Abstract
In order to evaluate the usefulness of p16 staining in predicting the outcome of histological low-grade squamous intraepithelial lesion/cervical intraepithelial neoplasia grade 1 (LSIL/CIN1) we prospectively recruited all the patients referred to colposcopy from 2003 to 2011 due to abnormal screening test results and diagnosed with LSIL/CIN1 at biopsy (n=507). All biopsies were stained for p16 and re-evaluated after three years by the same gynecological pathologist using the LAST criteria. Follow-up was conducted every 6 months and included a Pap test (liquid-based cytology), high-risk human papillomavirus testing (Hybrid Capture 2 test), and colposcopy. The mean follow-up was 28 months. An outcome diagnosis of HSIL was defined as a histological diagnosis of high-grade SIL/CIN (HSIL/CIN2-3). The diagnosis of LSIL/CIN1 was confirmed in 416 out of 507 biopsies (82%), whereas 58 (11%) were reclassified as negative and 33 (6%) as HSIL/CIN2-3. During follow-up, 86/507 women initially diagnosed with LSIL/CIN1 (17%) showed an outcome diagnosis of HSIL/CIN2-3, with the rate of HSIL final diagnosis of 3% (2/58) in the women with biopsies reclassified as negative, 17% (70/416) in the group with confirmed LSIL and 42% (14/33) in the women with biopsies reclassified as HSIL (P<0.001). p16 was positive in 245/507 patients (48%) and in 210/416 patients (50%) with confirmed LSIL/CIN1 at re-evaluation. Although positive p16 immunostaining was associated with risk of HSIL/CIN2-3 outcome in the multivariate analysis (Hazard ratio (HR) 1.9; 95% confidence interval (CI): 1.2–3.1; P=0.009) in the overall group of patients with LSIL/CIN1, this association was not verified in the subset of patients with confirmed LSIL/CIN1 after re-evaluation (HR: 1.6; 95% CI: 0.9–2.6; P=0.095). In conclusion, in LSIL/CIN1 lesions p16 should be limited to equivocal cases in which HSIL/CIN2 is included in the differential diagnosis since it has low value in clinical practice as a marker of progression of LSIL/CIN1.
Similar content being viewed by others
Main
Cervical cancer is a neoplasm caused by high-risk human papillomavirus (hrHPV) infection1 and arises from intraepithelial precursors, currently designated as squamous intraepithelial lesions or cervical intraepithelial neoplasias (SIL/CIN).2 High-grade SIL/CIN grades 2–3 (HSIL/CIN2-3) are considered the immediate precursor of cervical cancer.3 In contrast, about 80% of low-grade SIL/CIN1 (LSIL/CIN1) are transient lesions that generally spontaneously regress in 1–2 years.1, 4, 5, 6, 7 Accordingly, the management of HSIL/CIN2-3 generally involves the excision of the lesion and the transformation zone to prevent the development of cervical cancer, whereas the management of LSIL/CIN1 is conservative, being based on clinical follow-up.8, 9 However, 10 to 15% of LSIL/CIN1 lesions progress to HSIL/CIN2-3.1, 5, 10 Unfortunately, the clinical, cytological, virological and histological methods currently available cannot identify which LSIL/CIN1 lesions will show an HSIL/CIN2-3 outcome.
The p16INK4a (p16) tumor-suppressor protein has been shown to be a surrogate marker of hrHPV oncogenic activity.11, 12, 13, 14 The value of p16 in the diagnostic process is important, because it is detected in almost all HSIL/CIN2-3, whereas reactive mimickers, such as immature metaplasia or atrophy, are negative or show focal staining.5, 13, 15 Thus, p16 staining has achieved a major role in indisputably classifying a lesion as HSIL/CIN2-3 or as reactive.5, 13, 15
In contrast with the almost constantly positive results of HSIL/CIN2-3 the results of p16 staining in LSIL/CIN1 vary greatly, with some lesions being completely negative or showing focal staining, whereas others show a diffuse, basal, positive reaction.5, 11 This variability in staining has raised the question as to whether p16 overexpression in patients with LSIL/CIN1 could be correlated with the potential of ‘progression’. In the last decade, a number of studies have addressed this issue and have suggested that p16-positive LSIL/CIN1 lesions are at higher risk of showing a HSIL/CIN2-3 outcome diagnosis.4, 5, 16, 17 However, the number of cases included in these series was very small and consequently, the true value of p16 as a marker of ‘progression’ in these women remains uncertain. Thus, prospective, longitudinal studies focused on the prognostic value of p16 immunostaining in patients with biopsies showing LSIL/CIN1 are needed before definitive conclusions can be drawn.5, 16, 18, 19, 20, 21, 22
The aim of this study was to evaluate the usefulness of p16 staining in predicting the outcome of biopsy proven LSIL/CIN1 lesions, in a large prospective series of patients recruited at a single institution and who were strictly followed over a long period of time.
Materials and methods
Study Design and Patient Selection
Among the women referred to the Colposcopy Clinic of the Department of Obstetrics and Gynecology of the Hospital Clinic of Barcelona from January 2003 to October 2011, all the patients showing histologically confirmed LSIL/CIN1 as the most severe lesion at the initial evaluation were prospectively included in the study.
All the women had been referred because of an abnormal Pap test result (atypical squamous cells of any type or SIL of any grade). At the initial visit all patients underwent colposcopy examination and at least one biopsy for histological analysis (colposcopically directed biopsy and/or endocervical curettage). A cervical sample was collected from all of the patients using a cytobrush which was transferred to PreservCyt solution (Hologic Corp, Marlborough, MA, USA) for ThinPrep liquid-based cytology and high-risk HPV testing. Exclusion criteria were: (1) previous history of cervical cancer, (2) treatment for HSIL/CIN2-3 performed within the previous 3 years; (3) immunosuppression, (4) pregnancy, and (5) insufficient tissue showing LSIL/CIN1 for immunohistochemical analysis. All patients signed informed consent, and the study was approved by the Institutional Ethical Review Board.
Liquid-Based Cytology and hrHPV Testing
Thin-layer cytology slides were prepared using the Thin- Prep T2000 slide processor (Hologic) and stained with the Papanicolaou method. Cytology slides were evaluated by a cytotechnologist and reviewed by a pathologist using the revised Bethesda nomenclature.23
hrHPV testing was performed in the cervical specimens collected in PreservCyt solution using the commercially available Hybrid Capture 2 (HC2) system (Qiagen, Gaithersburg, MD, USA). All the samples were analyzed for the presence of hrHPV types (16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, and 68). A relative light units value of 1 (1.0 pg/ml) was used as the cutoff to classify and record each specimen as positive or negative.23, 24 The relative light units were recorded and used as a measure of viral load.23, 24
Histological Evaluation and Immunohistochemical Detection of p16
All the histological samples were fixed in 10% neutral buffered formalin and embedded in paraffin following routine procedures. The diagnosis of LSIL/CIN1 was based on hematoxylin and eosin (H&E), without knowledge of hrHPV status or the Pap test result. All the slides were reviewed by a gynecological pathologist (JO).
p16 was detected using the CINtec Histology Kit (clone E6H4, Roche-Mtm Laboratories, Heidelberg, Germany) following the manufacturer’s protocol. Immunohistochemistry was performed with the Autostainer Link 48 automated system (Dako Co, Carpinteria, CA, USA), using the EnVision system (Dako). Each series included a positive control, consisting of a HSIL/CIN3. Normal squamous ectocervical epithelium, which was present at least focally in all specimens, was used as a negative control. In all the cases the evaluation of p16 positivity was restricted to the area of the lesion. All the cases with no residual lesion in the additional sections of immunohistochemical staining were excluded from the analysis. Staining was scored as negative, focal, or diffuse on the basis of nuclear and/or cytoplasmic staining. Cases with complete absence of p16 staining were classified as negative. The immunostaining was scored as focal when either discontinuous staining of isolated basal cells or any type of staining of superficial and/or suprabasal layers was detected. Diffuse staining was defined as continuous staining of the basal and suprabasal cells in an area, independently of whether the superficial cells of the squamous epithelium were stained or not.5, 23 Although all types of positive results were recorded, only diffuse staining was considered as a positive reaction.
At least 3 years after the initial evaluation all the biopsies were re-evaluated by the same gynecological pathologist (JO). The re-evaluation was performed blindly to the results of hrHPV status and Pap test results and, although it was initially based on hematoxylin and eosin (H&E), the LAST criteria were used in this evaluation and in all cases with equivocal CIN1/CIN2 features p16 positivity was used to support a diagnosis of HSIL/CIN2.2
Follow-Up and Final Outcome
The first follow-up visit was performed at 3 months after the diagnosis. Thereafter, visits were scheduled every 6 months. At each follow-up a cervical sample was collected in PreservCyt and processed for liquid-based cytology and hrHPV testing, and all the patients underwent colposcopy. Directed biopsies and/or endocervical curettage were performed as indicated in the case of an HSIL result in the Pap test or significant changes in the colposcopy findings.
The final outcome of the patients was categorized as outcome diagnosis of HSIL, persistence or regression according to the results obtained at follow-up. Outcome diagnosis of HSIL was defined as the presence of histologically confirmed HSIL/CIN2-3 during the follow-up, independently of the Pap test or the hrHPV test result. All HSILs diagnosis were further subdivided into CIN2 and CIN3. Persistence was diagnosed on the basis of: (1) LSIL/CIN1 diagnosis by a colposcopically directed biopsy or endocervical curettage, independently of the Pap test result or hrHPV status; (2) one or more Pap tests showing atypical squamous cells or SIL of any grade with a negative biopsy, independently of the hrHPV test result; and (3) persistence of a positive hrHVP with a normal Pap test and/or normal biopsy results. Regression was diagnosed after one or more negative results in the hrHPV testing, with negative Pap test and biopsy results (if available).
All patients showing an HSIL/CIN2-3 diagnosis during follow-up underwent treatment with the loop electrosurgical excision procedure within 3 months after the diagnosis. Patients undergoing conization left the study after treatment and followed specific post-treatment controls.
Data Analysis
Statistical analysis was performed using the SPSS software (Version 18.0, SPSS, Inc, Chicago, IL, USA). The results are presented as absolute numbers and percentages or mean and standard deviation. χ2- or Fisher exact tests were used, as appropriate, for comparisons between categorical variables. The ANOVA test was used to compare quantitative variables between the different categories. A P-value below 0.05 was considered statistically significant. Univariate Cox models using the risk estimation as the hazard ratio (HR) and their 95% confidence intervals (95% CI) were used to analyze the prognostic factors putatively associated with progression to HSIL/CIN2-3. These models included age, positive hrHPV status, viral load in relative light units, HSIL Pap test result at initial visit and positive p16 immunostaining as covariates. Multivariate Cox regression models were applied to adjust group comparisons to possible confounders and evaluate interaction effects with regard to risk of progression. All analyses were performed for the whole study group (507 cases) and for the subset of patients with LSIL/CIN1 confirmed at re-evaluation.
Results
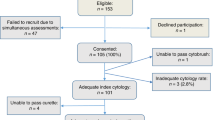
Six hundred and sixty-nine women were recruited for the study, 162 of whom met one of the exclusion criteria. Thus, 507 patients were finally included in the analysis. The mean age of the overall group was 33±10 years. The mean follow-up was 28± 25 months (range 3-132). Eighty-six patients (17%)showed an outcome diagnosis of HSIL/CIN2-3. Sixty-two (72%) of the HSIL/CIN2-3 final diagnosis were classified as CIN2 and 24 (28%) as CIN3. One hundred and forty-five women (29%) showed persistent LSIL/CIN1. Among this latter group, 58 (40%) had a histological diagnosis of LSIL/CIN1, 51 (35%) had persistent cytological SIL or atypical squamous cells (39 with a hrHPV positive test and 12 with a hrHPV negative test), and 36 (25%) had a persistent hrHPV-positive result with a normal Pap test. Finally, 276 women (54%) spontaneously regressed. Figure 1 shows the flow chart of the women initially recruited, those excluded and the causes of exclusion, as well as the patients who were finally enrolled and followed during the study.
In the histological re-evaluation the diagnosis of LSIL/CIN1 was confirmed in 416 out of 507 initial biopsies (82%). Fifty-eight biopsies (11%) were reclassified as negative and 33 (6%) as HSIL/CIN2-3. In the follow-up biopsies, the diagnosis of HSIL/CIN2-3 was confirmed in all the cases.
Table 1 summarizes the general characteristics (age, hrHPV status, Pap test result) of the patients at entry in the study according to their final outcome. Women with lesions showing an outcome diagnosis of HSIL/CIN2-3 were significantly older (P=0.039), more frequently positive for hrHPV (P=0.003) and had a higher incidence of an initial Pap test result of HSIL (P<0.001) than those with persistent or regressing lesions. The rate of lesions showing a final diagnosis of HSIL/CIN2-3 was 3% (2/58) in the women with biopsies reclassified as negative, 17% (70/416) in the group with confirmed LSIL, and 42% (14/33) in the women with biopsies reclassified as HSIL/CIN2 (P<0.001). No significant differences were observed among the different groups in terms of hrHPV load (P=0.070). The average time to HSIL-CIN2-3 diagnosis was 15±18 months. No significant differences in the time to HSIL/CIN2 and HSIL/CIN3 were observed (14±18 months vs 16±16; P=0.674). Interestingly, 69% of the patients showing an outcome diagnosis of HSIL/CIN2-3 had an HSIL cytology simultaneously with the LSIL/CIN1 histological diagnosis or were reclassified as HSIL/CIN2 at histological review using the LAST criteria. The time until HSIL/CIN2-3 diagnosis was shorter in women with an HSIL result in the Pap test performed at the initial visit (9±7 months vs 18±22; P=0.020).
Overall, p16 was diffusely positive in 230 LSIL/CIN1 lesions (45%), showed focal positivity in 123 (24%) and was negative in 154 biopsies (30%). Table 2 shows the results of the p16 staining in the initial biopsy and the final outcome after follow-up in the overall group, as well as in the three diagnostic categories defined after the histological re-evaluation. Overall, LSIL/CIN1 lesions showing an outcome diagnosis of HSIL/CIN2-3 showed a higher prevalence of positivity for p16 immunostaining compared with persistent or regressing LSIL/CIN1 lesions (71% (61/86) vs 44% (184/421); P=0.001). The rate of outcome diagnosis of HSIL of the women with LSIL/CIN1 staining positive for p16 was higher than that of those with p16-negative LSIL/CIN (25% (61/245) vs 9% (25/262); P<0.001). The rate of progression of the women with confirmed LSIL/CIN1 in the re-evaluation showing staining for p16 was higher than that of those with p16-negative LSIL/CIN1 (24% (47/210) vs 11% (23/206); P=0.006). No differences were observed between patients showing an outcome diagnosis of CIN2 or CIN3 in terms of the prevalence of positivity for p16 in the initial biopsy (data not shown). Eleven cases showed positive p16 immunostaining and a negative hrHPV test result: 3 progressed; 2 persisted; and 6 regressed. Figure 2 shows the initial biopsies (H&E and p16 immunostaining) of two patients who progressed to HSIL/CIN2-3, one with positive and another with negative p16 staining in the initial biopsy.
(a–d) LSIL/CIN1 lesion with positive p16 staining at the initial biopsy and presenting an outcome diagnosis of HSIL/CIN2-3. (a) Hematoxylin and eosin (H&E) stain of the initial biopsy; (b) positive p16 stain; (c) second biopsy showing HSIL/CIN2-3 (H&E); (d) positive p16 stain. (e–h) LSIL/CIN1 lesion with negative p16 staining at the initial biopsy and presenting an outcome diagnosis of HSIL/CIN2-3. (e) H&E stain of the initial biopsy; (f) negative p16 stain of the initial LSI/CIN1 lesion; (g) second biopsy showing HSIL/CIN2-3 (H&E); (h) positive p16 stain.
Table 3 shows the univariate and multivariate analysis for risk of progression. Age over 30 years, an HSIL result in the Pap test at the initial visit, a positive hrHPV test and positive p16 immunostaining in the LSIL/CIN1 biopsy were associated with the risk of outcome HSIL/CIN2-3 diagnosis. In the multivariate analysis the association with the risk of outcome diagnosis of HSIL/CIN2-3 persisted for all the factors except a positive hrHPV test. However, when the analysis with the same variables was restricted to the women with confirmed LSIL diagnosis /CIN1 in the re-evaluation, p16 immunostaining was not associated with increased risk of outcome diagnosis of HSIL/CIN2-3.
Discussion
Our study shows that, although the rate of HSIL/CIN2-3 outcome of women with p16 positive LSIL/CIN1 was twice/twofold that of those with p16 negative LSIL/CIN1, the difference diminished to non-significant values in the multivariate analysis on excluding biopsies reclassified as negative or HSIL/CIN2-3 from the analysis. Indeed, when only the SIL/CIN1 lesions confirmed after revision were included in the analysis positive p16 immunostaining was not associated with the risk of an outcome diagnosis of HSIL/CIN2-3 (HR 1.6 (95%CI: 0.9–2.6); P=0.095), suggesting that the usefulness of p16 as a marker of progression might be related to confounding factors such as underdiagnosis of HSIL/CIN2. Interestingly, more than half of the patients (69%) with an outcome diagnosis of HSIL/CIN2-3 had a HSIL cytology simultaneously with the LSIL/CIN1 histological diagnosis or were reclassified as HSIL/CIN2 at histological review using the LAST criteria,2 suggesting that HSIL/CIN2-3 which was histologically underdiagnosed or missed on colposcopic exam was already present. Moreover, the number of patients with diffuse p16 staining in the LSIL/CIN1 biopsy and an outcome diagnosis of HSIL/CIN2-3 was relatively low. On the other hand, p16-negative results do not exclude a HSIL/CIN2-3 outcome, as a low, albeit significant, number of patients with negative p16 staining showed HSIL/CIN2-3 in the follow-up. These results raise concern about the usefulness of p16 as a marker of progression in women with LSIL/CIN1 in clinical practice, and reinforce the LAST recommendations indicating that p16 should only be used in cases with equivocal CIN1/CIN2 features or in the differential diagnosis between CIN2/3 and its benign mimickers.2, 22 However, the cut-point between LSIL/CIN1 and HSIL/CIN2 is very subjective. As p16 is positive not only in LSIL/CIN1 cases upgraded to HSIL/CIN2 but also in a significant proportion of cases below this cut-point, there is concern as to the real limit at which a case should be considered as equivocal.
Only a few longitudinal studies, summarized in Table 4, have evaluated the value of p16 immunostaining in women with LSIL/CIN1. Some of these studies do not include hrHPV testing,4, 16 have a short follow-up,21 or include a very limited number of patients with LSIL/CIN1.25, 26 Other studies analyze specific groups, which were retrospectively selected based on the outcome.17, 27 Independently of their design, all these studies show that patients with LSIL/CIN1 with diffuse p16 staining are at higher risk of progression to HSIL/CIN2-3 and suggest the need for closer follow-up.5, 16, 17, 25, 26 However, all these studies reported a relative inaccuracy of p16 to predict the outcome of LSIL/CIN1, thereby questioning the usefulness of follow-up strategies modulated by p16.5, 22, 26
The main strength of our study is that it includes a large series of women with LSIL/CIN1. Moreover, all the patients were prospectively recruited and followed over a long period of time. Finally, a well-defined follow-up routine was established, which included liquid-based cytology, hrHPV testing, and colposcopy every 6 months with directed biopsies, with endocervical curettage being performed in the case of an HSIL result in the Pap test or significant changes in the colposcopy findings.
The rate of hrHPV-positive results in our patients with LSIL/CIN1 (86.4%) is in keeping with previous data showing that over 80% of LSIL/CIN1 lesions are positive for hrHPV.17 This percentage was slightly higher (96.5%) in the group of patients who showed an outcome diagnosis of HSIL/CIN2-3, being similar to the positive rates observed in women with HSIL/CIN2-3.24 In our study, an HSIL result in the Pap test performed at the initial visit was strongly associated with an outcome diagnosis of HSIL/CIN2-3 (OR=5.6), and interestingly, the time until HSIL/CIN2-3 diagnosis was also shorter in these women suggesting that these patients might have already had an underlying HSIL/CIN2-3 lesion not identified or underdiagnosed in the initial evaluation. Several studies have reported that about 15–20% of women showing LSIL/CIN1 on biopsy have a Pap test result of atypical glandular cells, or atypical squamous cells cannot exclude high-grade lesion, or HSIL.28 Close follow-up should be recommended in this subset of patients, as a significant number of cervical cancer occur in these women.28 In our study, an HSIL Pap test result in the initial visit was observed in 15% of the women. Finally, 6% of the biopsies initially diagnosed as LSIL/CIN1 were reclassified as HSIL/CIN 2-3. Inter- and intraobserver variability is a known inherent limitation of morphological diagnosis.2, 12 A diagnosis of HSIL in the re-evaluation was associated with progression, suggesting that these women indeed had high-grade lesions which had been underdiagnosed in the first evaluation.
This study has some possible limitations. No HPV typing data were available, and, therefore, the potential correlation between p16 staining, specific type of hrHPV, and progression of the LSIL/CIN1 lesions could not be assessed. However, a recent study concluded that the hrHPV subtype is a poor predictor of the behavior of a LSIL/CIN1 lesion.29 Another controversial issue is that the biopsy procedure might alter the natural evolution of lesions favoring spontaneous resolution, particularly of small lesions. However, previous studies using either cytology or biopsy have shown no effect of the biopsy on the short-term evolution of LSIL/CIN1 lesions.30 Besides this, in our study the rate of outcome diagnosis of HSIL/CIN2-3 was 17%, being slightly higher than that shown in other series5, 10, 18, 21, 25, 31 and suggesting that the effect of the biopsy procedure on increasing the rate of regression is very low if not null. A final possible limitation is related to the accuracy of colposcopy to guide biopsy sampling,32 which might miss an underlying HSIL/CIN2-3 at initial evaluation in a proportion of women. In spite of its possible limitations, colposcopy is currently considered the gold standard to guide biopsy sampling in these patients.32, 33 Recent guidelines recommend treatment for patients with persistent discordant results in the cytology and the colposcopy directed biopsy8, 9 due to the high risk of underdiagnosis of HSIL/CIN2-3.
In conclusion, our results show that p16 overexpression in biopsies from women with LSIL/CIN1 is a poor predictor of risk of progression to HSIL/CIN2-3 and has very low or no value as a marker of progression of LSIL/CIN1 in clinical practice. Our results are in agreement of the LAST recommendations, suggesting that the use of p16 in LSIL/CIN1 lesions should be limited to equivocal cases in which HSIL/CIN2 is included in the differential diagnosis.
References
Schiffman M, Castle PE, Jeronimo J et al. Human papillomavirus and cervical cancer. Lancet 2007;370:890–907.
Darragh TM, Colgan TJ, Thomas CJ et al. The lower anogenital squamous terminology standardization project for HPV-associated lesions: background and consensus recommendations from the College of American Pathologists and the American Society for Colposcopy and Cervical Pathology. Int J Gynecol Pathol 2013;32:76–115.
McCredie MR, Sharples KJ, Paul C et al. Natural history of cervical neoplasia and risk of invasive cancer in women with cervical intraepithelial neoplasia 3: a retrospective cohort study. Lancet Oncol 2008;9:425–434.
Cortecchia S, Galanti G, Sgadari C et al. Follow-up study of patients with cervical intraepithelial neoplasia grade 1 overexpressing p16Ink4a. Int J Gynecol Cancer 2013;23:1663–1669.
del Pino M, Garcia S, Fuste V et al. Value of p16(INK4a) as a marker of progression/regression in cervical intraepithelial neoplasia grade 1. Am J Obstet Gynecol 2009;201:488.
del Pino M, Torne A, Alonso I et al. Colposcopy prediction of progression in human papillomavirus infections with minor cervical lesions. Obstet Gynecol 2010;116:1324–1331.
Schlecht NF, Platt RW, Duarte-Franco E et al. Human papillomavirus infection and time to progression and regression of cervical intraepithelial neoplasia. J Natl Cancer Inst 2003;95:1336–1343.
Massad LS, Einstein MH, Huh WK et al. 2012 updated consensus guidelines for the management of abnormal cervical cancer screening tests and cancer precursors. Obstet Gynecol 2013;121:829–846.
Torne A, del Pino M, Cusidó M et al. Guia de cribado del cáncer de cuello de útero en España, 2014. Revista Española de Patologia 2015;47:1–43.
Ostor AG . Natural history of cervical intraepithelial neoplasia: a critical review. Int J Gynecol Pathol 1993;12:186–192.
Benevolo M, Mottolese M, Marandino F et al. Immunohistochemical expression of p16(INK4a) is predictive of HR-HPV infection in cervical low-grade lesions. Mod Pathol 2006;19:384–391.
Bergeron C, Ordi J, Schmidt D et al. Conjunctive p16INK4a testing significantly increases accuracy in diagnosing high-grade cervical intraepithelial neoplasia. Am J Clin Pathol 2010;133:395–406.
Klaes R, Friedrich T, Spitkovsky D et al. Overexpression of p16(INK4A) as a specific marker for dysplastic and neoplastic epithelial cells of the cervix uteri. Int J Cancer 2001;92:276–284.
von Knebel DM . New markers for cervical dysplasia to visualise the genomic chaos created by aberrant oncogenic papillomavirus infections. Eur J Cancer 2002;38:2229–2242.
van der Marel J, van Baars R, Alonso I et al. Oncogenic human papillomavirus-infected immature metaplastic cells and cervical neoplasia. Am J Surg Pathol 2014;38:470–479.
Hariri J, Oster A . The negative predictive value of p16INK4a to assess the outcome of cervical intraepithelial neoplasia 1 in the uterine cervix. Int J Gynecol Pathol 2007;26:223–228.
Negri G, Vittadello F, Romano F et al. p16INK4a expression and progression risk of low-grade intraepithelial neoplasia of the cervix uteri. Virchows Arch 2004;445:616–620.
Anschau F, Schmitt VM, Lambert AP et al. Transition of cervical carcinoma in situ to invasive cancer: role of p16 INK4a expression in progression and in recurrence. Exp Mol Pathol 2009;86:46–50.
Guedes AC, Brenna SM, Coelho SA et al. p16(INK4a) Expression does not predict the outcome of cervical intraepithelial neoplasia grade 2. Int J Gynecol Cancer 2007;17:1099–1103.
Jackson JA, Kapur U, Ersahin C . Utility of p16, Ki-67, and HPV test in diagnosis of cervical intraepithelial neoplasia and atrophy in women older than 50 years with 3- to 7-year follow-up. Int J Surg Pathol 2012;20:146–153.
Ozaki S, Zen Y, Inoue M . Biomarker expression in cervical intraepithelial neoplasia: potential progression predictive factors for low-grade lesions. Hum Pathol 2011;42:1007–1012.
Mills AM, Paquette C, Castle PE et al. Risk stratification by p16 immunostaining of CIN1 biopsies: a retrospective study of patients from the quadrivalent HPV vaccine trials. Am J Surg Pathol 2015;39:611–617.
Solomon D, Davey D, Kurman R et al. The 2001 Bethesda System: terminology for reporting results of cervical cytology. JAMA 2002;287:2114–2119.
Ordi J, Alonso I, Torne A et al. Human papillomavirus load in Hybrid Capture II assay: does increasing the cutoff improve the test? Gynecol Oncol 2005;99:313–319.
Liao GD, Sellors JW, Sun HK et al. p16INK4A immunohistochemical staining and predictive value for progression of cervical intraepithelial neoplasia grade 1: a prospective study in China. Int J Cancer 2014;134:1715–1724.
Pacchiarotti A, Ferrari F, Bellardini P et al. Prognostic value of p16-INK4A protein in women with negative or CIN1 histology result: a follow-up study. Int J Cancer 2014;134:897–904.
Negri G, Bellisano G, Zannoni GF et al. p16 ink4a and HPV L1 immunohistochemistry is helpful for estimating the behavior of low-grade dysplastic lesions of the cervix uteri. Am J Surg Pathol 2008;32:1715–1720.
Katki HA, Gage JC, Schiffman M et al. Follow-up testing after colposcopy: five-year risk of CIN 2+ after a colposcopic diagnosis of CIN 1 or less. J Low Genit Tract Dis 2013;17:S69–S77.
Razmpoosh M, Sansregret A, Oligny LL et al. Assessment of correlation between p16INK4a staining, specific subtype of human papillomavirus, and progression of LSIL/CIN1 lesions: first comparative study. Am J Clin Pathol 2014;142:104–110.
Youkeles L, Forsythe AB, Stern E . Evaluation of Papanicolaou smear and effect of sample biopsy in follow-up of cervical dysplasia. Cancer Res 1976;36:2080–2084.
Cox JT, Schiffman M, Solomon D . Prospective follow-up suggests similar risk of subsequent cervical intraepithelial neoplasia grade 2 or 3 among women with cervical intraepithelial neoplasia grade 1 or negative colposcopy and directed biopsy. Am J Obstet Gynecol 2003;188:1406–1412.
van der Marel J, van BR, Rodriguez A et al. The increased detection of cervical intraepithelial neoplasia when using a second biopsy at colposcopy. Gynecol Oncol 2014;135:201–207.
Massad LS, Collins YC . Strength of correlations between colposcopic impression and biopsy histology. Gynecol Oncol 2003;89:424–428.
Acknowledgements
We are grateful to Naiara Vega, Silvia Alòs, and Francisco M Perez for their technical assistance with the cytological evaluation and the HC2 test, and Ms. Ingrid Victoria for her help with the immunohistochemical studies. We thank Donna Pringle for the English revision of the manuscript. This work was partly supported by Instituto de Salud Carlos III (ISCIII)-Fondos de Investigación Sanitaria and ERDF ‘one way to Europe’ (PI12/01231, and PI12/01165).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
JO and AT have been temporary advisors to Roche-MTM Laboratories. The remaining authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Sagasta, A., Castillo, P., Saco, A. et al. p16 staining has limited value in predicting the outcome of histological low-grade squamous intraepithelial lesions of the cervix. Mod Pathol 29, 51–59 (2016). https://doi.org/10.1038/modpathol.2015.126
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/modpathol.2015.126
This article is cited by
-
Possible role of negative human papillomavirus E6/E7 mRNA as a predictor of regression of cervical intraepithelial neoplasia 2 lesions in hr-HPV positive women
Virology Journal (2022)
-
The value of Ki67 for the diagnosis of LSIL and the problems of p16 in the diagnosis of HSIL
Scientific Reports (2022)
-
Accuracy of HPV E6/E7 mRNA examination using in situ hybridization in diagnosing cervical intraepithelial lesions
Diagnostic Pathology (2021)
-
p16ink4 and cytokeratin 7 immunostaining in predicting HSIL outcome for low-grade squamous intraepithelial lesions: a case series, literature review and commentary
Modern Pathology (2016)
-
Moderne Biomarker bei Präkanzerosen der Cervix uteri
Der Pathologe (2016)