Abstract
Acute myeloid leukemia arising from chronic myelomonocytic leukemia is currently classified as acute myeloid leukemia with myelodysplasia-related changes, a high-risk subtype. However, the specific features of these cases have not been well described. We studied 38 patients with chronic myelomonocytic leukemia who progressed to acute myeloid leukemia. We compared the clinicopathologic and genetic features of these cases with 180 patients with de novo acute myeloid leukemia and 34 patients with acute myeloid leukemia following myelodysplastic syndromes. We also examined features associated with progression from chronic myelomonocytic leukemia to acute myeloid leukemia by comparing the progressed chronic myelomonocytic leukemia cases with a cohort of chronic myelomonocytic leukemia cases that did not transform to acute myeloid leukemia. Higher white blood cell count, marrow cellularity, karyotype risk score, and Revised International Prognostic Scoring System score were associated with more rapid progression from chronic myelomonocytic leukemia to acute myeloid leukemia. Patients with acute myeloid leukemia ex chronic myelomonocytic leukemia were older (P<0.01) and less likely to receive aggressive treatment (P=0.02) than de novo acute myeloid leukemia patients. Most cases showed monocytic differentiation and fell into the intermediate acute myeloid leukemia karyotype risk group; 55% had normal karyotype and 17% had NPM1 mutation. Median overall survival was 6 months, which was inferior to de novo acute myeloid leukemia (17 months, P=0.002) but similar to post myelodysplastic syndrome acute myeloid leukemia. On multivariate analysis of all acute myeloid leukemia patients, only age and karyotype were independent prognostic variables for overall survival. Our findings indicate that acute myeloid leukemia following chronic myelomonocytic leukemia displays aggressive behavior and support placement of these cases within the category of acute myeloid leukemia with myelodysplasia-related changes. The poor prognosis of these patients may be related to an older population and lack of favorable-prognosis karyotypes that characterize many de novo acute myeloid leukemia cases.
Similar content being viewed by others
Main
Chronic myelomonocytic leukemia is a hematopoietic malignancy characterized by peripheral blood monocytosis and displaying overlapping features of a myeloproliferative neoplasm and a myelodysplastic syndrome. In the initial French–American–British (FAB) classification,1 chronic myelomonocytic leukemia was considered to be a subtype of myelodysplastic syndrome, but in 1994, the FAB group divided it into two types, ‘proliferative’ (chronic myelomonocytic leukemia-myeloproliferative type) and ‘dysplastic’ (chronic myelomonocytic leukemia-myelodysplastic type), based on a peripheral blood white blood cell count cutoff of 13 000/μl.2 In 2001, the World Health Organization (WHO) subsequently classified chronic myelomonocytic leukemia within the group of myelodysplastic syndrome/myeloproliferative disease neoplasms, but no longer distinguished proliferative and dysplastic subtypes.3 Instead, the WHO recognized two subcategories (chronic myelomonocytic leukemia-1 and chronic myelomonocytic leukemia-2) based on the number of peripheral blood and bone marrow blasts, which appear to be the most important factors in predicting prognosis of chronic myelomonocytic leukemia patients.4, 5, 6
Chronic myelomonocytic leukemia progresses to acute myeloid leukemia in up to one third of cases.7, 8, 9 Acute myeloid leukemia arising in a patient with a history of chronic myelomonocytic leukemia is currently classified in the 2008 WHO classification as acute myeloid leukemia with myelodysplasia-related changes. This aggressive subtype comprises a heterogeneous group of acute myeloid leukemia cases that either arise from a previous myelodysplastic syndrome or myelodysplastic syndrome/myeloproliferative neoplasm (including chronic myelomonocytic leukemia), bear an myelodysplastic syndrome-related cytogenetic abnormality, or demonstrate significant multilineage dysplasia.10 Regardless of the etiology, acute myeloid leukemia with myelodysplasia-related changes confers a poor prognosis.11 Because of the relative rarity of chronic myelomonocytic leukemia (with an annual incidence estimated at 4 cases per 100 000 persons),4, 5 acute myeloid leukemia arising from chronic myelomonocytic leukemia is uncommon and has not been extensively studied. In particular, most studies examining ‘secondary acute myeloid leukemia’ have not differentiated between cases following chronic myelomonocytic leukemia and those following myelodysplastic syndrome, and thus the particular features and prognosis of acute myeloid leukemia following chronic myelomonocytic leukemia are not well described.12, 13, 14, 15, 16
In this retrospective, multi-institutional study, we evaluated the clinicopathologic and genetic features of 38 patients with acute myeloid leukemia arising after a prior diagnosis of chronic myelomonocytic leukemia. We examined the outcome of these patients in comparison with patients with de novo acute myeloid leukemia and acute myeloid leukemia arising from a myelodysplastic syndrome. In addition, we examined the clinicopathologic and genetic features that were associated with progression to acute myeloid leukemia in a larger group of chronic myelomonocytic leukemia patients, including the Revised International Prognostic Scoring System (IPSS-R) for myelodysplastic syndrome.17
Materials and methods
Patients
This retrospective study, performed with the approval of the Institutional Review Boards of all involved institutions, included 38 cases of acute myeloid leukemia, arising from patients with a documented diagnosis of chronic myelomonocytic leukemia (acute myeloid leukemia ex chronic myelomonocytic leukemia), originating from 4 institutions (18 from Massachusetts General Hospital, 7 from Weill Cornell Medical College, 7 from University of Pittsburgh Medical Center, and 6 from Institut Universitaire de Pathologie) diagnosed between 2000 and 2011. For comparison, 34 cases of acute myeloid leukemia arising from myelodysplastic syndrome (acute myeloid leukemia ex myelodysplastic syndrome) and 180 de novo acute myeloid leukemia cases were identified from one institution (Massachusetts General Hospital) over the same time period. The acute myeloid leukemia ex myelodysplastic syndrome cases followed diagnoses of refractory anemia with excess blasts (23 cases, including 11 cases of refractory anemia with excess blasts-1 and 12 cases of refractory anemia with excess blasts-2), refractory cytopenia with multilineage dysplasia (7 cases), myelodysplastic syndrome with isolated del(5q) (2 cases), refractory cytopenia with unilineage dysplasia (1 case), and refractory anemia with ring sideroblasts (1 case). As a control group for the time-to-progression analysis, 19 cases of chronic myelomonocytic leukemia (17 from Massachusetts General Hospital and 2 from Weill Cornell Medical College) with no documented transformation to acute myeloid leukemia over a period of at least 10 months (median follow-up 33 months) were identified between 2000 and 2011. Classification of all cases was according to the 2008 WHO classification criteria. Cases of therapy-related acute myeloid leukemia, myelodysplastic syndrome, or chronic myelomonocytic leukemia were excluded.
Clinical and Pathology Review
Clinical parameters were obtained from the medical record. The point of acute myeloid leukemia transformation was defined as the date of a bone marrow or blood sample documenting ⩾20% blasts (including promonocytes). Therapies administered for chronic myelomonocytic leukemia, myelodysplastic syndrome, and acute myeloid leukemia were recorded as: supportive care (transfusion support and anti-infectives); low-intensity therapy (low-dose cytotoxic chemotherapy or clinical trial therapies not involving induction chemotherapy); induction chemotherapy; or allogeneic stem cell transplant at any time point in the treatment course.
The following bone marrow parameters of acute myeloid leukemia and chronic myelomonocytic leukemia cases were evaluated by review of aspirate smears and/or core biopsy material: cellularity, reticulin fibrosis grade (modified Bauermeister, grade 0–4),18 myeloid/erythroid ratio, monocyte percentage, blast percentage (including promonocytes), and FAB classification (for acute myeloid leukemia cases). For the 38 acute myeloid leukemia ex chronic myelomonocytic leukemia cases, the acute myeloid leukemia bone marrow slides were reviewed by the authors in 31 cases and the preceding chronic myelomonocytic leukemia bone marrow slides were reviewed by the authors in 34 cases. For the 19 chronic myelomonocytic control cases, bone marrow slides were reviewed by the authors in 14 cases. In cases where slides were unavailable, information was obtained from review of the pathology reports. The diagnosis of acute myeloid leukemia ex chronic myelomonocytic leukemia was made by the presence of ≥20% blast equivalents (blasts plus promonocytes) in the bone marrow aspirate (26 cases), peripheral blood (8 cases), or bone marrow biopsy (2 cases, present in sheets); the diagnosis was made by biopsy of soft tissue showing myeloid sarcoma in 1 case and autopsy showing acute myeloid leukemia in 1 case.
For the chronic myelomonocytic leukemia and acute myeloid leukemia ex chronic myelomonocytic leukemia cases with available material, the bone marrow aspirate smears and core biopsy specimens were reviewed for erythroid, myeloid, and megakaryocyte dysplasia, as well as megakaryocyte number. The dysplasias were scored as follows: dysplastic cells comprising <10% of a lineage=0, 10–20%=1, 20–50%=2, and >50%=3. Dysplastic features of each lineage were defined according to the 2008 WHO classification recommendations.19 The megakaryocyte number was scored as follows: none or very rare megakaryocytes=0, <3 megakaryocytes per 40 × field=1, 3–5 per 40 × field=2, and >5 per 40 × field=3. These parameters were scored independently by two of the authors (RPH and ELC) and the final scores were the average of each observer.
Cytogenetics
Karyotypes were obtained from the pathology records and were reported using the International System for Human Cytogenetic Nomenclature. Cases were stratified according to the UK Medical Research Council20 karyotype risk grouping for acute myeloid leukemia. The chronic myelomonocytic leukemia karyotypes were also stratified according to the IPSS scoring system,21 the new Comprehensive Cytogenetic Scoring System (CCSS) for primary myelodysplastic syndrome,22 and the recently proposed cytogenetic risk stratification system for chronic myelomonocytic leukemia.23 The CCSS score was also used along with blood counts and bone marrow blast count to classify the chronic myelomonocytic leukemia cases according to the IPSS-R scoring system.17
NPM1 and FLT3 Mutation Analysis
NPM1 and FLT3 mutation status was obtained from available molecular diagnostic reports of acute myeloid leukemia, myelodysplastic syndrome, and chronic myelomonocytic leukemia cases. For the chronic myelomonocytic leukemia and acute myeloid leukemia ex chronic myelomonocytic leukemia cases with available paraffin-embedded material, NPM1 immunohistochemical staining was performed on deparaffinized bone marrow trephine sections24 using the FLEX monoclonal mouse anti-human nucleophosmin antibody, Clone 376 (ready-to-use; Dako North America, Carpinteria, CA, USA) incubated overnight at 4 °C after antigen retrieval. The secondary antibody Mouse HRP (incubated for 15 min at room temperature) and the DAB Chromogen from Dako kit (K4006) were used. Counterstain was with Hematoxylin-2 from Richard Allan Scientific (Fisher/Thermo no. 7231). The presence of cytoplasmic NPM1 staining was considered indicative of an NPM1 mutation.24 Nuclear C23 expression (C23 mouse monoclonal antibody; 1:60 dilution; Santa Cruz Biotechnology, Santa Cruz, CA, USA) was used as a control for all cases showing cytoplasmic NPM1 expression.
Statistical Analysis
Categorical variables were compared using a two-tailed Fisher’s exact-test or χ2 test. Continuous variables were compared using a two-tailed Wilcoxon rank-sum test. Survival analysis was done using the Kaplan–Meier method and the log-rank test was used to determine the significance of the difference between variable classes. Cox proportional hazard model was used to model the relationship between overall survival and other significant prognostic factors.
Results
Clinicopathologic Features of Acute Myeloid Leukemia Ex Chronic Myelomonocytic Leukemia
Clinical and pathologic features of the acute myeloid leukemia ex chronic myelomonocytic leukemia patients, as compared with the acute myeloid leukemia ex myelodysplastic syndrome and de novo acute myeloid leukemia cases, are summarized in Table 1. The acute myeloid leukemia ex chronic myelomonocytic leukemia patients were significantly older than the de novo acute myeloid leukemia patients (P<0.01), with a median age similar to the acute myeloid leukemia ex myelodysplastic syndrome population. There was no significant difference in the progression time to acute myeloid leukemia between the acute myeloid leukemia ex chronic myelomonocytic leukemia and acute myeloid leukemia ex myelodysplastic syndrome groups. The acute myeloid leukemia ex chronic myelomonocytic leukemia patients were treated with supportive care only (7 patients), low-intensity therapies (demethylating agents, 6 patients; hydroxyurea, 3 patients; low-dose cytarabine, 1 patient), induction chemotherapy (11 patients), or allogeneic stem cell transplant (6 patients); treatment of 4 patients was unknown. There were no significant differences in the types of therapies given for acute myeloid leukemia ex chronic myelomonocytic leukemia and acute myeloid leukemia ex myelodysplastic syndrome; however, patients with acute myeloid leukemia ex chronic myelomonocytic leukemia were significantly less likely to be treated with induction chemotherapy (P=0.02) or allogeneic stem cell transplant (P=0.01) compared with de novo acute myeloid leukemia patients.
Megakaryocyte dysplasia was frequently seen in the acute myeloid leukemia ex chronic myelomonocytic leukemia cases, with a median megakaryocyte dysplasia score of 2 (20–50% of cells dysplastic). In contrast, myeloid dysplasia (median score 0.5) and erythroid dysplasia (median score 1) were less prominent. The acute myeloid leukemia ex chronic myelomonocytic leukemia patients had a significantly higher peripheral WBC, absolute neutrophil count, and absolute monocyte count, and also higher bone marrow cellularity and bone marrow monocyte percentage than both the de novo acute myeloid leukemia and acute myeloid leukemia ex myelodysplastic syndrome patients. A total of 76% of acute myeloid leukemia ex chronic myelomonocytic leukemia were categorized as FAB M4 or M5, which was a significantly higher proportion compared with de novo acute myeloid leukemia (P=0.0004) and with acute myeloid leukemia ex myelodysplastic syndrome (P<0.0001). The median bone marrow blast count in acute myeloid leukemia ex chronic myelomonocytic leukemia was 40%, which was higher than acute myeloid leukemia ex myelodysplastic syndrome (P=0.02) but lower than de novo acute myeloid leukemia (P=0.01).
Cytogenetics and Mutation Analysis
The abnormal karyotypes for the acute myeloid leukemia ex chronic myelomonocytic leukemia patients and their precedent chronic myelomonocytic leukemia karyotypes are listed in Table 2. The karyotype changed upon conversion to acute myeloid leukemia in 4/24 patient samples (17%), including one where the chronic myelomonocytic leukemia karyotype was normal but the acute myeloid leukemia karyotype was abnormal. There was no significant difference in the incidence of adverse UK Medical Research Council karyotype between acute myeloid leukemia ex chronic myelomonocytic leukemia and acute myeloid leukemia ex myelodysplastic syndrome or de novo acute myeloid leukemia. In comparing the IPSS karyotype score21 and the new CCSS karyotype score22 for all 57 chronic myelomonocytic leukemia cases with available karyotypes, 3 patients moved from the IPSS good to the CCSS very good cytogenetic risk category (based on a –Y abnormality) and one moved from the IPSS poor to the CCSS very poor cytogenetic risk category (based on a highly complex karyotype). For the remaining patients, the risk grouping of the chronic myelomonocytic leukemia karyotypes did not change. Applying the chronic myelomonocytic leukemia cytogenetic scoring system of Such et al,23 2 cases moved from the IPSS good to an intermediate score, whereas 2 cases moved from the IPSS intermediate to high-risk score due to the presence of trisomy 8; both of the latter patients progressed to acute myeloid leukemia in 15 and 22 months.
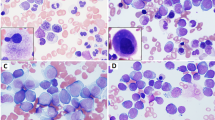
Five of the 29 acute myeloid leukemia ex chronic myelomonocytic leukemia cases (17%) and 15/69 tested de novo acute myeloid leukemia cases (22%) had the NPM1 mutation (P=0.8). Of the five acute myeloid leukemia ex chronic myelomonocytic leukemia cases with a NPM1 mutation, one had the NPM1 mutation detected in the precedent chronic myelomonocytic leukemia and three had precedent chronic myelomonocytic leukemia lacking detectable NPM1 mutation (Table 3); the NPM1 mutation status was unknown for one chronic myelomonocytic leukemia case. An additional acute myeloid leukemia case with unknown NPM1 mutation status had an NPM1 mutation detected in the precedent chronic myelomonocytic leukemia. All 11 samples that had both immunohistochemical and molecular testing for the NPM1 mutation performed had concordant results by both tests, consistent with prior reports;24 examples of NPM1 immunostaining patterns are shown in Figure 1. A FLT3 mutation was detected in 2/10 tested acute myeloid leukemia ex chronic myelomonocytic leukemia cases (20%, both internal tandem duplication (ITD) mutations) and 16/74 tested de novo acute myeloid leukemia cases (22%) (P=1.0). Of the 4 NPM1-mutated acute myeloid leukemia ex chronic myelomonocytic leukemia cases that had FLT3 testing performed, 1 had a FLT3 ITD mutation and 3 were negative. All of the acute myeloid leukemia ex chronic myelomonocytic leukemia cases with NPM1 and/or FLT3 mutation and available cytogenetic results had a normal karyotype. The 5 acute myeloid leukemia ex chronic myelomonocytic leukemia cases with NPM1 mutation had progressed to acute myeloid leukemia from chronic myelomonocytic leukemia in 0.5, 3, 3, 6, and 29 months; the acute myeloid leukemia case with unknown NPM1 mutation status but arising from NPM1-mutated chronic myelomonocytic leukemia had progressed to acute myeloid leukemia in 0.5 months.
Representative NPM1 immunohistochemistry. (a) NPM1-mutated chronic myelomonocytic leukemia case shows cytoplasmic NPM1 staining. (b) NPM1 wild-type chronic myelomonocytic leukemia case shows only nuclear NPM1 staining. (c) NPM1-mutated acute myeloid leukemia ex chronic myelomonocytic leukemia case from the same patient as (b) shows cytoplasmic NPM1 staining in blasts (Olympus DP25; magnification × 600).
Survival Analysis of Acute Myeloid Leukemia Ex Chronic Myelomonocytic Leukemia
The median follow-up time of surviving patients from the time of acute myeloid leukemia diagnosis was 17 months (range 11–43 months) for acute myeloid leukemia ex chronic myelomonocytic leukemia, 27 months (range 9–78 months) for acute myeloid leukemia ex myelodysplastic syndrome, and 27 months (range 2–106 months) for de novo acute myeloid leukemia. The median overall survival of the acute myeloid leukemia ex chronic myelomonocytic leukemia patients from the time of acute myeloid leukemia diagnosis was 6 months, similar to the acute myeloid leukemia ex myelodysplastic syndrome patients (9 months, P=0.37), but significantly inferior to de novo acute myeloid leukemia patients (17 months, P=0.002), as shown in Figure 2a. When the analysis was restricted to cases with an intermediate UK Medical Research Council cytogenetic risk grouping, median overall survival of the acute myeloid leukemia ex chronic myelomonocytic leukemia patients (13 months) was borderline inferior to the acute myeloid leukemia ex myelodysplastic syndrome patients (16 months, P=0.10) and significantly inferior to the de novo acute myeloid leukemia patients (24 months, P=0.007), as shown in Figure 2b. Among the 6 acute myeloid leukemia ex chronic myelomonocytic leukemia cases with NPM1 mutation detected in either the preceding chronic myelomonocytic leukemia or the acute myeloid leukemia, 2 patients died of disease at 2 and 12 months and 4 patients were alive at 1, 11, 14, and 43 months after acute myeloid leukemia diagnosis.
(a) Overall survival of acute myeloid leukemia ex chronic myelomonocytic leukemia (n=36), ex myelodysplastic syndrome (n=34), and de novo (n=179) patients from the time of acute myeloid leukemia diagnosis. Overall survival of the acute myeloid leukemia ex chronic myelomonocytic leukemia patients (median 6 months) is significantly shorter than the de novo acute myeloid leukemia patients (median 17 months, P=0.002), but similar to the acute myeloid leukemia ex myelodysplastic syndrome patients (median 9 months, P=0.37; log-rank test). (b) Overall survival of acute myeloid leukemia patients in the UK Medical Research Council intermediate karyotype risk group, comparing ex chronic myelomonocytic leukemia (n=22), ex myelodysplastic syndrome (n=18), and de novo (n=96) patients. Overall survival of the intermediate-risk acute myeloid leukemia ex chronic myelomonocytic leukemia patients (median 13 months) is significantly shorter than the intermediate-risk de novo acute myeloid leukemia patients (median 24 months, P=0.007) and borderline significantly shorter than the intermediate-risk acute myeloid leukemia ex myelodysplastic syndrome patients (median 16 months, P=0.10; log-rank test).
Univariate survival analysis
On univariate analysis of the acute myeloid leukemia ex chronic myelomonocytic leukemia group, increasing age tended to be associated with inferior overall survival (P=0.06), while prior chronic myelomonocytic leukemia-2, adverse acute myeloid leukemia UK Medical Research Council karyotype score, and prior treatment of chronic myelomonocytic leukemia (supportive care or hydroxyurea vs other treatments) did not affect overall survival. In contrast, in the acute myeloid leukemia ex myelodysplastic syndrome group, older age (P=0.02), an adverse acute myeloid leukemia UK Medical Research Council karyotype score (P=0.015), and any prior treatment of myelodysplastic syndrome other than supportive care (P=0.007) were all associated with inferior overall survival.
Multivariate survival analysis
The entire acute myeloid leukemia patient group (n=251 patients) was included in a multivariate model in order to identify factors independently associated with overall survival (Table 4). Variables included were: patient age at acute myeloid leukemia diagnosis; UK Medical Research Council karyotype score; acute myeloid leukemia group (ex chronic myelomonocytic leukemia, ex myelodysplastic syndrome, or de novo); and prior treatment of antecedent myelodysplastic syndrome or chronic myelomonocytic leukemia other than supportive care or hydroxyurea. Increasing age and increasing karyotype risk score were significantly associated with an inferior overall survival (P<0.0001). Neither a history of antecedent myelodysplastic syndrome or chronic myelomonocytic leukemia nor a history of prior therapy for myelodysplastic syndrome or chronic myelomonocytic leukemia was independently associated with overall survival. Similar results were obtained when treatment history was excluded from the multivariate analysis (data not shown).
Clinicopathologic Features and Time-to-Progression Analysis of Chronic Myelomonocytic Leukemia
We performed a time-to-progression analysis on 57 chronic myelomonocytic leukemia patients, including the 38 patients who progressed to acute myeloid leukemia and the 19 chronic myelomonocytic leukemia patients who did not progress to acute myeloid leukemia. Progression to acute myeloid leukemia was associated with bone marrow cellularity of ≥90% (P=0.003), increasing IPSS-R risk grouping as a continuous variable (P=0.004), a CCSS karyotype score of intermediate, poor, or very poor (P=0.006), a Such et al23 chronic myelomonocytic leukemia high-risk karyotype score (P=0.005), WBC >13 000/μl (P=0.03), and younger age as a continuous variable (P=0.04). Of note, the IPSS-R score was more strongly associated with acute myeloid leukemia progression than the original IPSS score (P=0.004 vs P=0.01, respectively). There was no significant difference in time to progression based on bone marrow reticulin grade, bone marrow monocyte or blast percentages, or the chronic myelomonocytic leukemia subcategory (chronic myelomonocytic leukemia-1 vs chronic myelomonocytic leukemia-2). There was a suggestion of an association between bone marrow blasts plus monocytes ≥10% and progression to acute myeloid leukemia, but this did not reach statistical significance (P=0.055). Statistical significance of the presence of an NPM1 mutation could not be evaluated because of the small number of cases with mutation, but both patients with NPM1 mutation identified at the time of chronic myelomonocytic leukemia diagnosis progressed to acute myeloid leukemia within 3 months.
Discussion
We analyzed the clinicopathologic and genetic features in a series of patients with acute myeloid leukemia arising after a prior diagnosis of chronic myelomonocytic leukemia. The acute myeloid leukemia ex chronic myelomonocytic leukemia cases retained ‘proliferative’ features, with a significantly higher peripheral WBC, absolute neutrophil count, absolute monocyte count, and higher bone marrow cellularity and monocyte percentage than either de novo acute myeloid leukemia or acute myeloid leukemia ex myelodysplastic syndrome cases. Most acute myeloid leukemia ex chronic myelomonocytic leukemia cases displayed monocytic differentiation (FAB M4 or M5), indicating preserved monocytic features from the antecedent chronic myelomonocytic leukemia.25 Similar to the acute myeloid leukemia ex myelodysplastic syndrome patients, acute myeloid leukemia ex chronic myelomonocytic leukemia patients tended to be older and were more often male than de novo acute myeloid leukemia patients. The acute myeloid leukemia ex chronic myelomonocytic leukemia patients were less likely to receive aggressive treatment (induction chemotherapy or induction chemotherapy and bone marrow transplant) for their acute myeloid leukemia than the de novo acute myeloid leukemia patients, which may be a reflection of either their older age or history of prior chronic myelomonocytic leukemia therapy.
Most of the acute myeloid leukemia ex chronic myelomonocytic leukemia cases fell into the intermediate UK Medical Research Council karyotype risk group, with fewer in the adverse risk group, and none in the favorable risk group. NPM1 and FLT3 mutations were identified in a significant subset of the acute myeloid leukemia ex chronic myelomonocytic leukemia cases (17 and 18%, respectively). Although more frequent than the reported 8% rate in acute myeloid leukemia with myelodysplasia-related changes,13 the NPM1 mutation rate in our series was similar to the 13% reported for a series of transformed myelodysplastic syndrome cases that included patients with chronic myelomonocytic leukemia.26 All the tested karyotypes of the acute myeloid leukemia ex chronic myelomonocytic leukemia cases with NPM1 mutation were normal, similar to strong association of NPM1 mutation with normal karyotype in de novo acute myeloid leukemia. In two of our NPM1-mutated acute myeloid leukemia ex chronic myelomonocytic leukemia cases, the NPM1 mutation was not detected in the antecedent chronic myelomonocytic leukemia; although NPM1 is generally thought to be a ‘founder mutation’ in acute myeloid leukemia,27 our data suggest that this mutation may occur as a secondary event in chronic myelomonocytic leukemia transformation to acute myeloid leukemia. Acquisition of NPM1 mutation has also been shown to occur during transformation of some myelodysplastic syndrome cases to acute myeloid leukemia.26
Although NPM1 and FLT3 mutations are two of the most common molecular abnormalities in acute myeloid leukemia, particularly within the cytogenetically normal group, they are relatively uncommon in myeloproliferative neoplasms and myelodysplastic syndromes.14, 28, 29, 30 Studies that evaluated chronic myelomonocytic leukemia specifically found the NPM1 mutation in 5–13% of patients.14 In one study, all three NPM1-mutated chronic myelomonocytic leukemia patients had rapid progression to overt acute myeloid leukemia, whereas in the other study, one of the two patients progressed to acute myeloid leukemia. It would be difficult to entirely exclude that these cases were actually early-stage acute myeloid leukemia (akin to CBF-rearranged acute myeloid leukemia with <20% blasts); however, the current WHO guidelines do not permit classification as such. Our findings are in line with these prior studies: we found the NPM1 mutation in 2/44 (5%) of our chronic myelomonocytic leukemia cases, and both progressed rapidly to overt acute myeloid leukemia (in 0.5 and 3 months) as compared with the entire chronic myelomonocytic leukemia cohort. Other somatic gene mutations that have been reported to occur in chronic myelomonocytic leukemia include TET2 (36–51%), ASXL1 (27–49%), SRSF2 (36%), CBL (10–22%), RUNX1 (9–21%), NRAS (4–22%), KRAS (7–12%), and JAK2 (2–10%);31, 32, 33 two of these, ASXL1 and RUNX1, have also been found with some frequency in a cohort of acute myeloid leukemia with myelodysplasia-related change cases (35 and 17%, respectively).13
In our series, we found that the acute myeloid leukemia ex chronic myelomonocytic leukemia patients had a similar survival to the acute myeloid leukemia ex myelodysplastic syndrome patients and a significantly worse survival than the de novo acute myeloid leukemia patients. This difference in survival from the de novo acute myeloid leukemia patients persisted even after restricting the analysis to the subset of patients with UK Medical Research Council acute myeloid leukemia intermediate cytogenetic risk group, and the survival of acute myeloid leukemia ex chronic myelomonocytic leukemia was similarly poor for both intermediate and adverse UK Medical Research Council cytogenetic risk groups. These observations suggest that adverse somatic gene mutations that characterize chronic myelomonocytic leukemia (such as ASXL1 and TET2) and are presumably retained in subsequent acute myeloid leukemia may exert a negative prognostic effect, despite a frequently normal or other intermediate-risk karyotype. On multivariate analysis of the series of acute myeloid leukemia cases as a whole, increasing age and high UK Medical Research Council karyotype risk group were significantly associated with a worse prognosis independent of the diagnosis of or treatment for an antecedent hematologic neoplasm (chronic myelomonocytic leukemia or myelodysplastic syndrome); both favorable karyotype risk (only present within de novo acute myeloid leukemia) and intermediate karyotype risk (present within all groups) acute myeloid leukemia patients exhibited superior overall survival to those with high-risk karyotype. Within the acute myeloid leukemia ex chronic myelomonocytic leukemia patient group, univariate survival analysis also identified increasing age as being associated with worse survival, similar to published results for de novo acute myeloid leukemia.34, 35 Although there were too few cases to attempt formal statistical comparisons regarding the NPM1 or FLT3 gene mutations, survival of the acute myeloid leukemia ex chronic myelomonocytic leukemia cases containing the NPM1 mutation were generally favorable compared with the other patients. This finding, as well as the rapid progression of NPM1-mutated chronic myelomonocytic leukemia patients to acute myeloid leukemia in our series and in another published series,24 suggests that cases diagnosed as chronic myelomonocytic leukemia with an NPM1 mutation may actually represent early de novo acute myeloid leukemia exhibiting dysplastic features and monocytosis mimicking chronic myelomonocytic leukemia.36 It may thus be useful to screen chronic myelomonocytic leukemia cases for NPM1 mutation and carefully follow cases with NPM1 mutation for possible rapid transformation to ‘bona fide’ acute myeloid leukemia.
We also examined clinicopathologic and genetic features that were associated with progression from chronic myelomonocytic leukemia to acute myeloid leukemia. Studies looking at the outcome of chronic myelomonocytic leukemia patients, performed at various time points along the evolution of the current chronic myelomonocytic leukemia classification scheme, have variably focused on survival or progression to acute myeloid leukemia as end points;8, 9, 37, 38, 39 in our study, we used time-to-progression analysis to evaluate factors associated with acute myeloid leukemia evolution. Although a potential bias in the follow-up of chronic myelomonocytic leukemia cases with aggressive features (such as increased WBC or blasts) could have influenced the exact time that acute myeloid leukemia progression was detected, review of the medical records revealed similar follow-up intervals among the chronic myelomonocytic leukemia patients who progressed vs those who did not progress (data not shown). We found that a peripheral WBC of >13 000/μl (which had been used to distinguish chronic myelomonocytic leukemia-myelodysplastic and chronic myelomonocytic leukemia-myeloproliferative subtypes in the 1994 FAB classification system2), as well as bone marrow cellularity of ≥90%, were associated with more rapid progression to acute myeloid leukemia. Contradictory to some of the previously published literature, there was no significant difference in time to progression based on the chronic myelomonocytic leukemia subcategory that focuses on blast count alone (chronic myelomonocytic leukemia-1 or chronic myelomonocytic leukemia-2), although we found that adding bone marrow monocytes to blast percentage was borderline associated with progression to acute myeloid leukemia. Our data suggest that considering ‘hyperproliferative features’ such as very high bone marrow cellularity, WBC, and monocyte count in addition to the blast count may improve the ability to predict rapid acute myeloid leukemia progression in chronic myelomonocytic leukemia patients. These hyperproliferative features may reflect specific underlying genetic aberrations: in one recent series, the ASXL1 mutation in chronic myelomonocytic leukemia was associated with both a high WBC and more rapid progression to acute myeloid leukemia,40 and RAS mutations in chronic myelomonocytic leukemia have also been associated with higher WBC and poorer survival.41 The results of our study suggest that the presence of an NPM1 mutation in chronic myelomonocytic leukemia also appears to be a harbinger of rapid progression to acute myeloid leukemia. We performed cytogenetic risk grouping of the chronic myelomonocytic leukemia cases based on the new CCSS for primary myelodysplastic syndrome (in which 5.3% of the test set patients had a diagnosis of chronic myelomonocytic leukemia). On univariate time-to-progression analysis, an intermediate or unfavorable (poor or very poor) CCSS karyotype risk score was strongly associated with more rapid progression to acute myeloid leukemia. Using the newly published IPSS-R scoring system that incorporates the CCSS karyotype score as well as a depth of cytopenias and bone marrow blast count, we found that a higher IPSS-R score predicted more rapid progression to acute myeloid leukemia in our cohort of chronic myelomonocytic leukemia patients.
In summary, our findings support the placement of acute myeloid leukemia ex chronic myelomonocytic leukemia in the category of acute myeloid leukemia with myelodysplasia-related changes in the 2008 WHO classification10 because of its significantly worse prognosis compared with de novo acute myeloid leukemia and frequent dysplastic morphology, particularly in megakaryocytes. This inferior prognosis of acute myeloid leukemia ex chronic myelomonocytic leukemia is not explained merely by a higher incidence of adverse karyotype compared with de novo acute myeloid leukemia, but rather appears to be related to an older patient population (treated less intensively when compared with the younger de novo acute myeloid leukemia cohort), absence of the favorable risk acute myeloid leukemia translocations inv(16), t(8;21), and t(15;17), and relatively poor outcome of acute myeloid leukemia ex chronic myelomonocytic leukemia patients with intermediate-risk karyotype. The inferior prognosis of these patients with intermediate-risk karyotype may reflect an adverse mutational risk profile, as chronic myelomonocytic leukemia has high incidence of TET2 and ASXL1 mutations, which have been recently shown to have an unfavorable prognostic impact on intermediate-risk karyotype acute myeloid leukemia.42 The outcome and classification of the subset of acute myeloid leukemia ex chronic myelomonocytic leukemia patients with mutated NPM1, which confers a favorable prognosis in de novo acute myeloid leukemia, warrants further study.
References
Bennett JM, Catovsky D, Daniel MT et al. Proposals for the classification of the myelodysplastic syndromes. Br J Haematol 1982;51:189–199.
Bennett JM, Catovsky D, Daniel MT et al. The chronic myeloid leukaemias: guidelines for distinguishing chronic granulocytic, atypical chronic myeloid, and chronic myelomonocytic leukaemia. Proposals by the French-American-British Cooperative Leukaemia Group. Br J Haematol 1994;87:746–754.
Vardiman JW, Pierre RV, Bain B et al. Chronic myelomonocytic leukaemia In: Jaffe ES, Harris NL, Stein H, Vardiman JW, (eds) World Health Organization Classification of Tumours. International Agency for Research on Cancer (IARC): Lyons, France, 2001, pp 49–52.
Beran M, Wen S, Shen Y et al. Prognostic factors and risk assessment in chronic myelomonocytic leukemia: validation study of the M.D. Anderson Prognostic Scoring System. Leuk Lymphoma 2007;48:1150–1160.
Onida F, Kantarjian HM, Smith TL et al. Prognostic factors and scoring systems in chronic myelomonocytic leukemia: a retrospective analysis of 213 patients. Blood 2002;99:840–849.
Germing U, Strupp C, Knipp S et al. Chronic myelomonocytic leukemia in the light of the WHO proposals. Haematologica 2007;92:974–977.
Orazi A, Bennett J, Germing U et al. Chronic myelomonocytic leukemia In: Swerdlow SH, Campo E, Harris NL, et al (eds) WHO Classification of Tumor of Haematopoietic and Lymphoid Tissues 4th edn International Agency for Research on Cancer: Lyon, 2008, pp 38–39.
Tefferi A, Hoagland HC, Therneau TM et al. Chronic myelomonocytic leukemia: natural history and prognostic determinants. Mayo Clin Proc 1989;64:1246–1254.
Fenaux P, Beuscart R, Lai JL et al. Prognostic factors in adult chronic myelomonocytic leukemia: an analysis of 107 cases. J Clin Oncol 1988;6:1417–1424.
Arber DA, Brunning RD, Orazi A et al. Acute myeloid leukaemia with myelodysplasia-related changes In: Swerdlow SH, Campo E, Harris NL, et al (eds). WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues 4th edn International Agency for Research on Cancer (IARC): Lyon, France, 2008, pp 124–126.
Arber DA, Stein AS, Carter NH et al. Prognostic impact of acute myeloid leukemia classification. Importance of detection of recurring cytogenetic abnormalities and multilineage dysplasia on survival. Am J Clin Pathol 2003;119:672–680.
Miesner M, Haferlach C, Bacher U et al. Multilineage dysplasia (MLD) in acute myeloid leukemia (AML) correlates with MDS-related cytogenetic abnormalities and a prior history of MDS or MDS/MPN but has no independent prognostic relevance: a comparison of 408 cases classified as “AML not otherwise specified” (AML-NOS) or “AML with myelodysplasia-related changes” (AML-MRC). Blood 2010;116:2742–2751.
Devillier R, Gelsi-Boyer V, Brecqueville M et al. Acute myeloid leukemia with myelodysplasia-related changes are characterized by a specific molecular pattern with high frequency of ASXL1 mutations. Am J Hematol 2012;87:659–662.
Bains A, Luthra R, Medeiros LJ et al. FLT3 and NPM1 mutations in myelodysplastic syndromes: frequency and potential value for predicting progression to acute myeloid leukemia. Am J Clin Pathol 2011;135:62–69.
Weinberg OK, Seetharam M, Ren L et al. Clinical characterization of acute myeloid leukemia with myelodysplasia-related changes as defined by the 2008 WHO classification system. Blood 2009;113:1906–1908.
Kosmider O, Delabesse E, de Mas VM et al. TET2 mutations in secondary acute myeloid leukemias: a French retrospective study. Haematologica 2011;96:1059–1063.
Greenberg PL, Tuechler H, Schanz J et al. Revised international prognostic scoring system for myelodysplastic syndromes. Blood 2012;120:2454–2465.
Manoharan A, Horsley R, Pitney WR . The reticulin content of bone marrow in acute leukaemia in adults. Br J Haematol 1979;43:185–190.
Brunning RD, Orazi A, Germing U et al. Myelodysplastic syndromes/neoplasms, overview In: SS H, Campo E, Harris NL, et al (eds) WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. International Agency for Research on Cancer (IARC): Lyon, France, 2008, pp 88–93.
Grimwade D, Hills RK, Moorman AV et al. Refinement of cytogenetic classification in acute myeloid leukemia: determination of prognostic significance of rare recurring chromosomal abnormalities among 5876 younger adult patients treated in the United Kingdom Medical Research Council trials. Blood 2010;116:354–365.
Greenberg P, Cox C, LeBeau MM et al. International scoring system for evaluating prognosis in myelodysplastic syndromes. Blood 1997;89:2079–2088.
Schanz J, Tuchler H, Sole F et al. New comprehensive cytogenetic scoring system for primary myelodysplastic syndromes (MDS) and oligoblastic acute myeloid leukemia after MDS derived from an international database merge. J Clin Oncol 2012;30:820–829.
Such E, Cervera J, Costa D et al. Cytogenetic risk stratification in chronic myelomonocytic leukemia. Haematologica 2011;96:375–383.
Falini B, Martelli MP, Bolli N et al. Immunohistochemistry predicts nucleophosmin (NPM) mutations in acute myeloid leukemia. Blood 2006;108:1999–2005.
Kern W, Bacher U, Haferlach C et al. Acute monoblastic/monocytic leukemia and chronic myelomonocytic leukemia share common immunophenotypic features but differ in the extent of aberrantly expressed antigens and amount of granulocytic cells. Leuk Lymphoma 2011;52:92–100.
Schnittger S, Bacher U, Haferlach C et al. Characterization of NPM1-mutated AML with a history of myelodysplastic syndromes or myeloproliferative neoplasms. Leukemia 2011;25:615–621.
Falini B, Martelli MP, Bolli N et al. Acute myeloid leukemia with mutated nucleophosmin (NPM1): is it a distinct entity? Blood 2011;117:1109–1120.
Caudill JS, Sternberg AJ, Li CY et al. C-terminal nucleophosmin mutations are uncommon in chronic myeloid disorders. Br J Haematol 2006;133:638–641.
Zhang Y, Zhang M, Yang L et al. NPM1 mutations in myelodysplastic syndromes and acute myeloid leukemia with normal karyotype. Leuk Res 2007;31:109–111.
Shiseki M, Kitagawa Y, Wang YH et al. Lack of nucleophosmin mutation in patients with myelodysplastic syndrome and acute myeloid leukemia with chromosome 5 abnormalities. Leuk Lymphoma 2007;48:2141–2144.
Muramatsu H, Makishima H, Maciejewski JP . Chronic myelomonocytic leukemia and atypical chronic myeloid leukemia: novel pathogenetic lesions. Semin Oncol 2012;39:67–73.
Jankowska AM, Makishima H, Tiu RV et al. Mutational spectrum analysis of chronic myelomonocytic leukemia includes genes associated with epigenetic regulation: UTX, EZH2, and DNMT3A. Blood 2011;118:3932–3941.
Kohlmann A, Grossmann V, Klein HU et al. Next-generation sequencing technology reveals a characteristic pattern of molecular mutations in 72.8% of chronic myelomonocytic leukemia by detecting frequent alterations in TET2, CBL, RAS, and RUNX1. J Clin Oncol 2010;28:3858–3865.
Appelbaum FR, Gundacker H, Head DR et al. Age and acute myeloid leukemia. Blood 2006;107:3481–3485.
Ganzel C, Rowe JM . Prognostic factors in adult acute leukemia. Hematol Oncol Clin North Am 2011;25:1163–1187.
Falini B, Nicoletti I, Martelli MF et al. Acute myeloid leukemia carrying cytoplasmic/mutated nucleophosmin (NPMc+ AML): biologic and clinical features. Blood 2007;109:874–885.
Worsley A, Oscier DG, Stevens J et al. Prognostic features of chronic myelomonocytic leukaemia: a modified Bournemouth score gives the best prediction of survival. Br J Haematol 1988;68:17–21.
Storniolo AM, Moloney WC, Rosenthal DS et al. Chronic myelomonocytic leukemia. Leukemia 1990;4:766–770.
Breccia M, Latagliata R, Mengarelli A et al. Prognostic factors in myelodysplastic and myeloproliferative types of chronic myelomonocytic leukemia: a retrospective analysis of 83 patients from a single institution. Haematologica 2004;89:866–868.
Gelsi-Boyer V, Trouplin V, Roquain J et al. ASXL1 mutation is associated with poor prognosis and acute transformation in chronic myelomonocytic leukaemia. Br J Haematol 2010;151:365–375.
Ricci C, Fermo E, Corti S et al. RAS mutations contribute to evolution of chronic myelomonocytic leukemia to the proliferative variant. Clin Cancer Res 2010;16:2246–2256.
Patel JP, Gonen M, Figueroa ME et al. Prognostic relevance of integrated genetic profiling in acute myeloid leukemia. N Engl J Med 2012;366:1079–1089.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Courville, E., Wu, Y., Kourda, J. et al. Clinicopathologic analysis of acute myeloid leukemia arising from chronic myelomonocytic leukemia. Mod Pathol 26, 751–761 (2013). https://doi.org/10.1038/modpathol.2012.218
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/modpathol.2012.218
Keywords
This article is cited by
-
Management and Outcomes of Blast Transformed Chronic Myelomonocytic Leukemia
Current Hematologic Malignancy Reports (2021)
-
Progression, transformation, and unusual manifestations of myelodysplastic syndromes and myelodysplastic-myeloproliferative neoplasms: lessons learned from the XIV European Bone Marrow Working Group Course 2019
Annals of Hematology (2021)
-
Update on the pathologic diagnosis of chronic myelomonocytic leukemia
Modern Pathology (2019)
-
Serotonin receptor type 1B constitutes a therapeutic target for MDS and CMML
Scientific Reports (2018)
-
So-called “blast phase” of chronic myelomonocytic leukemia: a plea for uniform terminology
Leukemia (2018)