Abstract
The molecular pathogenesis of gastroenteropancreatic neuroendocrine tumors is largely unknown. We hypothesize that gastroenteropancreatic neuroendocrine tumors are heterogeneous with regard to these signaling pathways and these differences could have a significant impact on the outcome of clinical trials. We selected 120 well-differentiated neuroendocrine tumors including tumors originating in pancreas (n=74), ileum (n=31), and rectum (n=15). Immunohistochemistry was performed on tissue microarrays using the following antibodies: NOTCH1, HES1, HEY1, pIGF1R, and FGF2. Gene profiling study was performed by using human genome U133A 2.0 array and data were analyzed. The gene profiling results were selectively confirmed by using quantitative reverse-transcription PCR. Initial immunohistochemical analysis showed NOTCH1 was uniformly expressed in rectal neuroendocrine tumors (100%), a subset of pancreatic neuroendocrine tumors (34%), and negative in ileal neuroendocrine tumors. Similarly, a downstream target of NOTCH1, HES1 was preferentially expressed in rectal neuroendocrine tumors (64%), a subset of pancreatic neuroendocrine tumors (10%), and uniformly negative in ileal neuroendocrine tumors. Messenger RNAs for NOTCH1, HES1, and HEY1 were 2.32-, 2.44-, and 2.39-folds, respectively, higher in rectal neuroendocrine tumors as compared with ileal neuroendocrine tumors. Global gene expression profiling showed 95 genes were differentially expressed in small intestinal vs rectal neuroendocrine tumors, with changes as high as 50-fold. These genes were concentrated in several signal transduction pathways including cancer endocrine pathway and cell growth/proliferation pathway. The differential expression of selected genes including ISL LIM homeobox 1, cathepsin B, glucagon, and tryptophan hydroxylase 1 were confirmed by qPCR and immunohistochemistry. Our results confirm the heterogeneity in signaling pathways of gastroenteropancreatic neuroendocrine tumors. NOTCH1 inhibitors are unlikely to provide benefit in ileal neuroendocrine tumors; conversely, their efficacy in rectal neuroendocrine tumors needs further study. Further analysis of signaling pathways is critical for designing clinical trials in gastroenteropancreatic neuroendocrine tumors.
Similar content being viewed by others
Main
Neuroendocrine tumors are a diverse group of neoplasms that have traditionally been divided according to their embryonic origins into foregut (esophagus, stomach, pancreas, and duodenum), midgut (superior mesenteric artery distribution including ileum, appendix, and ascending colon), and hindgut (inferior mesenteric artery distribution including descending colon and rectum).1, 2 Similar to the epithelial neoplasms of the gastrointestinal tract, there is significant morphological and immunohistochemical diversity across neuroendocrine tumors of the tubular gut. However, unlike epithelial neoplasms of the gastrointestinal tract little is known about the molecular heterogeneity or genetic underpinnings of neuroendocrine tumors of the tubular gut. One well-documented genetic alteration in neuroendocrine tumors of the gastrointestinal tract is inactivation of MEN1 gene. The genetic alterations are also location-specific events: MEN1 somatic mutations occur most frequently in foregut neuroendocrine tumors, 18q LOH almost exclusively in midgut and hindgut neuroendocrine tumors.3
Understanding the molecular pathology of neuroendocrine tumors is critical for developing medical therapy. The current medical therapies, including somatostatin analogs, interferon-α, and cytotoxic chemotherapy, have been somewhat effective in alleviating symptoms, but have done little in preventing neuroendocrine tumor progression.1, 4 There are more than 100 ongoing clinical trials on neuroendocrine tumor, targeting phosphatidylinositol 3′ kinase, mitogen-activated protein kinase, mTOR, and VEGFA pathways.5 However, there has been no systematic effort to explore the signaling transduction pathways in human neuroendocrine tumors, and even less is known about the variability in these signaling pathways across the gastrointestinal tract. For example, in spite of the success of sunitinib in patients with advanced neuroendocrine tumors, relatively little is known about the expression pattern and activation status of growth factor receptors such as VEGFA in human neuroendocrine tumors. Understanding these signaling pathways is particularly significant given the potential heterogeneity of these neoplasms.6
Although immunohistochemical and PCR-based approaches are sufficient to assess expression of selected proteins/genes, genome-wide microarray profiling provides a more comprehensive approach to the understanding of tumor signal transduction. Fresh-frozen tissue is a common source of high quality messenger RNA for microarray studies. Messenger RNA from formalin-fixed and paraffin-embedded tissue blocks was traditionally regarded as unsuitable for gene microarray applications because of degradation and chemical modification. This traditional view has recently been challenged, and several groups have reported quantitative gene expression and microarray profiling data from formalin-fixed and paraffin-embedded tissue blocks.7, 8
The goal of this study was to explore signal transduction pathways and gene expression profile in three common gastrointestinal neuroendocrine tumors: rectum, small intestine and pancreas, and assess the heterogeneity in the signaling pathways across these tumors. Identification of novel signaling pathway may uncover potential druggable targets. Identifying differences in signal transduction pathways among these neuroendocrine tumors provides important baseline data for evidence-based clinical trials for neuroendocrine tumors.
Materials and methods
Tissue Samples
All study cases were selected from the archives of the Department of Pathology, Massachusetts General Hospital between 2006 and 2009. All cases were routine diagnostic surgical specimens including biopsies and resections. Tissues were fixed in 4% buffered formalin, processed using standard procedures, and embedded in paraffin. All specimens were reviewed by an experienced gastrointestinal pathologist, and reclassification (if necessary) was based on criteria suggested by the WHO.6, 9
TMA Construction
In all, 120 consecutive selected specimens were included in the study, comprising 31 cases of neuroendocrine tumors from small intestine, 15 cases from rectum, and 74 pancreatic neuroendocrine tumors. Histological slides for each specimen included in the tissue microarray were reviewed, and the corresponding region on the paraffin block identified. Cores of 2 mm diameter were chosen for this study. The mean number of cores examined per sample was 1.6 (range 1–3).
Human Paraffin-Embedded Neuroendocrine Tumor mRNA Expression Profiling
RNA was extracted from formalin-fixed paraffin-embedded tissue blocks after macrodissection. Briefly, ‘donor’ region of neuroendocrine tumors, composed at least 95% of tumor cells, were identified under the microscope, and these were marked up on each glass slide and tissue block. Tissue cores of 1 mm diameter were extracted from each marked region by using tissue arrayer.
The Human Genome U133A 2.0 arrays (Affymetrix, Santa Clara, CA, USA) were used for microarray analysis. Human neuroendocrine tumors\RNA was purified using QIAzol lysis reagent and RNeasy mini kit (QIAGEN, Valencia, CA, USA). A total of 11 samples were analyzed, including 5 samples from small intestine and 6 samples from rectum. Each RNA sample was prepared individually and hybridized to a different microarray chip. Sample labeling and processing were performed according to the manufacture’s protocol. Double-stranded complementary DNA was synthesized, and biotinylated cRNA was prepared and then hybridized to the arrays. The arrays were then stained with streptavidin R-phycoerythrin (Molecular Probes, Eugene, OR, USA) using GeneChip Fluidics Station 400, and scanned with a GeneChip scanner 3000 (Affymetrix). The microarray data were analyzed using Array Studio (Omics Soft, Morrisville, NC, USA). The data was normalized with Robust Multiarray Averaging (RMA) method. Principal component analysis was used as quality control to detect outlier(s) among the samples. One small intestinal sample was detected as an outlier and was eliminated from further analysis. The normalized data of the two tissues were then analyzed for differential gene expression using one-way ANOVA. Changes in gene expression were represented as fold change, only the genes with statistically significant changes (P<0.01) in the one-way ANOVA test were reported.
Quantitative Real-Time PCR
RNA was extracted and purified as shown above. First-strand cDNA was generated from 1.0 μg of purified total RNA by using SuperScript II with Olgo (dt)12–18 primer (Invitrogen, Carlsbad, CA, USA). Gene-specific primers (attachment 1) were designed by using ABI Primer Express 2.0 software (Applied Biosystems, Foster City, CA, USA). PCR was run with 5 μl cDNA template in 20 μl reactions in triplicate on an ABI SDS 7900 using the ABI power SYBR Green Master Mix (Applied Biosystems), the concentration of each gene-specific primers was 0.2 μM. Gene-specific amplification was revealed by a single peak in the ABI Dissociation Curve software (Applied Biosystems). Average threshold cycle (Ct) values for cyclophilin (PPIA, used as house keeping gene) were used to normalize average Ct values of the interested genes. These values were used to calculate the average Ct values between groups, and the relative quantity (power of –ΔΔCt) was used to calculate fold change between each group. Statistical analyses were performed by paired Student’s t-test and P<0.05 was considered significantly different.
Histology and Immunohistochemical Stains
The histology of all tissue microarrays and tissue blocks were examined after hematoxylin and eosin stain on 5 μm thick sections. The antibodies and their titers employed in this study were listed in Table 1. Immunohistochemical stains were performed by using Ventana autostainer according to the manufacturer’s protocol. Slides were coded and positive/negative controls were included. Following routine deparaffinization, sections underwent heat-induced epitope retrieval using the Cell Conditioning 1 protocol at pH 8.5 (Ventana Medical System) and primary antibodies were applied for 60 min at room temperature. The slides were then incubated with either anti-Rabbit, anti-mouse, anti-goat, or anti-sheep-biotinalyted IgG separately and Strept-avidin-HRP, then developed in DAB substrate and counterstained by using hematoxylin. The slides were reviewed by two pathologists, and tumors were graded either as positive or negative.
Results
Immunohistochemistry
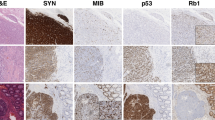
Expression of current neuroendocrine tumor therapeutic targets5 and related signaling factors were evaluated in our tissue microarrays. NOTCH1 protein level was significantly upregulated in rectal neuroendocrine tumors. High level expression of NOTCH1 was observed in all rectal neuroendocrine tumors, but small intestinal neuroendocrine tumors lacked NOTCH1 expression. NOTCH1 was expressed in 34% pancreatic neuroendocrine tumors (Figure 1 and Table 2). IGFR1 was expressed in 92.3% rectal neuroendocrine tumors but only in 30% pancreatic neuroendocrine tumors, whereas none of the intestinal neuroendocrine tumors stained for this marker. FGF2 was observed in 75% rectal neuroendocrine tumors, in 44% pancreatic neuroendocrine tumors, but only in 9.1% intestinal neuroendocrine tumors. The expression of the following proteins showed no differences across three types of neuroendocrine tumors evaluated: VEGFA, KDR, PDGFRB, NFKB, AKT, c-Kit/stem cell factor, β-catenin, cadherin, estrogen receptor, and androgen receptor. A summary of the immunohistochemical results is listed in Table 2.
To support the functional activity of NOTCH1 in rectal neuroendocrine tumors, expression of a downstream NOTCH1 effector, HES1, was also evaluated in our tissue microarrays. HES1 expression was observed in 65% of rectal neuroendocrine tumors, 10% pancreatic neuroendocrine tumors, and 0% small intestinal neuroendocrine tumors (Table 2). An attempt to evaluated HEY1 expression failed. The upregulation of NOTCH1 pathways in rectal neuroendocrine tumors was further confirmed by RT-PCR (see below).
Gene Expression Profiling of Neuroendocrine Tumors
Four small intestinal and five rectal neuroendocrine tumors formalin-fixed and paraffin-embedded tissue blocks were employed for the microarray study (Supplementary Table 1). Out of the 16 000 tested genes, 95 genes showed altered expression (P<0.01 and ≥2-fold) between the two types of neuroendocrine tumors. Among the differentially expressed genes, tryptophan hydroxylase 1 (TPH1), chromogranin A, and Dopa decarboxylase genes were higher in small intestinal neuroendocrine tumors. On the other hand, pancreatic polypeptide (PPY), glucagon, insulin LIM homeobox 1 (ISL1), cathepsin B (CTSB), and CD99 genes were differentially expressed in rectal neuroendocrine tumors. The changes of NOTCH1 and HEY1 genes detected by the profiling were <2-fold. Our genechip data showed no statistical difference in the mRNA levels of IGFR1 and FGF2 between rectal and intestinal neuroendocrine tumors.
The altered genes in our study primarily fell into six signal transduction pathways, as illustrated in Supplementary Figure 1 (Ingenuity Systems, Redwood City, CA, USA). The most relevant pathway is the cancer endocrine pathway. Out of the 35 tested genes in this pathway, 24 were altered in our study. The interaction of these gene targets is illustrated in Supplementary Figure 2.
Quantitative Real-Time PCR
Real-time PCR was performed to validate the differences between small intestinal and rectal neuroendocrine tumors identified on the microarray platform. We selected five genes to confirm the microarray and tissue microarray results, including insulin LIM homeobox 1, cathepsin B, NOTCH1, and HEY1 (Table 3). Specific primers were designed to cover a gene fragment of about 100 nt, and the gene products were sequenced to confirm their identity. As shown in Figure 2, the quantitative reverse-transcription PCR (qRT-PCR) data correlate almost perfectly with the microarray data (R2=0.97). However, the difference between the two tumor sites (small bowel and rectal) were wider on qRT-PCR than on the microarray data. In the microarray, cathepsin B and insulin LIM homeobox 1genes were upregulated by 5- and 18-fold in rectal neuroendocrine tumors, respectively. In contrast, the qRT-PCR indicated these two genes were 25- and 125-fold higher in rectal neuroendocrine tumors.
NOTCH1 mRNA detected by RT-PCR was 2.32-fold higher in rectal neuroendocrine tumors than in small intestinal neuroendocrine tumors; and HEY1 gene expression was 2.44-fold higher in rectal neuroendocrine tumors. In addition, qRT-PCR revealed that HES1 gene expression was 2.39-fold higher in rectal neuroendocrine tumors.
Validation of Expression Profiling Data by Immunohistochemistry
To further assess the validity of microarray and PCR results protein expression of four targets, ISL LIM homeobox 1, cathepsin B, glucagon, and tryptophan hydroxylase 1, were further evaluated on tissue microarrays (Table 4). As shown in Figure 3a and b, expression of tryptophan hydroxylase 1 protein was high in small intestinal neuroendocrine tumors. On the other hand, glucagon, cathepsin B, and ISL LIM homeobox 1 proteins were high in rectal neuroendocrine tumors (Figure 3a and b, Table 4). The protein expression pattern correlates well with the gene microarray study.
Discussion
Employing a variety of platforms, we show that there is broad site-dependent variability in the protein and gene expression patterns in gastrointestinal neuroendocrine tumors. Immunohistochemically, NOTCH1 is uniformly expressed in rectal neuroendocrine tumors (100%), in a subset of pancreatic neuroendocrine tumors (34%), and was negative in ileal neuroendocrine tumors. Similarly, a downstream target of NOTCH1, HES1, is preferentially expressed in rectal neuroendocrine tumors (64%), in a subset of pancreatic neuroendocrine tumors (10%), and uniformly negative in ileal neuroendocrine tumors. Messenger RNA for NOTCH1, HES1, and HEY1 were 2.32-, 2.44-, and 2.39-folds, respectively, higher in rectal neuroendocrine tumors as compared with ileal neuroendocrine tumors. Global gene expression profiling showed 96 genes were differentially expressed between ileal and rectal neuroendocrine tumors, with changes as high as 50-fold. These genes were concentrated in several signal transduction pathways including cancer endocrine pathway and cell growth/proliferation pathway. The differential expression of selected genes including ISL LIM homeobox 1, cathepsin B, glucagon, and tryptophan hydroxylase 1 were confirmed by immunohistochemistry and qPCR. Our results confirm the heterogeneity in signaling pathways of gastrointestinal neuroendocrine tumors. Furthermore, although rectal and small intestinal neuroendocrine tumors were relatively homogenous, pancreatic endocrine tumors were heterogeneous for the expression of these proteins. Some of the validated proteins and genes, including ISL LIM homeobox 1 and cathepsin B, may potentially provide clues to the origin of a metastatic neuroendocrine tumors.
Nakakura et al10 is among the first to show the importance of Notch signaling pathway in the gastrointestinal neuroendocrine tumor growth and differentiation. Forced overexpression of activated NOTCH1 in a neuroendocrine tumors cell line reduced serotonin concentration and repressed tryptophan hydroxylase 1 expression.10, 11 The oncogenic role of NOTCH1 is established in several tumors, including T-cell lymphoma, non-small cell lung cancer, cervical cancer, and pancreatic adenocarcinoma, to name a few.11 Conversely, a tumor suppressor role of NOTCH1 has been proposed for pulmonary neuroendocrine tumor, pancreatic endocrine neoplasms, and gastrointestinal neuroendocrine tumors.11, 12 A high level NOTCH1 expression in Merkel cell tumor does not support a tumor suppressor role of NOTCH1 in this cutaneous neuroendocrine carcinoma.13 In this context, it is important to note that Notch signaling inhibits endocrine differentiation by repressing expression of bHLH transcription factors. Forced activation of Notch during pancreatic development prevents the endocrine differentiation.14 Lack of Notch effector HES1 expression in mice results in excessive endocrine differentiation in lung, stomach, and intestine.15 Our study reveals the preferential expression of NOTCH1 and its downstream effectors, HES1 and HEY1, in rectal neuroendocrine tumors and a subset of pancreatic neuroendocrine tumors. This study correlated well with previous study by Nakakura et al12 and may explain the high level expression of tryptophan hydroxylase 1 in ileal neuroendocrine tumors, and conversely the low levels of tryptophan hydroxylase 1 in rectal neuroendocrine tumors. Although we have no proof to support such a hypothesis, one could speculate on a relationship between NOTCH1 and chromogranin A. The high level NOTCH1 expression in rectal neuroendocrine tumors, together with the relatively better 5-year survival of rectal neuroendocrine tumors, may suggest a tumor suppressor role of NOTCH1 in gastrointestinal neuroendocrine tumors.6, 16
Medical treatment of neuroendocrine tumors includes somatostatin analogs, traditional chemotherapy, and peptide receptor radionuclide therapy. Innovative therapeutics are, however, emerging and may prove especially useful treatment for metastatic neuroendocrine tumors.5, 17 Our results suggest several therapeutic targets including NOTCH1, IGFR1, and cathepsin B.18, 19 Our study also suggests that the expression of certain targets, such as PDGFRB and androgen receptor, may not be worth pursuing.
Microarray gene expression profiling from fresh or frozen tissue has proved to be a powerful tool in dissecting molecular pathogenesis and identifying of therapeutic targets.12 However, microarray analysis using formalin-fixed and paraffin-embedded tissue is a new and evolving field.8, 20 The technical challenges of formalin-fixed and paraffin-embedded tissue profiling are significant, formalin fixation leads to degradation and chemical modification of the RNA with extensive cross-links between various biomolecules in the tissue, thus, hindering analysis.21 However, significant improvements have been made in RNA isolation and amplification—a series of reports have suggested that gene profiling on formalin-fixed and paraffin-embedded tissue material is now feasible. One study7 concluded that although the sensitivity of formalin-fixed and paraffin-embedded tissue profiling was low (∼50%), when compared with frozen tissue, the specificity (95%) and positive predictive value (92%) were high. In another study,18 high correlation (R2 between 0.82 and 0.89) of profiling data between frozen and formalin-fixed and paraffin-embedded tissue tissue was identified in tumors from the lung and colon. Our formalin-fixed and paraffin-embedded tissue profiling study identified 96 differentially expressed genes between small intestinal and rectal neuroendocrine tumors. Some of these differences were anticipated and are consistent with existing literature in this field: ∼50-fold increase of pancreatic polypeptide and ∼40-fold increase of glucagon in rectal neuroendocrine tumors compared with ileal neuroendocrine tumors, as well as ∼30-fold increase of tryptophan hydroxylase expression in ileal neuroendocrine tumors. However, other significant differences emerged in this study—many of which have not been documented in the literature (Supplementary Table 1). To further validate the profiling data, we selected seven genes and performed RT-PCR using total RNA. Our results showed an almost perfect correlation (R2=0.97) between the microarray and qRT-PCR data. Together with other reports,8, 20 our results illustrated that formalin-fixed and paraffin-embedded tissue material is valuable in evaluating the molecular pathology of neuroendocrine tumors—a particularly useful resource when fresh-frozen tissue is scarce. However, our data also suggest limited sensitivity of the formalin-fixed and paraffin-embedded tissue profiling approach. Although both the formalin-fixed and paraffin-embedded tissue profiling and RT-PCR showed cathepsin B and ISL LIM homeobox 1 upregulation, the changes revealed by RT-PCR is about 10-fold higher in both targets.
To conclude, our results confirm the heterogeneity in signaling pathways of gastrointestinal neuroendocrine tumors. We demonstrate active NOTCH1 signaling in rectal neuroendocrine tumors; conversely, ileal neuroendocrine tumors lack such activity. NOTCH acts as an oncogene in some cancers but as a tumor suppressor in others—its role in solid tumors seems to be highly dependent on the cellular context. On the basis of these data NOTCH1 inhibitors are unlikely to provide benefit in ileal neuroendocrine tumors. Instead compounds that activate NOTCH1 may be effective in ileal neuroendocrine tumor. An ongoing trial is examining the role of Resveratrol (a compound that activates NOTCH1) in neuroendocrine tumors of the gastrointestinal tract. (http://clinicaltrials.gov/ct2/show/NCT01476592). In a recent study valproic acid (a NOTCH activator) treatment of gastrointestinal and pulmonary neuroendocrine tumor cell lines resulted in a dose-dependent inhibition of cancer cell growth and also increased levels of full-length NOTCH1 and the active NOTCH1 intracellular domain 9.22 This study underscores the need for a site-based critical assessment of signaling pathways, when designing clinical trials for gastrointestinal neuroendocrine tumors.
References
Poncet G, Faucheron JL, Walter T . Recent trends in the treatment of well-differentiated endocrine carcinoma of the small bowel. World J Gastroenterol 2010;16:1696–1706.
Landry CS, Brock G, Scoggins CR et al. A proposed staging system for rectal carcinoid tumors based on an analysis of 4701 patients. Surgery 2008;144:460–466.
Toumpanakis CG, Caplin ME . Molecular genetics of gastroenteropancreatic neuroendocrine tumors. Am J Gastroenterol 2008;103:729–732.
Kulke MH, Mayer RJ . Carcinoid tumors. N Engl J Med 1999;340:858–868.
Meeting proceedings of neuroendocrine tumors clinical trials planning meeting. 20–21 November 2009; Boston, MA.
Klöppel G, Perren A, Heitz PU . The gastroenteropancreatic neuroendocrine cell system and its tumors: the WHO classification. Ann N Y Acad Sci 2004;1014:13–27.
Linton KM, Hey Y, Saunders E et al. Acquisition of biologically relevant gene expression data by Affymetrix microarray analysis of archival formalin-fixed paraffin-embedded tumors. Br J Cancer 2008;98:1403–1414.
Roberts L, Bowers J, Sensinger K et al. Identification of methods for use of formalin-fixed, paraffin-embedded tissue samples in RNA expression profiling. Genomics 2009;94:341–348.
Modlin IM, Oberg K, Chung DC et al. Gastroenteropancreatic neuroendocrine tumours. Lancet Oncol 2008;9:61–72.
Nakakura EK, Sriuranpong VR, Kunnimalaiyaan M et al. Regulation of neuroendocrine differentiation in gastrointestinal carcinoid tumor cells by notch signaling. J Clin Endocrinol Metab 2005;90:4350–4356.
Kunnimalaiyaan M, Chen H . Tumor suppressor role of NOTCH1 signaling in neuroendocrine tumors. Oncologist 2007;12:535–542.
Missiaglia E, Dalai I, Barbi S et al. Pancreatic endocrine tumors: expression profiling evidences a role for AKT-mTOR pathway. J Clin Oncol 2010;28:245–255.
Panelos J, Batistatou A, Paglierani M et al. Expression of NOTCH1 and alteration of the E-cadherin/beta-catenin cell adhesion complex are observed in primary cutaneous neuroendocrine carcinoma (Merkel cell carcinoma). Mod Pathol 2009;22:959–968.
Murtaugh LC, Stanger BZ, Kwan KM et al. Notch signaling controls multiple steps of pancreatic differentiation. Proc Natl Acad Sci USA 2003;100:14920–14925.
Jensen J, Pedersen EE, Galante P et al. Control of endodermal endocrine development by Hes-1. Nat Genet 2000;24:36–44.
Anthony LB, Strosberg JR, Klimstra DS et alNorth American Neuroendocrine Tumor Society. The neuroendocrine tumor consensus guidelines for the diagnosis and management of gastrointestinal neuroendocrine tumors (neuroendocrine tumors): well-differentiated neuroendocrine tumors of the distal colon and rectum. Pancreas 2010;39:767–774.
Scherubl H . Local therapy of rectal carcinoids: a matter of debate. Arch Surg 2009;144:196.
Teigen K, McKinney JA, Haavik J et al. Selectivity and affinity determinants for ligand binding to the aromatic amino acid hydroxylases. Curr Med Chem 2007;14:455–467.
Chung CH, Pohlmann PR, Rothenberg ML et al. Insulin-like growth factor-1 receptor inhibitor, AMG-479, in cetuximab-refractory head and neck squamous cell carcinoma. Head Neck 2011;33:1804–1808.
Saleh A, Zain RB, Hussaini H et al. Transcriptional profiling of oral squamous cell carcinoma using formalin-fixed paraffin-embedded samples. Oral Oncol 2010;46:379–386.
Srinivasan M, Sedmak D, Jewell S . Effect of fixatives and tissue processing on the content and integrity of nucleic acids. Am J Pathol 2002;161:1961–1971.
Greenblatt DY, Vaccaro AM, Jaskula-Sztul R et al. Valproic acid activates NOTCH1 signaling and regulates the neuroendocrine phenotype in carcinoid cancer cells. Oncologist 2007;12:942–951.
Tomoo K . Development of cathepsin inhibitors and structure-based design of cathepsin B-specific inhibitor. Curr Top Med Chem 2010;10:696–707.
Brennan C, Momota H, Hambardzumyan D et al. Glioblastoma subclasses can be defined by activity among signal transduction pathways and associated genomic alterations. PLoS One 2009;4:e7752.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on Modern Pathology website
Rights and permissions
About this article
Cite this article
Wang, H., Chen, Y., Fernandez-Del Castillo, C. et al. Heterogeneity in signaling pathways of gastroenteropancreatic neuroendocrine tumors: a critical look at notch signaling pathway. Mod Pathol 26, 139–147 (2013). https://doi.org/10.1038/modpathol.2012.143
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/modpathol.2012.143
Keywords
This article is cited by
-
From microbiota toward gastro-enteropancreatic neuroendocrine neoplasms: Are we on the highway to hell?
Reviews in Endocrine and Metabolic Disorders (2021)
-
The oncogenic effects of HES1 on salivary adenoid cystic carcinoma cell growth and metastasis
BMC Cancer (2018)
-
Insulinoma-associated protein 1 (INSM1) is a useful marker for pancreatic neuroendocrine tumor
Medical Molecular Morphology (2018)
-
Clinical characteristics, risk factors and outcomes of asymptomatic rectal neuroendocrine tumors
Surgical Endoscopy (2017)
-
Guidelines for biomarker testing in gastroenteropancreatic neuroendocrine neoplasms: a national consensus of the Spanish Society of Pathology and the Spanish Society of Medical Oncology
Clinical and Translational Oncology (2014)