indicate a highly variable percentage of multifocal tumors identified as clonally related (up to 70%) and all reports agree that multifocal tumors may arise either as metastases from a single tumor or as independent tumors. Discrepancy between clinical and molecular classification of originally presumed cases of multiple primary lung cancers ranged in different series from 18 to 30%. Recent recommendations for routine molecular profiling of lung adenocarcinoma resulted in a widespread use of targeted mutational profiling to find actionable oncogenic mutations and gene rearrangements.11 They also provided justification for implementation of genomic analysis for simultaneous tumor lineage profiling with potentially improved accuracy of T staging of multiple lung nodules along with detection of potentially actionable genomic alterations. In contrast, molecular profiling of squamous cell carcinoma is not routinely performed, and morphological comparisons and subtyping is even more challenging than in lung adenocarcinoma. The outcome data using molecular approach are controversial and probably influenced by different analytical methods, patient selection and treatment. Overall, synchronous lung cancers show a relatively good outcome particularly in patients without mediastinal lymph node involvement.
The aim of this study was to assess possible impact of histological assessment and mutational profiling performed in clinical practice on staging of surgically resected synchronous multifocal non-small cell lung carcinomas.
Materials and methods
Patient Selection
A total of 60 patients, each with two surgically resected synchronous, multifocal adenocarcinoma (N=42) or squamous cell carcinoma (N=18), occurring in the same lobe (pT3) or ipsilateral different lobes (pT4), were selected for the study. All cases were considered to represent intrapulmonary metastases according to Martini and Melamed criteria.3 Clinical information, including gender, age, tumor stage, smoking history and CT findings, were obtained from the electronic medical records. Survival data were collected through the UPMC Network Cancer Registry. The study was conducted under an exemption approved by the University of Pittsburgh Institutional Review Board (PRO 09010191).
Morphological subtyping of lung adenocarcinomas and squamous cell carcinoma was performed according to the 2015 World Health Organization classification criteria.12 Tumors showing similar morphological features were considered to be intrapulmonary metastases and those with different morphology were considered to be multiple primary tumors.
Molecular Analyses
Tumor targets from all tumor nodules (N=120) were manually microdissected. DNA was isolated from each target using the DNeasy Tissue Kit (Qiagen, Valencia, CA, USA) according to the manufacturer’s instructions. Mutation analyses were performed by bidirectional Sanger sequencing analysis as previously described.13
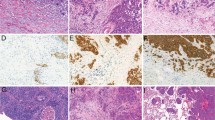
FISH analysis of MET amplification was performed using standard methods with the dual-color MET SpectrumOrange/CEP7 SpectrumGreen probe (Vysis, Downers Grove, IL, USA) and Paraffin Pretreatment Reagent Kit (Vysis).14 MET gene amplification was defined as a ratio between MET gene copy numbers and chromosome 7 >2. FISH analyses for ALK and ROS1 rearrangements were performed according to previously reported methods.15 At least 60 cells were scored for each case and control.
Statistical Analysis
Overall survival was calculated in months from the date of a diagnostic procedure until death. Univariate models examining group differences in overall survival were plotted and evaluated using Kaplan–Meier survival curves and the log-rank test. Statistical tests were two-sided. Analyses were conducted using the SPSS software, version 23 (IBM, Armonk, NY, USA).
Results
Patient Characteristics
Clinical characteristics of the 60 study patients are summarized in Table 1. There were 42 patients with adenocarcinomas and 18 with squamous cell carcinomas. All cases were classified as intrapulmonary metastases by Martini–Melamed criteria. Tumors occurred in the same lobe in 48 patients (33 adenocarcinoma, 15 squamous cell carcinoma) and in the ipsilateral, different lobes in 12 patients (9 adenocarcinoma, 3 squamous cell carcinoma). Lymph node metastases were present in 24 patients (16 adenocarcinoma and 8 squamous cell carcinoma).
Morphological and Molecular Assessment
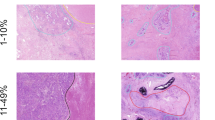
Concordance between Martini–Melamed classification of adenocarcinoma as intrapulmonary metastases and histological subtyping was identified in 21 of the 42 patients (50%) (P<0.0001). Molecular alterations were identified in 27 of the 42 patients (64%) (Table 2). Of the 42 successfully tested adenocarcinoma, KRAS mutations were identified in 22 adenocarcinoma cases (52%; 21 codon 2, 1 codon 61), EGFR mutations in 2 (5%; 1 exon 19, 1 exon 20), MET amplification in 2 (5%) and ALK rearrangement in 1 (2%). No ROS1 rearrangements were identified. KRAS point mutations were identical in both tumors from 11 patients and different in the remaining 11 cases. Discordance between the presence and absence of MET amplification was found in two cases. One case showed a discordant ALK rearrangement. Fifteen cases of adenocarcinoma (36%) were wild type for eight tested genes. Overall, 12 patients (44%) with identified molecular alterations were considered to have intrapulmonary metastases and 15 (56%) to have separate primary tumors. We observed a high agreement between histology and molecular profile in predicting tumor clonal relationship. Concordance between histological and molecular staging was observed in 24 of the 27 cases (89%) with detected oncogenic mutations or rearrangements (Figure 1). Two cases with disagreement between histology and molecular classification were classified molecularly as intrapulmonary metastases (same KRAS mutations) while morphology favored independent primary tumors (Figure 2). The third case showed discordant molecular results (one tumor nodule harbored a KRAS mutation and the second nodule was wild type for KRAS) favoring independent primaries, while morphology favored intrapulmonary metastases.
The interpretation of a ‘wild type’ adenocarcinoma is uncertain. These cases could be considered to be clonally related or molecularly inconclusive. If ‘wild-type’ cases were considered to be clonally related, then 27 patients (64%) could be considered to have intrapulmonary metastases and 15 patients (36%) separate primary tumors. In contrast to adenocarcinomas with detected oncogenic mutations/rearrangements, concordance between histology and molecularly ‘wild-type’ adenocarcinoma was less common and observed in 10 of the 15 cases (67%).
Of the 18 squamous cell carcinoma cases that were classified as intrapulmonary metastases according to the Martini and Melamed criteria, only 9 cases (50%) were morphologically similar and thus interpreted as intrapulmonary metastases (P<0.0001). Molecular alterations were identified in 8 of the 18 patients (44%) (Table 3). A PIK3CA exon 9 mutation was detected in a single tumor from one patient. Tumors from 7 patients were found to have p16 deletions (one tumor in 3 patients, both tumors in 4 patients).
Similar to adenocarcinoma, ‘wild-type’ cases could be considered as clonally related and in that case only one case would be interpreted as separate primary carcinoma. The other approach would be to consider these cases molecularly indeterminate because of a limited number of analyzed genes. Of cases that showed molecular alterations, 4 cases (57%) were considered intrapulmonary metastases. Concordance between molecular and histological staging of squamous cell carcinoma with molecular alterations was seen in 4 of the 7 cases (57%). Of the three cases with disagreement between histology and molecular classification, two were interpreted molecularly as intrapulmonary metastases and one as an independent primary tumor. One case that was histologically interpreted as intrapulmonary metastasis showed discordant p16 FISH results between two tumor nodules. Similar to adenocarcinoma, a concordance between histology and molecular ‘wild-type’ squamous cell carcinoma was observed in 5 of the 8 cases (63%).
Survival Analysis
Of the 60 patients with multiple lung cancers, 26 died between 3 and 88 months after diagnosis, and 34 were alive as of 5–185 months after diagnosis. The median survival time for all study patients was 25 months (range 2–138 months). Cases with molecularly indeterminate results were excluded from survival analysis. Univariate survival analyses failed to demonstrate significant differences in overall and cancer-specific survival in patients with adenocarcinoma and squamous cell carcinoma restaged according to histology and/or molecular profile (Figure 3). Lymph node metastases (N1/N2 vs N0) (P=0.03) and age >65 years (P=0.05) were associated with shorter overall survival. Squamous cell carcinoma with p16 deletion showed shorter overall survival when compared with squamous cell carcinoma without p16 deletion (P=0.05). Univariate analysis did not show prognostic significance for gender, histology, type of surgery, tumor size, angiolymphatic invasion, visceral pleural invasion or smoking history.
Discussion
Staging of multifocal lung cancers in the seventh edition of AJCC staging is based on the Martini–Melamed criteria established in 1975.3 However, the staging manual recognizes that these criteria may not be clinically optimal. Therefore, an option was given to pathologists to include their morphological impressions, immunohistochemistry results and molecular studies into pathological staging. However, no detailed recommendations are given as to which histological criteria or molecular studies should be used. Furthermore, it is not defined how to incorporate molecular studies into the staging of multifocal lung cancers.
Our study focused on lung adenocarcinoma and squamous cell carcinoma that were, according to Martini–Melamed criteria, considered to be intrapulmonary metastases. Similar to previously published studies, in our study histo-molecular characterization correlated with Martini–Melamed criteria in about 50% of the cases, including both adenocarcinoma and squamous cell carcinoma.4, 5, 9, 16, 17 This is not surprising as morphological subtying of lung adenocarcinoma has changed in the past decade, and the proposed detailed approach has been implemented in the 2015 World Health Organization classification of lung tumors.18 Adenocarcinoma of the lung is morphologically heterogenous and careful comparison of morphological subtypes between two tumors is potentially useful in staging of synchronous tumors. One limitation of this approach is that the reproducibility of histological subtyping between different pathologists is only fair to moderate according to the IASLC Pathology Committee reproducibility study.19 One could argue that the IASLC reproducibility study did not reflect daily routine practice as it relied on review of a representative image of a particular histological subtype rather than review of the entire tumor sections. Recently, Homer20 reported the results of a voluntary survey of the Pulmonary Pathology Society that showed major disagreement in the approach to staging of multifocal lung cancer. The results of this survey should be interpreted with caution as only five clinical scenarios were presented and no details about morphological or other potential criteria were published. We suspect that intraobserver variability, rather than interobserver variability, may potentially have a role in the staging of multifocal cancers in an individual case. As demonstrated by Noguchi et al,21 pathologists' education of diagnostic criteria should ultimately lead to significant improvement in diagnostic reproducibility.
Our study includes the largest number of multifocal primary lung squamous cell carcinoma reported to date. In contrast to adenocarcinoma, morphology of squamous cell carcinoma is less heterogenous. The 2015 World Health Organization classification of lung tumors recognizes three types of squamous cell carcinoma: keratinizing, non-keratinizing, and basaloid.14 We used this simple subtyping scheme and essentially demonstrated a very similar disagreement with Martini–Melamed criteria as for adenocarcinoma. In our opinion, this is an encouraging observation indicating a potential application of the WHO subtyping of squamous cell carcinoma. In contrast to adenocarcinoma, the IASLC reproducibility study of poorly differentiated non-small cell carcinoma demonstrated great agreement among pulmonary pathologists in recognizing features of squamous cell carcinoma, such as keratinization, pearl formation and intercellular bridges. This suggests a potential role of this simple approach in staging of multifocal squamous cell carcinoma.22 Girard et al5 demonstrated that histological subtyping of multifocal lung carcinoma is a superior predictor of tumor time to progression when compared with Martini–Melamed3 and molecular criteria. Our study failed to demonstrate prognostic significance of morphological restaging. We believe that this is the result of a very selected, homogenous group of surgically resected multifocal lung cancers in our study. Patients in our cohort had only one surgical procedure to resect both tumors. Patients in the study by Girard et al5 had multiple surgical procedures, but neither type of surgery nor the time interval between the two interventions were reported.
Our study illustrates the difficulties in the interpretation of genotypic data. The assumption is that the matched driver mutations or gene rearrangements define tumors as clonally related. The discordance between tumor genotype and Martini–Melamed stage was seen in 56% of lung adenocarcinomas. This is similar to the previously published studies regardless of methodological approaches.2, 6, 7, 10, 23, 24, 25 The presence of discordant mutations most likely can be used as an indicator of a different clone. Earlier studies indicated a broad range of discordance (25–49%) in driver mutations (EGFR, KRAS) between primary and metastatic lung tumors that in part can be explained by different methodological approaches, although authors mostly suggested intratumoral heterogeneity.7, 24, 26 Yatabe et al27 showed that the co-founding factors in the interpretation of targeted PCR-based mutation assays are related to the co-existence of gene amplification, contamination with normal tissue, tumor cell content and assay sensitivity rather than heterogeneity of somatic mutations. More recently, Vignot et al28 demonstrated a high concordance rate (94%) for driver somatic alterations between primary lung tumors and matched metastases using next-generation sequencing approach. All of these observations support the assumption that somatic mutations can potentially be used as a marker of clonal relationship. However, it is known that the same mutations can occur in different tumor types and that is why correlation with tumor morphology becomes very important.29, 30 Our study demonstrated a high concordance of 89% between adenocarcinoma morphology and molecular assessment of the clonal relationship. The concordance for squamous cell carcinoma was much lower (56%) because of a relatively low frequency of identified molecular alterations and lack of morphological heterogeneity. One of the shortcomings of our study may be the small number of analyzed genes that may have led to a relatively large number of molecularly inconclusive cases (36% of adenocarcinomas and 47% of squamous cell carcinomas). Recent more comprehensive approaches also reported inconclusive results.31 Murphy et al31 proposed that the assessment of DNA rearrangements by next-generation DNA sequencing might be a better approach as identifier of lineage than single-nucleotide mutations, but unfortunately no survival data were provided to support the merit of this suggestion. At this time, use of next-generation sequencing in the detection of gene rearrangements is limited, complex and more suitable as a research tool. Furthermore, a relative rarity of gene rearrangements in lung adenocarcinoma would make a targeted testing less practical. In contrast to adenocarcinoma, squamous cell carcinoma is not routinely subjected to molecular testing in clinical practice. Therefore, it was difficult to select appropriate mutations or genes subject to copy number changes that would occur in a significant portion of cases. We selected PIK3CA mutations and p16 deletions as these are two of the most common genetic alterations in squamous cell carcinoma based on the TCGA data (47 and 72%, respectively).32 In our study, only one tumor harbored a PIK3CA mutation (5%), similar to the frequency of 4% reported by Rekhtman et al33 in their study of surgically resected squamous cell carcinoma. The prevalence of PIK3CA mutations in multifocal squamous cell carcinoma has not been reported previously, and therefore, we are uncertain about the significance of our results. On the other hand, p16 deletions were detected in 41% of squamous cell carcinomas and were associated with shorter survival. It is striking that many studies, including ours, failed to demonstrate correlation between clonality defined by genotype and outcomes.9, 10 Therefore, it is uncertain whether different mutations identify separate primary cancers or just a different clone within single tumor. Large prospective studies with precisely defined treatment management could potentially provide the answer.
In summary, our study demonstrates the difficulties in pathological staging of multifocal lung cancers based on morphology and genotype. Comparing morphology of multiple tumors is feasible and more useful in adenocarcinomas than squamous cell carcinomas. Although utilizing genotypic data for pathological staging seems appealing, demonstration of driver mutations alone is not sufficient to establish a clonal relationship. It is essential to correlate genotypic data with morphology and other clinical data including imaging studies. This multidisciplinary approach in the classification of multifocal lung tumors should be prospectively validated in routine clinical practice.
References
Chang YL, Wu CT, Lee YC . Surgical treatment of synchronous multiple primary lung cancers: experience of 92 patients. J Thorac Cardiovasc Surg 2007;134:630–637.
Arai J, Tsuchiya T, Oikawa M et al. Clinical and molecular analysis of synchronous double lung cancers. Lung Cancer 2012;77:281–287.
Martini N, Melamed MR . Multiple primary lung cancers. J Thorac Cardiovasc Surg 1975;70:606–612.
Dacic S, Ionescu DN, Finkelstein S et al. Patterns of allelic loss of synchronous adenocarcinomas of the lung. Am J Surg Pathol 2005;29:897–902.
Girard N, Ostrovnaya I, Lau C et al. Genomic and mutational profiling to assess clonal relationships between multiple non-small cell lung cancers. Clin Cancer Res 2009;15:5184–5190.
Huang J, Behrens C, Wistuba I et al. Molecular analysis of synchronous and metachronous tumors of the lung: impact on management and prognosis. Ann Diagn Pathol 2001;5:321–329.
Kalikaki A, Koutsopoulos A, Trypaki M et al. Comparison of EGFR and K-RAS gene status between primary tumours and corresponding metastases in NSCLC. Br J Cancer 2008;99:923–929.
Takamochi K, Oh S, Matsuoka J et al. Clonality status of multifocal lung adenocarcinomas based on the mutation patterns of EGFR and K-ras. Lung Cancer 2012;75:313–320.
Warth A, Macher-Goeppinger S, Muley T et al. Clonality of multifocal nonsmall cell lung cancer: implications for staging and therapy. Eur Respir J 2012;39:1437–1442.
Wu C, Zhao C, Yang Y et al. High discrepancy of driver mutations in patients with NSCLC and synchronous multiple lung ground-glass nodules. J Thorac Oncol 2015;10:778–783.
Lindeman NI, Cagle PT, Beasley MB et al. Molecular testing guideline for selection of lung cancer patients for EGFR and ALK tyrosine kinase inhibitors: guideline from the College of American Pathologists, International Association for the Study of Lung Cancer, and Association for Molecular Pathology. Arch Pathol Lab Med 2013;137:828–860.
Travis WD, Noguchi M, Yatabe Y et al. Adenocarcnioma. In: Travis WD, Brambilla E, Burke AP et al (eds). The World Health Organization Classification of Tumours of the Lung, Pleura, Thymus and Heart, 4th edn. IARC: Lyon, France, 2015, pp 46–51.
Li Z, Dacic S, Pantanowitz L et al. Correlation of cytomorphology and molecular findings in EGFR+, KRAS+, and ALK+ lung carcinomas. Am J Clin Pathol 2014;141:420–428.
Kris MG, Johnson BE, Berry LD et al. Using multiplexed assays of oncogenic drivers in lung cancers to select targeted drugs. JAMA 2014;311:1998–2006.
Dacic S, Luvison A, Evdokimova V et al. RET rearrangements in lung adenocarcinoma and radiation. J Thorac Oncol 2014;9:118–120.
Girard N, Deshpande C, Azzoli CG et al. Use of epidermal growth factor receptor/Kirsten rat sarcoma 2 viral oncogene homolog mutation testing to define clonal relationships among multiple lung adenocarcinomas: comparison with clinical guidelines. Chest 2010;137:46–52.
Girard N, Deshpande C, Lau C et al. Comprehensive histologic assessment helps to differentiate multiple lung primary nonsmall cell carcinomas from metastases. Am J Surg Pathol 2009;33:1752–1764.
Travis WD, Brambilla E, Noguchi M et al. International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society International Multidisciplinary Classification of Lung Adenocarcinoma. J Thorac Oncol 2011;6:244–285.
Thunnissen E, Beasley MB, Borczuk AC et al. Reproducibility of histopathological subtypes and invasion in pulmonary adenocarcinoma. An international interobserver study. Mod Pathol 2012;25:1574–1583.
Homer RJ . Pathologists' staging of multiple foci of lung cancer: poor concordance in absence of dramatic histologic or molecular differences. Am J Clin Pathol 2015;143:701–706.
Noguchi M, Minami Y, Iijima T et al. Reproducibility of the diagnosis of small adenocarcinoma of the lung and usefulness of an educational program for the diagnostic criteria. Pathol Int 2005;55:8–13.
Thunnissen E, Noguchi M, Aisner S et al. Reproducibility of histopathological diagnosis in poorly differentiated NSCLC: an international multiobserver study. J Thorac Oncol 2014;9:1354–1362.
Daniele L, Cassoni P, Bacillo E et al. Epidermal growth factor receptor gene in primary tumor and metastatic sites from non-small cell lung cancer. J Thorac Oncol 2009;4:684–688.
Schmid K, Oehl N, Wrba F et al. EGFR/KRAS/BRAF mutations in primary lung adenocarcinomas and corresponding locoregional lymph node metastases. Clin Cancer Res 2009;15:4554–4560.
Wang X, Wang M, MacLennan GT et al. Evidence for common clonal origin of multifocal lung cancers. J Natl Cancer Inst 2009;101:560–570.
Cortot AB, Italiano A, Burel-Vandenbos F et al. KRAS mutation status in primary nonsmall cell lung cancer and matched metastases. Cancer 2010;116:2682–2687.
Yatabe Y, Matsuo K, Mitsudomi T . Heterogeneous distribution of EGFR mutations is extremely rare in lung adenocarcinoma. J Clin Oncol 2011;29:2972–2977.
Vignot S, Frampton GM, Soria JC et al. Next-generation sequencing reveals high concordance of recurrent somatic alterations between primary tumor and metastases from patients with non-small-cell lung cancer. J Clin Oncol 2013;31:2167–2172.
Ciriello G, Miller ML, Aksoy BA et al. Emerging landscape of oncogenic signatures across human cancers. Nat Genet 2013;45:1127–1133.
Alexandrov LB, Nik-Zainal S, Wedge DC et al. Signatures of mutational processes in human cancer. Nature 2013;500:415–421.
Murphy SJ, Aubry MC, Harris FR et al. Identification of independent primary tumors and intrapulmonary metastases using DNA rearrangements in non-small-cell lung cancer. J Clin Oncol 2014;32:4050–4058.
Cancer Genome Atlas Research Network. Comprehensive genomic characterization of squamous cell lung cancers. Nature 2012;489:519–525.
Rekhtman N, Paik PK, Arcila ME et al. Clarifying the spectrum of driver oncogene mutations in biomarker-verified squamous carcinoma of lung: lack of EGFR/KRAS and presence of PIK3CA/AKT1 mutations. Clin Cancer Res 2012;18:1167–1176.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Schneider, F., Derrick, V., Davison, J. et al. Morphological and molecular approach to synchronous non-small cell lung carcinomas: impact on staging. Mod Pathol 29, 735–742 (2016). https://doi.org/10.1038/modpathol.2016.66
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/modpathol.2016.66
This article is cited by
-
Morphological and genetic heterogeneity of synchronous multifocal lung adenocarcinoma in a Chinese cohort
BMC Cancer (2021)
-
Concomitant EGFR mutation and ALK rearrangement in multifocal lung adenocarcinoma: a case report
Diagnostic Pathology (2020)
-
Histopathological and molecular study for synchronous lung adenocarcinoma staging
Virchows Archiv (2020)
-
Prognostic factors for pN2 non-small cell lung cancer: a comprehensive evidence from 73 studies involving 23,772 patients*
Oncology and Translational Medicine (2020)
-
Clonal Origin Evaluated by Trunk and Branching Drivers and Prevalence of Mutations in Multiple Lung Tumor Nodules
Molecular Diagnosis & Therapy (2020)