Abstract
Multilocular cystic renal cell carcinoma is a rare renal cell carcinoma with an excellent prognosis. To clarify the relationship with typical clear cell renal cell carcinoma, we evaluated 15 cases of multilocular cystic renal cell carcinomas diagnosed according to the 2004 WHO classification. Von Hippel Lindau (VHL) gene mutations were determined by whole genome amplification and direct sequencing. Carbonic anhydrase 9 (CAIX), a hypoxia-inducible factor (HIF) target, paired box gene 2 (PAX2), cyclin-dependent kinase inhibitor p27 and glycogen synthase kinase 3-β (GSK3β) were immunohistochemically evaluated as members of the VHL protein (pVHL)- and phosphatase and tensin homolog (PTEN)-controlled pathways. VHL mutations were identified in 3 of 12 (25%) tumors. Inactivated GSK3β, decreased PTEN expression and PAX2 positivity were observed in the vast majority of the multilocular cystic renal cell carcinomas. Strong nuclear staining of p27 was seen in 14 of 15 cases. Compared with multilocular cystic renal cell carcinomas, expression frequencies of PAX2, p-GSK3β, PTEN and CAIX were similar in a set of low-grade, early-stage clear cell renal cell carcinomas, whereas only 30% had strong p27 positivity. These results are consistent with the hypothesis that multilocular cystic renal cell carcinomas are related at the molecular level with clear cell renal cell carcinomas. Maintenance of a strong subcellular p27 expression in all multilocular cystic renal cell carcinomas analyzed may in part explain the excellent prognosis of these tumor patients.
Similar content being viewed by others
Main
Multilocular cystic renal cell carcinoma is a rare renal carcinoma type showing characteristic cystic growth. It constitutes <2% of all renal cell carcinomas. According to the 2004 World Health Organization (WHO) classification of tumors, it is defined by morphologic criteria as a specific tumor type. Patients diagnosed with this tumor have an excellent outcome, as there are no reports of this tumor ever recurring or metastasizing to date.1 Multilocular cystic renal cell carcinoma is potentially a subtype of clear cell renal cell carcinoma because the cysts are lined by single layers of clear cell epithelial cells. Recently, Halat et al2 reported a high frequency of chromosomal loss of 3p similar to the one observed in clear cell renal cell carcinoma. As a chromosome 3p deletion is considered the hallmark of cytogenetic abnormality found in clear cell renal cell carcinoma, it was concluded that multilocular cystic renal cell carcinoma is a subtype of this tumor entity. However, chromosomal loss of the 3p arm has also been reported in some non-clear cell renal cell carcinomas and is a frequent finding in cancers of the lung, cervix, head and neck, esophagus and pancreas (http://www.progenetix.net/progenetix/). Importantly, tumor development in sporadic clear cell renal cancer is linked with inactivation of the von Hippel Lindau (VHL) gene by chromosome 3p loss in combination with mutation, or hypermethylation of the second VHL allele on the 3p arm. In multilocular cystic renal cell carcinomas, no data exist about the presence of VHL mutations, representing an additional hallmark of clear cell renal cell carcinoma.
Alteration of pathways regulated by the von Hippel-Lindau protein (pVHL) due to mutation of its coding gene is the main characteristic molecular feature of all hereditary and most sporadic clear cell renal cell carcinomas. pVHL is a multifunctional protein that acts as an adapter for different molecular and subcellular complexes.3 Recently, we comprehensively analyzed pVHL- and phosphatase and tensin homolog (PTEN)-controlled pathways in >800 clear cell renal cell carcinoma patients.4 It was shown that the expression status of p27, PTEN, carbonic anhydrase 9 (CAIX) and phosphorylated ribosomal protein S6 is crucial for the biological behavior of sporadic clear cell renal cell carcinoma.
In this study, we sequenced VHL and investigated the expression status of PTEN, phosphorylated (inactivated) glycogen synthase kinase 3-β (GSK3β) and paired box gene 2 (PAX2), all of which represent critical components for cyst formation5, 6, 7, 8 as well as of the tumor suppressor p27 and the hypoxia-inducible factor (HIF)-target CAIX in 15 multilocular cystic renal cell carcinomas. The results were compared with a control group of early-stage, low-grade clear cell renal cell carcinomas.
Materials and methods
Tumor Specimens
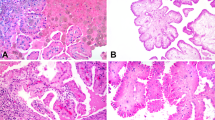
HE sections of 15 formalin-fixed and paraffin-embedded multilocular cystic renal cell carcinomas were reviewed by two pathologists (EC and HM). Areas with nodular aggregates containing clear cells in the cyst walls are shown in Figure 1a. All tumors were diagnosed with the Fuhrman differentiation grade 1. The control group consisted of 40 pT1 and pT2 clear cell renal cell carcinomas with low Fuhrman differentiation grade (1 and 2) from a recently published study.4
HE section of a multilocular cystic renal cell carcinoma showing cysts and thin septae (a, middle panel). Septae lined by cuboid clear cells without a solid component (a, upper and lower panels). HE section of the same multilocular cystic renal cell carcinoma after punching (b, middle panel). Circular punched areas are highlighted by square boxes (overview in middle panel) and magnifications of two punched areas are shown (b, upper and lower panels). Sections were scanned using the NanoZoomer (zoom factor: × 40; Hamamatsu Photonics K.K., Hamamatsu City, Japan).
DNA Extraction and Whole Genome Amplification
For DNA extraction the marked HE slides were used to punch three tissue cylinders (diameter 0.6 mm) from each of the 15 paraffin blocks. Punched areas of one multilocular cystic renal cell carcinoma case are shown in Figure 1b. DNA was extracted using the DNeasy Tissue Kit (Qiagen, Hilden, Germany). Whole genome amplification of the yielded DNA was performed using the WGA kit (Sigma, St Louis, MO, USA). The quality of the amplified DNA was determined on a 1.5% agarose gel. DNA quantity was measured with the Nanodrop (Thermo Fisher Scientific, Waltham, MA, USA).
VHL Sequencing
PCR and sequencing of VHL were performed as previously described,9 with slight modifications. Only one PCR step with 40 cycles was carried out per exon. Exons 2 and 3 were amplified using forward primer 5′-ACCGGTGTGGCTCTTTAACA-3′, reverse primer 5′-TCCTGTACTTACCACAACAACCTT-3′ and forward primer 5′-GAGACCCTAGTCTGTCACTGAGG-3′, reverse primer 5′-TCATCAGTACCATCAAAAGCTGA-3′, respectively. The obtained sequences were compared with the NCBI sequence AF010238 using NCBI's Blast 2 Sequences. VHL point mutations were validated by a second separate whole genome amplification and sequence analysis.
Immunohistochemistry
Paraffin sections (2.5 μm) were transferred to glass slides and treated using Ventana Benchmark XT (Tuscon, AZ, USA) or BOND-MAX (Leica Microsystems, Wetzlar, Germany) automated systems. Immunostainings were performed using mouse anti-CAIX (ab15086, 1:300 dilution; Abcam, Cambridge, UK), rabbit anti-phospho-Ser9 GSK3β (no. 9323, clone 5B3, 1:100 dilution; Cell Signaling Technology, Danvers, MA, USA), rabbit anti-p27 (sc528, 1:100 dilution; Santa Cruz Biotechnology, Santa Cruz, CA, USA), mouse anti-PTEN (M3627, 1:200 dilution; DAKO A/S, Glostrup, Denmark) and rabbit anti-PAX2 (71-6000, 1:100 dilution; Zymed Laboratories, South San Francisco, CA, USA). Nuclear (PAX2), nuclear and cytoplasmic (PTEN, p27), cytoplasmic (phospho-Ser 9 GSK3β) and membranous expression (CAIX) were defined positive if at least 5% of tumor cells showed weak (+1) or strong (+2) staining.
Statistical Analysis
Contingency table analysis and χ2 tests were used for the analysis of statistical differences between protein expression patterns in multilocular cystic renal cell carcinomas and low-grade, early-stage clear cell renal cell carcinomas.
Results
Whole Genome Amplification
The yield of DNA after extraction from paraffin-embedded tissue varied between 0.22 and 2.18 μg. The starting amount of DNA for whole genome amplification was 75 ng. Figure 2 shows an example of four whole genome amplified samples on an agarose gel for quality control. The amounts of DNA after amplification and purification ranged between 0.87 and 8.68 μg, which is a 12- to 121-fold level of amplification.
VHL Mutation Analysis
Although the starting amount of DNA for the amplification was the same for all 15 samples, the quality and quantity of the PCR products of three samples were not sufficient for VHL sequence analysis. VHL mutations were identified in 3 of 12 (25%) analyzable multilocular cystic renal cell carcinoma patients. Two patients had missense mutations in exon 1 (c.194C>T/ p.Ser65Leu) and in exon 2 (c.461C>T/p.Pro154Leu), respectively. Two frameshift mutations were identified in a third patient, one in exon 1 (c.201_203delCTCinsG/p.Asn67LysfsX64) and the second in exon 3 (c.641_642delGA/p.XfsX).
Immunohistochemistry
Strong expression of CAIX and p27 was seen in the vast majority of the multilocular cystic renal cell carcinomas. In all, 80% of the tumors were PAX2 positive. In comparison with normal tubular epithelial cells in which PTEN was highly expressed and p-GSK3β was absent (data not shown), decreased PTEN expression and inactivated GSK3β was observed in 14 of 15 cases. The results of the immunohistochemical analysis are listed in Table 1. Examples of four multilocular cystic renal cell carcinomas with strong nuclear PAX2, cytoplasmic p-GSK3β, combined nuclear/cytoplasmic p27 and membranous CAIX positivity are shown in Figure 3. Besides p27 (nuclear and cytoplasmic) and PTEN (nuclear), no significant differences in CAIX, p-GSK3β, PAX2 and cytoplasmic PTEN expression frequencies were observed between multilocular cystic renal cell carcinomas and early-stage, low-grade clear cell renal cell carcinoma. The results are listed in Table 2.
Discussion
Our data demonstrate the existence of VHL mutations in multilocular cystic renal cell carcinoma. Furthermore, the downregulation of PTEN, the inactivation of GSK3β and the upregulation of PAX2 were identified as indicators for the dysregulation of the pVHL and PTEN-controlled pathways. With the exception of p27, the expression frequencies of the remaining proteins were comparable between multilocular cystic renal cell carcinoma and clear cell renal cell carcinoma and confirm the close relationship.
Cystic lesions are found not only in multilocular cystic renal cell carcinoma but also in cystic nephroma, extensively cystic clear cell renal cell carcinoma, tubulocystic carcinoma, mixed epithelial and stroma tumor,10 clear cell papillary renal cell carcinoma11 and tubulocystic carcinoma.12 Cystic nephroma, mixed epithelial and stroma tumor as well as clear cell papillary renal cell carcinoma also have clear cells lining the septum. Given the broad range of tumors with clear cells in the kidney, the histogenetic relationship of multilocular clear cell renal cell carcinoma to one of the cystic renal tumor entities with clear cells requires molecular analyses. Little is known about the molecular genetic characteristics of multilocular cystic renal cell carcinoma. Halat et al2 recently identified chromosome 3p deletions in 14 of 19 (74%) multilocular cystic renal cell carcinomas. Loss of the chromosome 3p is a consistent finding in clear cell renal cell carcinoma and is considered one of the primary events in the pathogenesis of this tumor type.1 Inactivation of VHL is mostly because of mutation of the second VHL allele, located at chromosome 3p25, and is specific for sporadic clear cell renal cell carcinoma and for tumors associated with the von Hippel Lindau syndrome. VHL mutations have never been described in multilocular cystic renal cell carcinoma.
In this study, we performed direct sequencing of VHL for mutations on samples from multilocular cystic renal cell carcinoma, and VHL mutations were identified in three cases. We used a whole genome amplification step, because the yield of DNA extracted from the tumor cells in the cyst walls was frequently insufficient. The frequency of validated VHL mutations was 25%, which is somewhat below the mutation rates reported in clear cell renal cell carcinoma (reviewed in Gossage et al13). The two missense mutations, c.194C>T and c.461C>T, have been described in both sporadic and hereditary clear cell renal cell carcinomas.14, 15 Possible effects of the first point mutation on pVHL binding partners are unknown; however, on the second mutation, Hansen et al16 showed that exon 2 mutants may exhibit an impaired degradation of HIF-α as these pVHL mutants display defects in substrate binding. The two frameshift mutations found in one patient were validated by two separate whole genome amplifications. In several studies, clear cell renal cell carcinoma cases with two or more VHL mutations have been described.17, 18, 19 It is conceivable that in this tumor both parental VHL alleles were affected, whereas chromosome 3p was retained.
Amplifying whole genome DNA extracted from only a few thousand micro-dissected and formalin-fixed tumor cells is accompanied by the high risk of producing artificial mutations.20, 21 As a consequence, several whole tissue sections would be required for a micro-dissection to yield sufficient amounts of DNA to reliably perform a PCR. To decrease the risk of PCR artifacts and to avoid wasting of valuable multilocular cystic renal cell carcinoma tissue, we punched directly in the cyst walls using a hollow needle with a diameter of 0.6 mm. We cannot exclude that some of the tissue biopsies were contaminated with non-tumor cells, thus preventing a higher VHL mutation rate more similar to that observed in clear cell renal cell carcinoma. This is supported by our observation that 13 of 15 multilocular cystic renal cell carcinoma showed a strong expression of CAIX, a HIF target that is considerably upregulated because of loss of the pVHL function.22 The existence of VHL mutations is a clear indicator that multilocular cystic renal cell carcinoma is a subtype of clear cell renal cell carcinoma.
Loss of the pVHL function has an important role in the initiation of clear cell renal cell carcinoma. However, additional signaling pathways are required for further tumor progression. It is unclear why multilocular cystic renal cell carcinoma is associated with such an excellent prognosis. Recently, novel mechanisms of tumor initiation have been identified in clear cell renal cell carcinoma because of the hereditary von Hippel-Lindau syndrome. A model for a cyst-dependent and a cyst-independent pathway was proposed23 and extended to sporadic clear cell renal cell carcinoma. The disruption of the maintenance of the primary cilia in the renal tubules due to combined inactivation of pVHL and GSK3β is an important step for the cyst-dependent progression pathway.7 Recent findings strongly suggest that additional deregulating factors are required to develop cysts. In mice, combined deletion of the two tumor-suppressor genes, VHL and PTEN, caused benign genital tract tumors with regions of cyst adenoma that were histologically identical to lesions found in VHL patients.24 pVHL and PTEN also cooperatively suppress kidney cyst formation.5 Combined conditional inactivation of VHL and PTEN in the mouse kidney elicits cyst formation. Interestingly, in two-thirds of our multilocular cystic renal cell carcinomas, nuclear staining of PTEN was absent, whereas only 17.5% of the low-grade, early-stage clear cell renal cell carcinomas were PTEN negative. These data suggest the dysregulation of pVHL, GSK3β and, probably, nuclear import of PTEN as common molecular mechanisms that are important to produce the phenotype characteristic for multilocular cystic renal cell carcinoma.
It is important to note that the risk of metastasis is proportional to the number of carcinoma cells present. Multilocular cystic renal cell carcinomas contain rather small numbers of carcinoma cells (most of their volumes are spaces filled with fluid), whereas clear cell renal cell carcinomas are composed of significantly more tumor cells.
On the molecular level, we have previously demonstrated that the expression status of p27, PTEN, CAIX, phospho-S6 as well as PAX2 characterizes a subgroup of sporadic clear cell renal cell carcinoma with a better prognosis.4 In contrast to multilocular cystic renal cell carcinoma patients, who have an extremely good prognosis after surgery,25 a substantial fraction of clear cell renal cell carcinoma patients die because of metastases. This implies that pathways, including those mediated by EGFR,26, 27 with the tumor-progressing potential of clear cell renal cell carcinoma are kept under control in multilocular cystic renal cell carcinoma. One of these pathways appears to be regulated by p27, as almost all multilocular cystic renal cell carcinomas expressed this tumor suppressor in both the nucleus and the cytoplasm. The dominant presence of p27 in multilocular cystic renal cell carcinoma indicates a negatively controlling role on pathways that are important in different malignant tumors in which the antagonists of p27, such as AKT,28 SKP229 and SRC,30 are highly active. The abundant expression of p27 in multilocular cystic renal cell carcinoma may partly explain the high rate of surgical cure of these patients. A recent study that demonstrated nuclear and cytoplasmic p27 expression primarily in low-grade and early-stage clear cell renal cell carcinomas supports this hypothesis.4
It was shown that clear cell renal cell carcinomas with strong expression of CAIX have a favorable outcome.4, 31 Furthermore, Patard et al32 was able to divide patients with clear cell renal cell carcinoma into three prognostic groups. Patients with a poor prognosis had no VHL mutations and low CAIX levels, whereas, those with either a VHL mutation or high CAIX expression had an intermediate prognosis. Finally, patients who had VHL mutations and a high CAIX expression had a good prognosis. It is therefore tempting to speculate that the latter group of clear cell renal cell carcinoma is molecularly closely related to multilocular cystic renal cell carcinoma.
Nuclear positivity of PAX2 was seen in the majority of the multilocular cystic renal cell carcinoma specimens. PAX2 is a member of the PAX gene family of developmental transcription factors and is known to be involved in cyst formation. Patients with polycystic kidney disease and juvenile cystic kidneys abundantly express PAX2 in cystic and hyperproliferative epithelial cells.6, 8 In addition, it was shown that a reduced PAX2 gene dosage significantly inhibited renal cyst growth in mice.33 In renal cancer cell lines, loss of the pVHL function leads to a strong reactivation of the PAX2 gene34 that is normally silenced in the mature epithelium of the glomeruli, the proximal and distal tubules of the kidney. Furthermore, in clear cell renal cell carcinoma, PAX2 expression mainly occurs in low-grade tumors and significantly correlates with a better outcome.4, 34 These data suggest that PAX2 is a pVHL-dependent factor that contributes to cyst formation in multilocular cystic renal cell carcinoma.
In summary, the results of our study suggest that cyst formation in multilocular cystic renal cell carcinoma is caused by molecular mechanisms similar or even identical to those seen in VHL-associated clear cell renal cell carcinoma. As PAX2, p27 as well as pVHL-dependent CAIX expression correlate with low differentiation grade and/or a favorable outcome in clear cell renal cell carcinoma, these proteins may represent potential markers of the benign behavior of multilocular cystic renal cell carcinoma. The combined deregulation of the pVHL/HIF axis and PTEN alone appear to be important for multilocular cystic renal cell carcinoma development but not sufficient to drive metastatic processes. The abundance of p27 is a characteristic feature of multilocular cystic renal cell carcinoma and potentially implies that this protein acts as a metastasis suppressor. Our observations, together with the recently published finding of frequent chromosome 3p deletions,2 further support the concept that multilocular cystic renal cell carcinoma is a subtype of clear cell renal cell carcinoma. The term ‘multilocular cystic clear cell renal cell carcinoma’ would better reflect its relationship with clear cell renal cell carcinoma.
References
Eble JN, Sauter G, Epstein JI, et al. Pathology and Genetics. Tumours of the Urinary System and Male Genital Organs. IARC Press: Lyon, 2004.
Halat S, Eble JN, Grignon DJ et al. Multilocular cystic renal cell carcinoma is a subtype of clear cell renal cell carcinoma. Mod Pathol 2010;23:931–936.
Frew IJ, Krek W . pVHL: a multipurpose adaptor protein. Sci Signal 2008;1:pe30.
Dahinden C, Ingold B, Wild P, et al. Mining tissue microarray data to uncover combinations of biomarker expression patterns that improve intermediate staging and grading of clear cell renal cell cancer. Clin Cancer Res 2010;16:88–98.
Frew IJ, Thoma CR, Georgiev S, et al. pVHL and PTEN tumour suppressor proteins cooperatively suppress kidney cyst formation. EMBOJ 2008;27:1747–1757.
Stayner C, Iglesias DM, Goodyer PR, et al. Pax2 gene dosage influences cystogenesis in autosomal dominant polycystic kidney disease. Hum Mol Genet 2006;15:3520–3528.
Thoma CR, Frew IJ, Hoerner CR, et al. pVHL and GSK3beta are components of a primary cilium-maintenance signalling network. Nat Cell Biol 2007;9:588–595.
Winyard PJ, Risdon RA, Sams VR, et al. The PAX2 transcription factor is expressed in cystic and hyperproliferative dysplastic epithelia in human kidney malformations. J Clin Invest 1996;98:451–459.
Schraml P, Frew IJ, Thoma CR, et al. Sporadic clear cell renal cell carcinoma but not the papillary type is characterized by severely reduced frequency of primary cilia. Mod Pathol 2009;22:31–36.
Moch H . Cystic renal tumors: new entities and novel concepts. Adv Anat Pathol 2010;17:209–214.
Gobbo S, Eble JN, Grignon DJ, et al. Clear cell papillary renal cell carcinoma: a distinct histopathologic and molecular genetic entity. Am J Surg Pathol 2008;32:1239–1245.
Amin MB, MacLennan GT, Gupta R, et al. Tubulocystic carcinoma of the kidney: clinicopathologic analysis of 31 cases of a distinctive rare subtype of renal cell carcinoma. Am J Surg Pathol 2009;33:384–392.
Gossage L, Eisen T . Alterations in VHL as potential biomarkers in renal-cell carcinoma. Nat Rev Clin Oncol 2010;7:277–288.
Gnarra JR, Tory K, Weng Y, et al. Mutations of the VHL tumour suppressor gene in renal carcinoma. Nat Genet 1994;7:85–90.
Whaley JM, Naglich J, Gelbert L, et al. Germ-line mutations in the von Hippel-Lindau tumor-suppressor gene are similar to somatic von Hippel-Lindau aberrations in sporadic renal cell carcinoma. Am J Hum Genet 1994;55:1092–1102.
Hansen WJ, Ohh M, Moslehi J, et al. Diverse effects of mutations in exon II of the von Hippel-Lindau (VHL) tumor suppressor gene on the interaction of pVHL with the cytosolic chaperonin and pVHL-dependent ubiquitin ligase activity. Mol Cell Biol 2002;22:1947–1960.
Banks RE, Tirukonda P, Taylor C, et al. Genetic and epigenetic analysis of von Hippel-Lindau (VHL) gene alterations and relationship with clinical variables in sporadic renal cancer. Cancer Res 2006;66:2000–2011.
Schraml P, Struckmann K, Hatz F, et al. VHL mutations and their correlation with tumour cell proliferation, microvessel density, and patient prognosis in clear cell renal cell carcinoma. J Pathol 2002;196:186–193.
van Houwelingen KP, van Dijk BA, Hulsbergen-van de Kaa CA, et al. Prevalence of von Hippel-Lindau gene mutations in sporadic renal cell carcinoma: results from The Netherlands cohort study. BMC Cancer 2005;5:57.
Akbari M, Hansen MD, Halgunset J, et al. Low copy number DNA template can render polymerase chain reaction error prone in a sequence-dependent manner. J Mol Diagn 2005;7:36–39.
Sieben NL, ter Haar NT, Cornelisse CJ, et al. PCR artifacts in LOH and MSI analysis of microdissected tumor cells. Hum Pathol 2000;31:1414–1419.
Mandriota SJ, Turner KJ, Davies DR, et al. HIF activation identifies early lesions in VHL kidneys: evidence for site-specific tumor suppressor function in the nephron. Cancer Cell 2002;1:459–468.
Thoma CR, Frew IJ, Krek W . The VHL tumor suppressor: riding tandem with GSK3beta in primary cilium maintenance. Cell Cycle 2007;6:1809–1813.
Frew IJ, Minola A, Georgiev S, et al. Combined VHLH and PTEN mutation causes genital tract cystadenoma and squamous metaplasia. Mol Cell Biol 2008;28:4536–4548.
Suzigan S, Lopez-Beltran A, Montironi R, et al. Multilocular cystic renal cell carcinoma: a report of 45 cases of a kidney tumor of low malignant potential. Am J Clin Pathol 2006;125:217–222.
Moch H, Sauter G, Buchholz N, et al. Epidermal growth factor receptor expression is associated with rapid tumor cell proliferation in renal cell carcinoma. Hum Pathol 1997;28:1255–1259.
Moch H, Sauter G, Gasser T, et al. EGF-r gene copy number gains detected by fluorescence in situ hybridization in renal cell carcinoma. J Pathol 1998;184:424–429.
Liang J, Slingerland JM . Multiple roles of the PI3K/PKB (Akt) pathway in cell cycle progression. Cell Cycle 2003;2:339–345.
Carrano AC, Eytan E, Hershko A, et al. SKP2 is required for ubiquitin-mediated degradation of the CDK inhibitor p27. Nat Cell Biol 1999;1:193–199.
Chu I, Sun J, Arnaout A, et al. p27 phosphorylation by Src regulates inhibition of cyclin E-Cdk2. Cell 2007;128:281–294.
Leibovich BC, Sheinin Y, Lohse CM, et al. Carbonic anhydrase IX is not an independent predictor of outcome for patients with clear cell renal cell carcinoma. J Clin Oncol 2007;25:4757–4764.
Patard JJ, Fergelot P, Karakiewicz PI, et al. Low CAIX expression and absence of VHL gene mutation are associated with tumor aggressiveness and poor survival of clear cell renal cell carcinoma. Int J Cancer 2008;123:395–400.
Ostrom L, Tang MJ, Gruss P, et al. Reduced Pax2 gene dosage increases apoptosis and slows the progression of renal cystic disease. Dev Biol 2000;219:250–258.
Luu VD, Boysen G, Struckmann K, et al. Loss of VHL and hypoxia provokes PAX2 up-regulation in clear cell renal cell carcinoma. Clin Cancer Res 2009;15:3297–3304.
Acknowledgements
The project was supported by the Swiss National Science Foundation to HM (3238BO-103145) and the Zurich Cancer League to HM.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Teichman, A., Compérat, E., Behnke, S. et al. VHL mutations and dysregulation of pVHL- and PTEN-controlled pathways in multilocular cystic renal cell carcinoma. Mod Pathol 24, 571–578 (2011). https://doi.org/10.1038/modpathol.2010.222
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/modpathol.2010.222
Keywords
This article is cited by
-
Clear cell renal cell carcinoma with cystic component similar to multilocular cystic renal neoplasm of low malignant potential: a rare pattern of cyst-dependent progression from multilocular cystic renal neoplasm of low malignant potential
Diagnostic Pathology (2023)
-
Comparison of survival between unilocular cystic and purely solid renal cell carcinoma
Scientific Reports (2022)
-
Predominantly cystic clear cell renal cell carcinoma and multilocular cystic renal neoplasm of low malignant potential form a low-grade spectrum
Virchows Archiv (2018)
-
Unlike in clear cell renal cell carcinoma, KRAS is not mutated in multilocular cystic clear cell renal cell neoplasm of low potential
Virchows Archiv (2015)
-
Clear cell papillary renal cell carcinoma: differential diagnosis and extended immunohistochemical profile
Modern Pathology (2013)