Abstract
The diagnosis of malignant peripheral nerve sheath tumor is challenging, particularly in the sporadic setting. Inactivation of the polycomb repressive complex 2 (PRC2), resulting from inactivating mutations of its constituents SUZ12 or EED1, has recently been identified in 70–90% of malignant peripheral nerve sheath tumors. Homozygous PRC2 inactivation results in loss of histone H3K27 trimethylation (H3K27me3). PRC2 inactivation promotes tumor progression and may render patients sensitive to epigenetic-based targeted therapies. H3K27me3 loss has not yet been validated as a diagnostic marker. We evaluated immunohistochemistry for H3K27me3 in 100 malignant peripheral nerve sheath tumors (70 sporadic, 10 neurofibromatosis type 1-associated, 10 radiation-associated, 10 epithelioid) and 200 other spindle cell neoplasms representing potential mimics (20 each monophasic synovial sarcoma, leiomyosarcoma, dedifferentiated liposarcoma, malignant solitary fibrous tumor, low-grade fibromyxoid sarcoma, cellular schwannoma, spindle cell melanoma, unclassified postradiation sarcoma; 10 each atypical neurofibroma, spindle cell rhabdomyosarcoma, gastrointestinal stromal tumor, fibrosarcomatous dermatofibrosarcoma protuberans). In total, 51 (51%) malignant peripheral nerve sheath tumors, including 34 (49%) sporadic, 7 (70%) neurofibromatosis type 1-associated, and 10 (100%) radiation-associated, but no epithelioid malignant peripheral nerve sheath tumors, were negative for H3K27me3. An additional 6 (6%) tumors showed heterogeneous H3K27me3 expression. Among the 90 sporadic, neurofibromatosis type 1-associated, and radiation-associated malignant peripheral nerve sheath tumors, complete H3K27me3 loss was observed in 29% of low-grade, 59% of intermediate-grade, and 83% of high-grade tumors (low vs intermediate/high grade, P=0.0003). Among other tumor types, 4 (20%) unclassified postradiation sarcomas were negative for H3K27me3, whereas all other neoplasms were positive. Loss of H3K27me3 is highly specific for malignant peripheral nerve sheath tumor (although only modestly more sensitive than S-100 protein and SOX10) and may be a useful diagnostic marker. Our findings suggest that PRC2 inactivation in malignant peripheral nerve sheath tumor may occur during progression to higher grades.
Similar content being viewed by others
Main
Accounting for ~5% of soft tissue sarcomas, malignant peripheral nerve sheath tumors occur either sporadically or in the setting of neurofibromatosis type 1 (each accounting for ~50% of cases); 10% of tumors in both patient groups arise following radiation therapy.1 They most often arise in the limbs, followed by the trunk/retroperitoneum and head and neck region of middle-aged to elderly adults. Most malignant peripheral nerve sheath tumors pursue an aggressive clinical course with a 5-year survival rate of 35–50%.2 In the absence of effective systemic therapy, surgery remains the mainstay of treatment. The lifetime risk of developing malignant peripheral nerve sheath tumor is estimated to be 5–10% for patients with neurofibromatosis type 1.3
An identifiable origin from a peripheral nerve or a neurofibroma, evidence of Schwann cell differentiation by immunohistochemistry or ultrastructural examination, or the development of a spindle cell sarcoma in a patient with neurofibromatosis type 1 represent diagnostic criteria. Histologically, a fascicular growth pattern with varying cellularity, myxoid areas, perivascular condensation of tumor cells, and spindle cells with tapering, hyperchromatic nuclei and scant cytoplasm are characteristic of malignant peripheral nerve sheath tumor. Up to 10–15% of malignant peripheral nerve sheath tumors show heterologous differentiation, most often rhabdomyosarcomatous (the so-called malignant Triton tumor). Epithelioid malignant peripheral nerve sheath tumor, in contrast, is usually unassociated with neurofibromatosis type 1, superficially located, and may occasionally arise from schwannomas.
Only a subset of malignant peripheral nerve sheath tumors express Schwann cell markers such as S-100 protein (40–50%, often only in a minority of tumor cells), SOX10 (30%), or glial fibrillary acidic protein (GFAP) (30%).4 Therefore, the diagnosis of sporadic malignant peripheral nerve sheath tumor often relies on recognition of characteristic histologic features and exclusion of histologic mimics. For the purposes of this study, we have separated sporadic malignant peripheral nerve sheath tumor cases into ‘tier 1’ when the diagnosis was certain (i.e., immunohistochemistry supported the histologic diagnosis) and ‘tier 2’ when histology was typical but supportive immunohistochemical findings were lacking (see Materials and Methods). Depending on the clinical setting and tumor site, the differential diagnosis of malignant peripheral nerve sheath tumor may include benign Schwann cell neoplasms such as cellular schwannoma and atypical neurofibroma, as well as other spindle cell sarcomas including monophasic synovial sarcoma, leiomyosarcoma, dedifferentiated liposarcoma, malignant solitary fibrous tumor, low-grade fibromyxoid sarcoma, spindle cell rhabdomyosarcoma, spindle cell gastrointestinal stromal tumor, and fibrosarcomatous dermatofibrosarcoma protuberans, and other neuroectodermal neoplasms, most notably spindle cell melanoma.
NF1 mutations and CDKN2A inactivation are found in the majority of malignant peripheral nerve sheath tumors.5, 6 CDKN2A inactivation is an early event in the development of malignant peripheral nerve sheath tumor, occurring during progression from conventional to atypical neurofibroma.5 In addition, inactivation of the polycomb repressive complex 2 (PRC2), resulting from mutually exclusive inactivating mutations of its constituents SUZ12 or EED1, was recently identified in 70–90% of malignant peripheral nerve sheath tumors.6, 7, 8 PRC2 inactivation leads to loss of trimethylation at lysine 27 of histone H3 (H3K27me3).6, 7, 8, 9 Inactivation of PRC2 was shown to be associated with progression from plexiform neurofibroma to malignant peripheral nerve sheath tumor by amplifying RAS-driven transcription.7, 10 Functional studies confirm that H3K27me3 restoration, and thereby reduced transcription of PRC2-regulated genes, inhibits cell growth in malignant peripheral nerve sheath tumor, suggesting a cooperative effect of inactivated PRC2 and NF1 and CDKN2A mutations in malignant peripheral nerve sheath tumor development and progression.7 Based on these findings, H3K27me3 may be used as a biomarker to predict functional PRC2 activity in malignant peripheral nerve sheath tumor.6, 7
To validate the potential diagnostic value of loss of H3K27me3, we evaluated immunohistochemistry for H3K27me3 in 100 cases of malignant peripheral nerve sheath tumor, including different clinical subgroups, and 200 other spindle cell neoplasms, focusing on histologic mimics.
Materials and methods
Cases were retrieved from the surgical pathology files of Brigham and Women's Hospital and the consult files of one of the authors (CDMF). Representative hematoxylin and eosin (H&E)-stained slides were reviewed by two of the authors (IMS and JLH). After rereview of 116 cases initially diagnosed as malignant peripheral nerve sheath tumor, 16 cases were excluded from the study because the morphologic features were felt not to be typical. In total, 100 cases of malignant peripheral nerve sheath tumor, including 70 sporadic, 10 neurofibromatosis type 1-associated, 10 radiation-associated, and 10 epithelioid were included. Altogether, 31 malignant peripheral nerve sheath tumors were classified as low grade (31%), 36 as intermediate grade (36%), and 33 as high grade (33%) according to the FNCLCC grading system. Eight sporadic malignant peripheral nerve sheath tumors showed heterologous differentiation (osteocartilaginous in 5 cases and rhabdomyosarcomatous in 3 cases). Two neurofibromatosis type 1-associated malignant peripheral nerve sheath tumors harbored heterologous rhabdomyosarcomatous differentiation, and one radiation-associated malignant peripheral nerve sheath tumor each showed glandular and chondro-osseous differentiation. As mentioned in the introduction, 47 sporadic malignant peripheral nerve sheath tumors were designated ‘tier 1’ cases with typical morphology and supportive immunohistochemical staining (i.e., expression of S-100 protein, SOX10, and/or GFAP), and 23 cases were designated ‘tier 2’, lacking immunohistochemical support for the diagnosis of malignant peripheral nerve sheath tumor but showing classic histology. Potential histologic mimics were carefully excluded by immunohistochemistry in the latter group of tumors. In addition, 200 other spindle cell neoplasms were evaluated, including 20 monophasic synovial sarcomas, 20 leiomyosarcomas, 20 dedifferentiated liposarcomas, 20 malignant solitary fibrous tumors, 20 low-grade fibromyxoid sarcomas (10 with associated sclerosing epithelioid fibrosarcoma), 20 cellular schwannomas, 10 atypical neurofibromas, 10 spindle cell rhabdomyosarcomas, 10 gastrointestinal stromal tumors, 10 cases of fibrosarcomatous dermatofibrosarcoma protuberans, 20 spindle cell melanomas, and 20 unclassified postradiation sarcomas.
Immunohistochemistry for H3K27me3 was performed on 4-μm-thick formalin-fixed paraffin-embedded whole tissue sections following pressure cooker antigen retrieval (Target Retrieval Solution, pH 6.1; Dako, Carpinteria, CA, USA) using a rabbit polyclonal antibody directed against the trimethylated lysine 27 residue of the N-terminal portion of histone H3 (1:500 dilution; 07-449; Millipore, Billerica, MA, USA). Appropriate positive (normal skeletal muscle, colon, and skin) and negative controls (a high-grade malignant peripheral nerve sheath tumor with known loss of H3K27me3) were used throughout. Extent of staining was scored as positive, heterogeneous (i.e., expression lost in a scattered subset of tumor cells), and negative (complete absence of staining).
This study was performed with the approval of the Institutional Review Board at Brigham and Women's Hospital.
Results
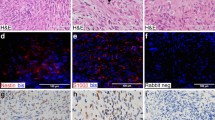
The results of immunohistochemical staining for H3K27me3 in 100 malignant peripheral nerve sheath tumors and 200 other benign and malignant spindle cell neoplasms are shown in Tables 1 and 2, respectively. In many tumors within most diagnostic categories, a small subset of nuclei (<5%) lacked H3K27me3 staining; this finding is of unknown significance. In total, 51 of 100 (51%) malignant peripheral nerve sheath tumors were negative for H3K27me3, including 34 of 70 sporadic (49%) (Figure 1), 7 of 10 (70%) neurofibromatosis type 1-associated (Figure 2), 10 of 10 (100%) radiation-associated, and 0 of 10 (0%) epithelioid malignant peripheral nerve sheath tumors (Figure 3). Among the sporadic tumors, 21 of 47 (44%) ‘tier 1’ and 13 of 23 (56%) ‘tier 2’ malignant peripheral nerve sheath tumors (i.e., without other supportive immunohistochemistry) were negative for H3K27me3. In the group of 90 sporadic, neurofibromatosis type 1-associated, and radiation-associated malignant peripheral nerve sheath tumors (excluding epithelioid malignant peripheral nerve sheath tumor owing to distinct biology), complete H3K27me3 loss was observed in 29% of low-grade, 59% of intermediate-grade, and 83% of high-grade tumors. H3K27me3loss was more frequent in intermediate- and highgrade (42 of 59; 71%) compared with low-grade (9 of 31; 29%) malignant peripheral nerve sheath tumors (P=0.0003). In addition, a heterogeneous staining pattern with a subset of tumor cells (~20–40%) showing loss of staining for H3K27me3 was observed in 3 of 10 (30%) neurofibromatosis type 1-associated and in 3 of 70 (4%) sporadic malignant peripheral nerve sheath tumors. Among the other tumor types evaluated, 4 of 20 (20%) unclassified postradiation spindle cell sarcomas showed negative staining for H3K27me3 (Figure 4), and 2 additional cases (10%) showed a heterogeneous staining pattern. Of the four H3K27me3-negative unclassified postradiation sarcomas, one was composed of fascicles of uniform spindle cells with vesicular chromatin and small nucleoli with a prominent lymphocytic infiltrate; one was composed of nondescript ovoid to short spindle cells with a haphazard architecture in a dense, collagenous stroma; one was composed of sheets of pleomorphic and epithelioid cells; and one was composed of hyperchromatic spindle cells with tapering nuclei and scattered pleomorphic cells with focally myxoid stroma. Only the latter tumor showed Schwann cell-like cytomorphology (but was negative for Schwann cell markers) (see Figure 4a). All other neoplasms exhibited diffuse nuclear H3K27me3 expression (Figures 5, 6, 7).
The majority of low-grade malignant peripheral nerve sheath tumors (a; hematoxylin and eosin (H&E)) show positive staining for histone H3 lysine 27 trimethylation (H3K27me3) (b). In contrast, more than half of all high-grade malignant peripheral nerve sheath tumors (c and e; H&E) exhibit loss of H3K27me3 expression (d and f); vascular endothelial cells and scattered inflammatory cells serve as positive internal controls.
Atypical neurofibromas containing scattered cells with degenerative nuclear nuclei atypia (a; hematoxylin and eosin (H&E)), and cellular schwannomas, in this case with a typical hyalinized blood vessel (c; H&E), show strong and diffuse expression of histone H3 lysine 27 trimethylation (H3K27me3) (b and d).
Discussion
Proteins of the polycomb group are transcriptional repressors that modify chromatin, regulate cell fate, and promote cancer development by epigenetic modifications.9, 11 In malignant peripheral nerve sheath tumor, inactivation of PRC2 promotes cell proliferation and tumor growth.6 PRC2 is composed of SUZ12, one EED isoform, and EZH1 or EZH2; this complex catalyzes the trimethylation of lysine 27 of histone H3,12 a well-described mark of epigenetic silencing.
We detected loss of H3K27me3 expression by immunohistochemistry in 51% of all malignant peripheral nerve sheath tumors (as well as heterogeneous staining in an additional 6% of tumors) and in 20% of radiation-associated unclassified spindle cell sarcomas, but in none of the other tumor types evaluated. These findings suggest that H3K27me3 loss is a highly specific marker for malignant peripheral nerve sheath tumor (albeit only modestly sensitive) and may be useful in differential diagnosis, particularly for tumors that lack expression of supportive Schwann cell markers (i.e., S-100 protein, SOX10, and GFAP) and among high-grade spindle cell sarcomas. Of note, negative staining for H3K27me3 was observed in 56% of malignant peripheral nerve sheath tumor cases designated ‘tier 2’ for the purposes of this study (i.e., those with classic histology but without immunohistochemical support), a similar rate as in tumors that expressed Schwann cell markers. Immunohistochemistry for H3K27me3 may therefore be used as a diagnostic marker for malignant peripheral nerve sheath tumor (potentially independent of Schwann cell markers) in the presence of typical morphologic features.
The biologic function of H3K27me3 in the pathogenesis of sarcomas is currently being investigated. Depending on the cellular context, the PRC2 proteins SUZ12, EED, and EZH2 are able to exert different functions in human cancer. EZH2 is known to be upregulated in various tumor types, including prostate13 and breast cancer,14 in which its overexpression correlates with aggressive behavior and poor outcome, as well as in lymphomas, lung cancer, and glioblastoma.15 EZH2 has been shown to be upregulated in poorly differentiated synovial sarcoma, a histologic variant associated with particularly aggressive behavior and unfavorable clinical outcome compared with monophasic and biphasic subtypes, resulting in increased expression of H3K27me3.16 In alveolar rhabdomyosarcomas with PAX3-FOXO1 fusion, EZH2 was also found to be overexpressed, leading to increased proliferation and survival and offering a potential target for EZH2 inhibitor therapies.17 Both oncogenic and tumor suppressor functions have been attributed to EZH2; it is likely that a delicately balanced gene dosage of the polycomb group proteins together with biologic context are crucial for stem cell homeostasis and determine oncogenic or tumor suppressor functions.9, 11 In contrast, EED and SUZ12 thus far appear to act predominantly as tumor suppressors;7, 8, 9 however, additional studies are required to clarify the role of each polycomb group protein in different cellular contexts.
The majority of malignant peripheral nerve sheath tumors harbor NF1 and CDKN2A mutations, which activate RAS signaling and dysregulate coordinated cell cycle progression, respectively. It has been suggested that PRC2 inactivation by mutations in either of its constituents might exert cooperative effects with NF1 and CDKN2A mutations.7 PRC2 inactivation might also promote tumor progression by further modulating already altered RAS signaling through an epigenetic switch.7, 10 Based on our findings, PRC2 inactivation may occur at some point during progression to higher grade malignant peripheral nerve sheath tumors, as H3K27me3 expression was lost significantly more frequently in intermediate/high-grade compared with low-grade tumors, but was retained in all atypical neurofibromas evaluated.
PRC2 inactivation has been reported in up to 88% of malignant peripheral nerve sheath tumors as detected by DNA and RNA sequencing analyses,6 which is a higher frequency than loss of H3K27me3 observed in our series (51%). As previously noted by Lee et al.,6 immunohistochemistry for H3K27me3 reliably detects homozygous inactivation of PRC2. However, heterozygous mutations of PRC2 components do not result in loss of H3K27me3 expression.6 The authors conclude from RNA levels, which correspond to H3K27me3 expression in malignant peripheral nerve sheath tumors with heterozygous inactivation, that DNA sequencing alone cannot predict PRC2 functional status in malignant peripheral nerve sheath tumors and that H3K27me3 immunohistochemistry might in fact be more accurate.6 In this previous study, 46 of 52 (88%) malignant peripheral nerve sheath tumors were found to harbor either SUZ12 (n=27) or EED (n=19) mutations.6 However, these included both heterozygous and homozygous (biallellic) inactivating mutations. Biallelic inactivation of SUZ12 or EED was identified in 65% of malignant peripheral nerve sheath tumor cases in this study, which is more in line with the rate of H3K27me3 loss observed in our study. In the present study, all radiation-associated malignant peripheral nerve sheath tumors (including 1 low-grade, 3 intermediate-grade, and 6 high-grade) exhibited loss of H3K27me, which is similar to a previously reported PRC2 mutation rate of 90%, all of which showed homozygous PRC2 inactivation.6
Interestingly, we detected a heterogeneous (mosaic) staining pattern with loss of expression in a subset of tumor cells (20–40%) in 30% of neurofibromatosis type 1-associated malignant peripheral nerve sheath tumors, 6% of sporadic malignant peripheral nerve sheath tumors, and 10% of radiation-associated unclassified spindle cell sarcomas. One possible explanation for this heterogeneous staining pattern might be that only a subset of tumor cells with heterozygous PRC2 subunit mutations (positive for H3K27me3) has acquired an additional genomic alteration that inactivates the second allele (negative for H3K27me3), likely loss of heterozygosity, which has not yet been completely selected for during tumor progression.
In our study, the group of neurofibromatosis type 1-associated malignant peripheral nerve sheath tumors showed a higher rate of H3K27me3 loss than sporadic cases (70% vs 49%). This observation is in accordance with previous studies, which reported PRC2 inactivation predominantly in malignant peripheral nerve sheath tumors with NF1 microdeletions (79%) vs non-microdeletions (34%).7 The investigators suggested that SUZ12 inactivation potentiated the effects of NF1 mutations by amplifying RAS signaling.7 Co-occurrence of genomic NF1 alterations and PRC2 complex inactivating mutations (i.e., SUZ12 mutations) has also been observed in melanoma and glioblastoma.7 However, all spindle cell melanomas in our study showed positive staining for H3K27me3, suggesting that immunohistochemistry for H3K27me3 may help distinguish malignant peripheral nerve sheath tumor from melanoma. In contrast, all epithelioid malignant peripheral nerve sheath tumors retained H3K27me3 expression; this rare malignant peripheral nerve sheath tumor variant is not associated with neurofibromatosis type 1 and arises in a different biologic context, namely, often accompanied by loss of INI1 (SMARCB1) expression and occasionally from a schwannoma.18, 19 Although genomic studies are lacking, is seems unlikely that NF1 and CDKN2A mutations would be found in this tumor type—PRC2 inactivation would therefore not be relevant for tumor progression. The fact that 20% of radiation-associated unclassified spindle cell sarcomas were negative for H3K27me, with another 10% showing heterogeneous loss of staining, suggests that some of these sarcomas might in fact represent malignant peripheral nerve sheath tumors. For the clinical management of these patients, it may be of value to be able to identify malignant peripheral nerve sheath tumors among these sarcomas to select patients who might be candidates for novel targeted therapeutic approaches.
Similar to alveolar rhabdomyosarcomas, therapies using EZH2 inhibiting agents have recently been evaluated in xenograft models and in vitro studies of malignant peripheral nerve sheath tumors; targeted therapy directed against EZH2 inhibited tumor cell growth and prolonged survival.15 Beyond the potential application of EZH2 inhibitors, PRC2 inactivation in malignant peripheral nerve sheath tumor also seems to render these tumors sensitive to BRD4 targeted therapies.7 Further investigations are needed to confirm efficacy of these approaches in patients; however, H3K27me3 loss might serve not only as a useful diagnostic tool but also as a predictive biomarker in malignant peripheral nerve sheath tumor.
In summary, H3K27me3 loss detected by immunohistochemistry may serve as a useful diagnostic marker in the distinction of malignant peripheral nerve sheath tumor from other benign and malignant spindle cell neoplasms, although it shows low sensitivity in low-grade and modest sensitivity in intermediate-grade tumors. H3K27me3 loss is more common in neurofibromatosis type 1-associated compared with sporadic malignant peripheral nerve sheath tumors and is particularly frequent in higher grade and radiation-associated tumors. Detection of H3K27me3 loss in previously unclassified radiation-associated sarcomas suggests a subset of these tumors may represent malignant peripheral nerve sheath tumors. The value of H3K27me3 as a predictive marker for sensitivity to epigenetic-based therapies remains to be determined.
References
Antonescu CR, Scheithauer BW, Woodruff JM . Malignant tumors of the peripheral nerves. In: AFIP Atlas of Tumor Pathology Series 4: Tumors of the Peripheral Nervous System. Volume 19. American Registry of Pathology: Silver Spring, MD, USA, 2013, pp 381–385.
Zou C, Smith KD, Liu J et al. Clinical, pathological, and molecular variables predictive of malignant peripheral nerve sheath tumor outcome. Ann Surg 2009;249:1014–1022.
Widemann BC . Current status of sporadic and neurofibromatosis type 1-associated malignant peripheral nerve sheath tumors. Curr Oncol Rep 2009;11:322–328.
Karamchandani JR, Nielsen TO, van de Rijn M et al. Sox10 and S100 in the diagnosis of soft-tissue neoplasms. Appl Immunohistochem Mol Morphol 2012;20:445–450.
Beert E, Brems H, Daniels B et al. Atypical neurofibromas in neurofibromatosis type 1 are premalignant tumors. Genes Chromosomes Cancer 2011;50:1021–1032.
Lee W, Teckie S, Wiesner T et al. PRC2 is recurrently inactivated through EED or SUZ12 loss in malignant peripheral nerve sheath tumors. Nat Genet 2014;46:1227–1232.
De Raedt T, Beert E, Pasmant E et al. PRC2 loss amplifies Ras-driven transcription and confers sensitivity to BRD4-based therapies. Nature 2014;514:247–251.
Zhang M, Wang Y, Jones S et al. Somatic mutations of SUZ12 in malignant peripheral nerve sheath tumors. Nat Genet 2014;46:1170–1172.
Sauvageau M, Sauvageau G . Polycomb group proteins: multi-faceted regulators of somatic stem cells and cancer. Cell Stem Cell 2010;7:299–313.
Baude A, Lindroth AM, Plass C . PRC2 loss amplifies Ras signaling in cancer. Nat Genet 2014;46:1154–1155.
Lee SC, Miller S, Hyland C et al. Polycomb repressive complex 2 component Suz12 is required for hematopoietic stem cell function and lymphopoiesis. Blood 2015;126:167–175.
Cao R, Wang L, Wang H et al. Role of histone H3 lysine 27 methylation in Polycomb-group silencing. Science 2002;298:1039–1043.
Varambally S, Dhanasekaran SM, Zhou M et al. The polycomb group protein EZH2 is involved in progression of prostate cancer. Nature 2002;419:624–629.
Kleer CG, Cao Q, Varambally S et al. EZH2 is a marker of aggressive breast cancer and promotes neoplastic transformation of breast epithelial cells. Proc Natl Acad Sci USA 2003;100:11606–11611.
Zhang P, Yang X, Ma X et al. Antitumor effects of pharmacological EZH2 inhibition on malignant peripheral nerve sheath tumor through the miR-30a and KPNB1 pathway. Mol Cancer 2015;14:55.
Changchien YC, Tatrai P, Papp G et al. Poorly differentiated synovial sarcoma is associated with high expression of enhancer of zeste homologue 2 (EZH2). J Transl Med 2012;10:216.
Ciarapica R, De SM, Carcarino E et al. The Polycomb group (PcG) protein EZH2 supports the survival of PAX3-FOXO1 alveolar rhabdomyosarcoma by repressing FBXO32 (Atrogin1/MAFbx). Oncogene 2014;33:4173–4184.
Hornick JL, Dal CP, Fletcher CD . Loss of INI1 expression is characteristic of both conventional and proximal-type epithelioid sarcoma. Am J Surg Pathol 2009;33:542–550.
Jo VY, Fletcher CD . Epithelioid malignant peripheral nerve sheath tumor: clinicopathologic analysis of 63 cases. Am J Surg Pathol 2015;39:673–682.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Schaefer, IM., Fletcher, C. & Hornick, J. Loss of H3K27 trimethylation distinguishes malignant peripheral nerve sheath tumors from histologic mimics. Mod Pathol 29, 4–13 (2016). https://doi.org/10.1038/modpathol.2015.134
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/modpathol.2015.134
This article is cited by
-
A distal ileum malignant peripheral nerve sheath tumour after abdominal radiation therapy: case report of a rare tumour
International Cancer Conference Journal (2023)
-
Sinonasal mucosal melanoma with smooth muscle differentiation: a potential pathological diagnostic pitfall
Diagnostic Pathology (2022)
-
Unusual split green-orange signals in USP6 fluorescence in situ hybridization in a malignant peripheral nerve sheath tumor with a novel NF1-SCIMP fusion: a potential diagnostic pitfall
Virchows Archiv (2022)
-
Update from the 5th Edition of the World Health Organization Classification of Head and Neck Tumors: Soft Tissue Tumors
Head and Neck Pathology (2022)
-
Leiomyosarcoma of the Nasal Cavity and Paranasal Sinuses: A Case Report and Comprehensive Review of the Literature
Head and Neck Pathology (2022)