Abstract
Traditionally regarded as simple foot soldiers of the innate immune response limited to the eradication of pathogens, neutrophils recently emerged as more complex cells endowed with a set of immunoregulatory functions. Using a model of invasive pneumococcal disease, we highlighted an unexpected key role for neutrophils as accessory cells in innate interleukin (IL)-17A production by lung resident Vγ6Vδ1+ T cells via nucleotide-binding oligomerization domain receptor, pyrin-containing 3 (NLRP3) inflammasome-dependent IL-1β secretion. In vivo activation of the NLRP3 inflammasome in neutrophils required both host-derived and bacterial-derived signals. Elaborately, it relies on (i) alveolar macrophage-secreted TNF-α for priming and (ii) subsequent exposure to bacterial pneumolysin for activation. Interestingly, this mechanism can be translated to human neutrophils. Our work revealed the cellular and molecular dynamic events leading to γδT17 cell activation, and highlighted for the first time the existence of a fully functional NLRP3 inflammasome in lung neutrophils. This immune axis thus regulates the development of a protective host response to respiratory bacterial infections.
Similar content being viewed by others
Introduction
The Gram-positive bacterium Streptococcus pneumoniae (pneumococcus) is often present in the mucosa lining of the nasopharynx. In healthy individuals, colonization by the pneumococcus is usually associated with mild symptoms or may even be asymptomatic. However, under certain circumstances (e.g., in immunocompromised hosts and/or with virulent strains), colonization can result in life-threatening diseases (such as community-acquired pneumonia, meningitis, and sepsis).1, 2 Pneumococcal-related mortality is currently increasing, and recent estimates suggest that the pneumococcus is responsible for at least a million deaths worldwide each year.3 Invasive pneumococcal disease accounts for most of these cases, of which 35% are caused by serotypes 1, 5, and 14.4 Although several vaccines have been developed to control this infection, pneumococcal disease has by no means been eradicated. Innate immune responses are critically involved in the host’s early attempts to contain or eliminate bacterial pathogens, and also provide support for a subsequent adaptive immune response (if required).
The cytokine interleukin-17A (IL-17A) is a particularly important pro-inflammatory innate factor that regulates immune responses at mucosal barriers.5 IL-17A has a key role in the host’s anti-microbial defenses in particular through the indirect recruitment of neutrophils via induction of chemokines.6 In this context, most of the data on T helper 17-related cytokines in protective acquired host responses to pneumococcal infection have been collected using colonizing strains.7, 8 However, IL-17 contribution to the outcome of an acute infection (leading to invasive disease) has not been characterized.
Gamma-delta (γδ) T cells reportedly produce large amounts of IL-17A9, 10 early on in a variety of immune responses.11, 12 Mouse IL-17-producing γδT (γδT17) cells are characterized by the lack of surface expression of CD27—a thymic regulator that determines the cytokine profile (IFN-γ- vs. IL-17A-production) of γδT cells in the periphery.13 We recently reported that a substantial proportion of γδT cells in the lung (20–30% under resting conditions) express high CD3 levels and preferentially produce IL-17A in response to various stimuli.14 In the lung, we unexpectedly found this phenotype to be restricted to the innate-like Vγ6Vδ1+ T-cell subset.
Neutrophils and alveolar macrophages (AMs) have long been recognized as innate effector cells during acute infection. They facilitate extracellular pathogen clearance by mediating phagocytosis and the release of lytic enzymes, antimicrobial peptides, and reactive oxygen species.15, 16 Moreover, recent evidence indicates that the production of a wide array of inflammatory mediators (such as cytokines and/or chemokines) enable these cells to have potential accessory functions.17, 18, 19 For example, neutrophils express various pattern recognition receptors including inflammasome components.20 Thus, it was recently demonstrated that ex vivo cultured neutrophils produce the pro-inflammatory cytokine IL-1β through the nucleotide-binding oligomerization domain receptor, pyrin-containing 3 (NLRP3) inflammasome.21, 22, 23 The physiological relevance of this activity remains to be determined.
Recently, we and others have shown that tumor necrosis factor-α (TNF-α) can be instrumental in the transcriptional priming of NLRP3 inflammasome activity in vivo and in vitro by upregulating protein levels of proIL-1β in various myeloid cell populations (including neutrophils).24, 25 tumor necrosis factor-α is an early, ubiquitous cytokine that is massively produced during infections and exerts important biological functions by regulating a wide range of genes in target cells. By binding to two structurally distinct receptors (namely TNF-R1 (CD120a) and TNF-R2 (CD120b)),26 TNF-α contributes to pleiotropic activities during inflammation such as cell survival/death and the induction of the production of other cytokines.27
In the present study of lung infection by an invasive strain of S. pneumoniae serotype 1, we demonstrate that the IL-17A produced by γδT cells (and especially the resident Vγ6Vδ1+ subset) mediates the neutrophilia that is critical for bacterial containment and clearance. Most importantly, we discovered an unexpected but critical accessory role for NLRP3-dependent, neutrophil-derived IL-1β in γδT17 cell activation. The neutrophils’ accessory function required AM-mediated TNF-α and the bacterial-derived toxin pneumolysin (Ply) to promote NLRP3 activity. Taken as a whole, our data highlight the spatial and sequential organization of innate immune cell activation. This constitutes an important new pathway in the early development of the host’s innate immune response to bacterial infection of the respiratory tract.
Results
Pulmonary Vγ6Vδ1+ T cells are important in anti-pneumococcal innate defense through IL-17A-mediated neutrophilia
We first investigated the role of innate IL-17A in host resistance to invasive pneumococcal infection. A lack of IL-17A resulted in a significantly lower survival rate, enhanced bacterial growth in the lungs and systemic dissemination, and less accumulation of neutrophils in the infected lungs (Figure 1a–c). Since neutrophils have been shown to exert potent, direct, anti-pneumococcal activities,28 we confirmed (using an anti-Ly6G antibody) that neutrophil depletion critically impaired mouse survival (Supplementary Figure 1a online). Flow cytometry analysis revealed that only innate lymphocytes (i.e., innate lymphoid cells, type I natural killer T cells and γδT cells) produced significant levels of IL-17A (Figure 1d and Supplementary Figure 1b). However, γδT cells were by far the main producers of this cytokine (Figure 1d). Based on Vγ usage, we determined that Vγ6Vδ1+ T cells constituted the prime source of IL-17A (Figure 1e). Importantly, the fluorescence-activated cell sorting (FACS) results confirmed our recent genomic findings14 whereby lung CD3bright γδT cells uniformly expressed the invariant Vγ6Vδ1 T cell receptor (TCR) (Figure 1e). In addition to Vγ6Vδ1+ T cells’ intrinsic specialized functions, we next postulated that the cells’ location of γδT subsets within the lung compartment could also account for their variable ability to respond to infection. A FACS-based approach29 indicated that non-Vγ6Vδ1+ (CD3dim) T cells preferentially populated the vascular compartment (Figure 1f and Supplementary Figure 1c) whereas Vγ6Vδ1+ T cells were almost exclusively represented in the interstitial area (i.e., in proximity to respiratory tract pathogens) (Figure 1f). It is noteworthy that γδT compartmentalization was hardly affected upon infection (Supplementary Figure 1c).
IL-17A and γδT cells are essential for the control of S. pneumoniae infection. (a–e) Age- and sex-matched WT C57BL/6 or IL-17A-deficient mice were infected i.n with S.p. (5 × 105 CFU: 1 × LD50) (a) Survival of mice was monitored daily (n=10). (b) Number of CFU was determined 60 h post infection (p.i.) in the lungs (left panel) and in the spleen (right panel). Individual counts of one representative experiment out of two (n=7–10) are shown. (c) Absolute number of pulmonary neutrophils was assessed by FACS (CD11b+ Ly6G+) 12 h p.i. The mean±s.e.m. of one experiment out of two is shown (n=4–5). (d) Absolute number of pulmonary IL-17A-producing lymphocyte subsets was assessed by intracellular FACS staining 12 h p.i. The mean±s.e.m. of two experiments (n=8) is shown. (e) IL-17A+ γδT cells from infected mice were screened by FACS for Vγ chain usage 12 h p.i. A pie chart representing means of two experiments is shown (n=6) (left panel). A representative FACS plot of pulmonary γδT cells is presented in the middle panel. CD3bright vs. CD3dim γδT cells were labeled in presence or not of the 17D1 mAb (right panel). (f) Means±s.e.m. of CD45+ vs. CD45− populations in naive lungs from three experiments (n=6) are represented in the upper panel. A representative FACS plot of γδT cell subsets for anti-CD45 (in vivo) labeling is shown in the lower panel. (g,h) WT and Tcrd−/− mice were infected with S.p. (5 × 105 CFU). (g) Survival of mice was monitored daily (n=7–8). (h) Number of CFU was determined 60 h p.i. in the lungs and in the spleen (upper panel). Individual counts of two experiments (n=16–17) are shown. Absolute number of neutrophils in lungs of control or S.p.-infected WT or Tcrd−/− mice (lower panel) was assessed by FACS 12 h p.i. and represented as in panel (c). The mean±s.e.m. of two experiments (n=7–8) is shown. *P<0.05; **P<0.01; ***P<0.001. IL-17, interleukin-17; FACS, fluorescence-activated cell sorting; WT, wild-type.
These findings prompted us to investigate the impact of a γδT cell deficiency on the host’s response to a pneumococcal infection. When compared with wild-type (WT) mice, S. pneumoniae-infected Tcrd−/− mice died sooner and displayed a greater bacterial burden and lower levels of neutrophilia (Figure 1g–h). In summary, γδT cells in general (and resident Vγ6Vδ1+ T cells in particular) are major sources of IL-17A during the early stages of S. pneumoniae infection and might contribute to bacterial containment by mediating neutrophil recruitment.
IL-23 and IL-1R signaling are required for IL-17A production by γδT cells during S. pneumoniae infection
IL-17 production by innate γδT cells can occur in response to activating cytokines (such as IL-23 and IL-1β), even in the absence of concomitant TCR ligation.30 Interestingly, IL-23 and IL-1β are produced early in the course of pneumococcal infection.31, 32 In experiments with Il23a−/− and Il1r1−/− mice, we evaluated IL-23 and IL-1β’s contribution to IL-17A production by lung γδT cells. As expected, disruption of IL-23 or IL-1R signaling dramatically impaired the capacity of γδT cells to produce IL-17A following pneumococcal infection (Figure 2a). This impairment was accompanied by a severe relative reduction in neutrophil recruitment to the lung (Figure 2b). It is noteworthy that the frequency of CD3bright Vγ6Vδ1+ T cells was lower in Il23a−/− mice than in WT and Il1r1−/− mice (Supplementary Figure 2). Taken as a whole, these results demonstrate that both IL-23 and IL-1β are required for IL-17A production by γδT cells and subsequent neutrophilia during S. pneumoniae infection.
γδT17 cell activation relies on IL-23 and IL-1R signaling. (a,b) C57BL/6 WT, Il1r1−/− or Il23a−/− mice were infected with S.p. and pulmonary cells were analyzed by flow cytometry 12 h p.i. (a) Representative FACS plots of IL-17A production by γδT cells from two experiments are shown (upper panel). Means±s.e.m. of pulmonary IL-17A-positive γδT cell frequency is shown in the lower panel. (b) Absolute number of pulmonary neutrophils was analyzed. The mean±s.e.m. for two experiments (n=6) is shown. NS, not significant; *P<0.05; **P<0.01. IL-17, interleukin-17; FACS, fluorescence-activated cell sorting; WT, wild-type.
Neutrophils are a prime source of IL-1β following pneumococcal infection of the lungs
Given the importance of IL-1β in γδT17 cell activation, we next attempted to determine the cellular sources of this cytokine following pneumococcal challenge. IL-1β is first expressed as biologically inactive proIL-1β form. Given that IL-1β is predominantly derived from the myeloid lineage, we used flow cytometry to analyze the lung myeloid cells’ ability to express proIL-1β in response to pneumococcal infection. Although S. pneumoniae infection led to proIL-1β upregulation in all tested cell populations as early as 6 h post-infection (Figure 3a and Supplementary Figure 3a), we found that neutrophils were the primary source of this procytokine (Figure 3a and Supplementary Figure 3b). To determine whether or not neutrophils could produce bioactive IL-1β in response to S. pneumoniae, we cultured purified lung neutrophils from naive and infected mice. Interestingly, we found that lung neutrophils could produce bioactive IL-1β (Figure 3b) but neither IL-12p40 nor IL-23p19 (not shown) in response to infection. It is noteworthy that we only detected low levels of IL-1β in the supernatants of neutrophil-depleted cell cultures (Figure 3b). Furthermore, neutrophil depletion was associated with significantly lower levels of IL-1β in the lung tissue of infected mice (Figure 3c). Thus, our results highlight neutrophils as an important source of IL-1β in the early response to pneumococcal lung infection.
Neutrophils are a major and early source of IL-1β in response to pneumococcal infection. (a,b) WT C57BL/6 mice were infected i.n with S.p. (a) Kinetics of proIL-1β expression by pulmonary neutrophils was assessed by flow cytometry. Means±s.e.m. of frequency of proIL-1β-positive cells among myeloid population is represented at different kinetics following infection (upper left panel). The mean±s.e.m. of absolute number of proIL-1β+ pulmonary myeloid cells is shown in the upper right panel. Representative FACS plots of proIL-1β+ neutrophils are shown in the lower panel. Data presented are from two experiments (n=6). (b) 4 × 105 magnetic-activated cell sorting-enriched pulmonary neutrophils from either naive or S.p.-infected (12 h p.i.) mice were incubated for 3 h at 37 °C in complete media. Levels of IL-1β levels in culture supernatants from the positive and negative fractions were measured by ELISA. Means±s.e.m. of biological replicates from two experiments are represented (n=6). (c) Levels of IL-1β in whole lungs of controls or neutrophil-depleted mice were assessed 12 h p.i. The mean±s.e.m. of two experiments (n=4–10) is shown. NS: not significant; *P<0.05; **P<0.01; ***P<0.001. IL-17, interleukin-17; ELISA, enzyme-linked immunosorbent assay; FACS, fluorescence-activated cell sorting; WT, wild-type.
Neutrophil priming requires AM-produced TNF-α
To investigate the general mechanisms involved in IL-1β maturation and secretion by lung neutrophils, we first addressed the mechanisms underlying the priming step leading to proIL-1β upregulation. Synthesis of proIL-1β requires nuclear factor-kappa B (NF-κB)- and activator protein-1-dependent gene transcription in response to bacterial components and cytokines.33, 34 We and others have demonstrated that TNF-α is sufficient to upregulate proIL-1β protein levels in myeloid cells in vitro and in vivo.24, 25 TNF-α is produced soon after pneumococcal challenge and is predominantly detected in the airways (Figure 4a and Supplementary Figure 4a). In line with the sole presence of AMs in the airways at 6 h post-infection (Supplementary Figure 4b), intracellular staining revealed that AMs (but not other myeloid cells) were able to produce TNF-α (Figure 4b). Moreover, the specific depletion of AMs (Supplementary Figure 4c) abrogated early-stage TNF-α production in the lungs of infected mice (Figure 4c). To determine whether or not TNF-α is instrumental in neutrophil priming in our model, we gave the mice an intraperitoneal dose of anti-TNF-α mAb prior to bacterial inoculation. TNF-α neutralization impaired priming in neutrophils and other myeloid populations as judged by decreased intracellular levels of proIL-1β proteins (Figure 4d and Supplementary Figure 4d). Anti-TNF-α treatment was also associated with lower relative and absolute counts of lung γδT17 cells (Figure 4e) and with lower levels of neutrophilia (Figure 4e). Furthermore, IL-1β levels in the lungs were low after AM depletion (Figure 4f).
proIL-1β upregulation in neutrophils is mediated through a TNF-α/TNFR1 pathway. (a,b) TNF-α production was assessed at different kinetics following pneumococcal infection. (a) Levels of TNF-α protein in whole lungs were assessed by ELISA. The mean±s.e.m. of three experiments (n=5–9) is shown. (b) Representative FACS plots of intracellular TNF-α+ alveolar macrophages at different kinetics were represented (left panel) and means±s.e.m. of frequency of TNF-α+ myeloid cell populations over time after S.p. infection were shown in the right panel (n=5–9). (c) Control or AM-depleted mice were infected i.n. with S.p. Levels of TNF-α in whole lung were quantified 12 h p.i. by ELISA. The mean±s.e.m. of TNF-α protein detected from two experiments is shown (n=6–10). (d,e) Ig control- or anti-TNF-α mAb-treated mice were challenged with S.p. and killed 12 h p.i. (d) ProIL-1β expression by neutrophils was determined by intracellular FACS staining. A representative plot is shown in the left panel. Means±s.e.m. of frequency (middle panel) and absolute number (right panel) of proIL-1β+ neutrophils from two experiments are represented (n=5–6). (e) Means±s.e.m. of frequency (left panel) and absolute number (middle panel) of IL-17A+ γδT cells are shown. Means±s.e.m. of absolute number of pulmonary neutrophils are shown in the right panel. Data are pooled from two experiments (n=6). (f) Control liposomes or clodronate liposomes-treated mice were infected or not i.n. with S.p. Levels of IL-1β in whole lung were quantified 12 h p.i. by ELISA. The mean±s.e.m. of IL-1β protein detected from two experiments is shown (n=6–10). (g) WT or Tnfrsf1a−/− mice were infected with S.p. and killed 12 h p.i. ProIL-1β expression by neutrophils was determined by intracellular FACS staining. Means±s.e.m. of frequency of proIL-1β+ (left panel) and total (right panel) neutrophils from two experiments were represented (n=6–14). (h) 2 × 105 FACS-sorted pulmonary neutrophils from naive mice were cultured for 3 h at 37° in complete media in presence of recombinant mouse TNF-α at the indicated concentrations. Then cells were analyzed by FACS for proIL-1β expression. Individual biological replicates and means±s.e.m. from two experiments were represented (n=3–5). *P<0.05; **P<0.01; ***P<0.001. AM, alveolar macrophage; IL-17, interleukin-17; ELISA, enzyme-linked immunosorbent assay; FACS, fluorescence-activated cell sorting; WT, wild-type; TNF-α, tumor necrosis factor-α.
TNF-α can exert its biological activity through the recruitment of TNF-R1 and TNF-R2 receptors. We showed that neutrophils from TNF-R1-deficient mice failed to be primed (Figure 4g). Consequently, neutrophilia was severely affected in the absence of TNF-α signaling (Figure 4g). Lastly, the in vitro incubation of sorted naive lung neutrophils with TNF-α triggered dose-dependent upregulation of proIL-1β (Figure 4h). Overall, these data demonstrate that neutrophil priming (proIL-1β synthesis) is strongly regulated by AM-derived TNF-α during the early stages of pneumococcal infection.
IL-1β secretion by lung neutrophils is dependent on the NLRP3 inflammasome
It was originally suggested that the release of IL-1β by neutrophils was dependent on the cleavage of proIL-1β into mature IL-1β by serine proteases (e.g., elastase and proteinase 3).35, 36 Recent data also suggest that neutrophils can express functional inflammasomes which may produce bioactive IL-1β.21, 22 In agreement with the latter observations, we found that the levels of IL-1β in the lung tissue of S. pneumoniae-infected mice were lower in Casp1/11-deficient mice than in WT controls—indicating a key role for inflammasome signaling in this model of infection (Figure 5a). Compared with WT mice, pneumococcal infection in mice lacking NLRP3 inflammasome components (Nlrp3−/− or Asc−/−) resulted in significantly lower IL-1β production (Figure 5a). Moreover in vivo treatment with the selective NLRP3 inflammasome inhibitor MCC95037 was associated with significantly lower levels of IL-1β in the lungs of S. pneumoniae-infected mice (Figure 5b). To assess signs for NLRP3 inflammasome priming in neutrophils, we analyzed mRNA and protein samples from lung neutrophils purified from either naïve or S. pneumoniae-infected mice. We observed high levels of NLRP3 protein expression (Figure 5c) and Nlrp3 mRNA transcripts in neutrophils from infected mice (Supplementary Figure 5a). We next measured NLRP3 inflammasome activity in neutrophils purified from naïve or infected mice. Interestingly, treatment with MCC950 abrogated ex vivo IL-1β production by lung neutrophils from challenged mice (Figure 5d). Furthermore, a Western blot analysis of purified neutrophils from infected mice showed clear evidence of IL-1β maturation (Figure 5c).
NLRP3 inflammasome contributes to IL-1β production by neutrophils. (a) IL-1β levels production were assessed in whole lung from PBS or infected WT, Nlrp3−/−, Asc−/− and Casp1/11−/− mice. The mean±s.e.m. for two experiments is shown (n=6–10). (b) PBS or S.p.-infected WT mice have been treated or not with MCC950 (1 mg/mouse i.n., 2 h p.i.) and killed 12 h p.i. Lungs were harvested, homogenized and IL-1β production was quantified in homogenates by ELISA. The mean±s.e.m. for two experiments is shown (n=6–12). (c,d) Pulmonary neutrophils from PBS or S.p.-infected (12 h p.i.) mice were purified by FACS sorting. (c) Lysates from neutrophils were blotted for β-actin, IL-1β, and NLRP3 expression. (d) Neutrophils were cultured for 3 h at 37 °C in complete media in presence or absence of MCC950 (10 μM). Levels of IL-1β in supernatants were analyzed by ELISA. The mean±s.e.m. of IL-1β concentrations from two experiments is shown. (e) WT mice were i.n. infected with parental and mutant strains of D39 (4 × 106 CFU per mouse). Mice were killed 12 h p.i. and IL-1β was measured in lung homogenates. The mean±s.e.m. of IL-1β concentrations from two experiments is shown (n=8–9). (f,g) 2 × 105 pulmonary neutrophils from WT, Nlrp3−/−, and Casp1/11−/− naive mice were incubated in complete RPMI media at 37 °C with or without recombinant TNF-α (100 ng ml−1) for 3 h and pneumolysin was added for an additional 90 min (500 ng ml−1). IL-1β secretion in supernatants was quantified by ELISA. The mean±s.e.m. of IL-1β concentrations from two experiments is shown. *P<0.05; **P<0.01; ***P<0.005. ELISA, enzyme-linked immunosorbent assay; FACS, fluorescence-activated cell sorting; IL-17, interleukin-17; NLRP3, nucleotide-binding oligomerization domain receptor, pyrin-containing 3; PBS, phosphate-buffered saline; WT, wild-type; TNF-α, tumor necrosis factor-α.
Along with many other bacterial pore-forming toxins, the S. pneumoniae virulence factor pneumolysin (Ply) is thought to be an NLRP3 activator.38 When comparing the development of immune responses to a parental strain and a Ply-deficient strain (D39Δply) of pneumococcus (Supplementary Figure 5b), we detected significantly lower levels of IL-1β in the lungs of mice infected with the mutant strain (Figure 5e). Ply complementation restored the levels of IL-1β (Figure 5e and Supplementary Figure 5b). We next used a recombinant Ply to mimic in vivo NLRP3 inflammasome activation. As is thought to be the case in vivo, the addition of Ply induced IL-1β production by TNF-α-primed WT neutrophils (but not by unprimed neutrophils) (Figure 5f). However, lung neutrophils from either Nlrp3−/− or Casp1/11−/− mice failed to respond to the same stimuli (Figure 5g). Collectively, these data indicate that IL-1β production by neutrophils is governed by NLRP3 inflammasome-dependent caspase-1 activity.
TNF-α and Ply are sufficient to elicit NLRP3 inflammasome-dependent IL-1β secretion by human neutrophils
To investigate whether TNF-α and Ply also induced IL-1β secretion by human neutrophils, we prepared total leukocytes from the peripheral blood of healthy donors, primed the cells with TNF-α for 3 h, and then stimulated the cells with Ply for an additional 90 min. In line with the data from the mouse experiments, standalone treatments failed to induce IL-1β production by human leukocytes but the TNF-α/Ply combination resulted in IL-1β secretion (Figure 6a). This effect was NLRP3 inflammasome-dependent, since MCC950 treatment fully abrogated IL-1β secretion (Figure 6a). We next studied the ability of peripheral blood neutrophils from healthy donors (Supplementary Figure 6a) to produce NLRP3 inflammasome-dependent IL-1β. TNF-R1 was expressed on human neutrophils (Supplementary Figure 6b) and treatment with Ply induced IL-1β release by TNF-α-primed neutrophils in an NLRP3 inflammasome-dependent manner (Figure 6b). Thus, our data suggest that the NLRP3 inflammasome is active in inducing the production of IL-1β by human neutrophils via a TNF-α/Ply-dependent mechanism.
Human neutrophils produce NLRP3 inflammasome-dependent IL-1β in response to TNF-α and Ply. (a) 4 × 105 leukocytes from peripheral blood of non-smokers healthy donors were seeded in complete medium with recombinant human TNF-α with or without MCC950 (10 μM) for 3 h and then stimulated with ply (500 ng ml−1) for an extra 90 min. Supernatants were collected and tested for IL-1β. The means±s.e.m. of IL-1β concentrations from four donors out of six is shown. (b) 2.5 × 105 purified neutrophils from peripheral blood of non-smokers healthy donors were cultured in complete medium with recombinant human TNF-α with or without MCC950 (10 μM) for 3 h and then stimulated with ply (500 ng ml−1) for an extra 90 min. Supernatants were collected and tested for IL-1β. The means±s.e.m. of IL-1β concentrations from four donors out of nine is shown. IL-17, interleukin-17; NLRP3, nucleotide-binding oligomerization domain receptor, pyrin-containing 3; TNF-α, tumor necrosis factor-α.
Neutrophil-derived IL-1β is involved in γδT17 cell activation following pneumococcal infection
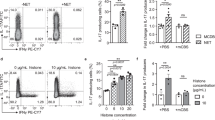
To establish whether or not neutrophil-derived IL-1β was linked to critical γδT17 cell-dependent immune responses in the containment/elimination of S. pneumoniae, we analyzed the γδT cells’ ability to produce IL-17A after neutrophil depletion. Interestingly, neutrophil depletion reduced IL-17A production (Figure 7a) in early-stage infection. It is noteworthy that neutrophils do not have a critical role in the containment of bacteria during the earliest phase of infection (Supplementary Figure 7a). Moreover, purified naive lung CD27− γδT cells that were cultured in conditioned media from S. pneumoniae-infected lung neutrophil cultures produced IL-17A (Figure 7b). As expected, IL-17A secretion was only seen for the CD27− γδT cell fraction but not for their CD27+ counterparts (Supplementary Figure 7b). In vitro neutralization of IL-1β abrogated IL-17A production by γδT cells—indicating that this effect was fully dependent on IL-1β and did not require cell-cell contact (Figure 7b). Of note, IL-17A production by γδT cells was only achieved when recombinant IL-23 was added to the culture medium. Taken as a whole, our results indicate that S. pneumoniae induces rapid IL-1β secretion by neutrophils. This secretion contributes strongly to IL-17A production by lung γδT cells.
IL-17A production by γδT17 cells is dependent on neutrophil-derived IL-1β. (a) IL-17A production by pulmonary γδT cells was assessed in S.p.-infected control or neutrophil-depleted mice 6 h p.i. Representative FACS plots of IL-17A+ γδT cells are shown in the upper panel. The mean±s.e.m. of IL-17A+ γδT cell frequency from two experiments (n=6) is shown in the lower panel. (b) FACS-sorted pulmonary CD27− γδT cells were incubated at 37 °C for 20 h with complete and conditioned media (1:1 ratio) from non-sensitized neutrophils (NSN) or pneumococcus-sensitized neutrophils (PSN). In some conditions, anti-IL-1β mAb (5 μg ml−1), rIL-1β (1 ng ml−1), and rIL-23 (1 ng ml−1) were added to the culture. Conditioned media are supernatants of cultured purified pulmonary neutrophils from pooled naive (NSN) or S.p.-infected (PSN) mice. Neutrophils from infected mice have been purified 12 h p.i. IL-17A levels in the supernatants were analyzed by ELISA. The mean±s.e.m. of IL-17A concentrations from three experiments (n=3–5) is shown. *P<0.05; **P<0.01. IL-17, interleukin-17; ELISA, enzyme-linked immunosorbent assay; FACS, fluorescence-activated cell sorting.
Discussion
Given that the early immune events following respiratory bacterial infection are critical in the development of an effective host response against the pathogen, we decided to investigate the functions and activation mechanisms of γδT17 cells during pneumococcal infection. Our present results show that in addition to the canonical phagocytic functions of AMs and neutrophils, these cells are key components in the initiation and regulation of the innate immune response. Indeed, we highlighted a “three-component system” in which AMs (via TNF-α secretion) and neutrophils (via NLRP3 inflammasome-dependent IL-1β production) are critical players in γδT17 cell activation.
We found that CD3bright Vγ6Vδ1+ T cells produced the largest amounts of IL-17A during the early stages of pneumococcal infection. Remarkably, these cells express high levels of IL-1β and IL-23 receptors that we found essential for activation in response to pneumococcus.14 Furthermore, our present results also show that Vγ6Vδ1+ T cells are located in the lung’s interstitial compartment and therefore stand on the front line in the host’s defense against microbes. In contrast, other recognized IL-17A producers (such as Vγ4+ T cells and type I NK1.1− natural killer T cells) are mainly found in the vasculature (not shown). Interestingly, vaginal Vγ6Vδ1+ T cells are resident in the epithelium.39 Our data suggest that their pulmonary counterparts have a similar location. Nevertheless, more specific tools will be required to determine the precise location of Vγ6Vδ1+ T cells within the interstitial compartment. Moreover, the decrease in the proportion of Vγ6Vδ1+ T cells in the lung of IL-23p19-deficient mice might indicate a requirement for this cytokine in the subset’s maintenance in the periphery. Shibata et al.40 demonstrated that STAT3 was not essential for the development of γδT17 cells. Hence, we postulate that IL-23 is important for the maintenance of Vγ6Vδ1+ T cells in the periphery. Further investigations will be required to define the interactions between the various factors (e.g., IL-23, and the microbiota) involved in lung Vγ6Vδ1+ T-cell homeostasis.14
Other innate immune cells from the lymphoid compartment respond to the IL-23/IL-1β combination by producing IL-17A.12 Thus, it is likely that this AM/neutrophil pathway might be responsible (through IL-1β secretion) for the activation of lymphocytes such as natural killer T cells, mucosal-associated invariant T cells, and innate lymphoid cells in other respiratory disorders.
From a mechanistic perspective, we demonstrated that NLRP3 inflammasome activation in neutrophils depends on AM-derived TNF-α for priming and bacterial Ply for NLRP3 activation. It is already been shown that Ply is involved in NLRP3 activation in neutrophils in vitro.21 Recently the same group of researchers suggested that during S. pneumoniae corneal infection, adenosine triphosphate (ATP) can also activate the NLRP3 inflammasome in neutrophils via engagement of the P2X7 receptor.41 In vitro studies have previously shown that ATP can activate NLRP3 in neutrophils.22, 23, 42 However, ATP seems to have low activity on neutrophilic NLRP3 inflammasome compared to other common NLRP3 activators.22, 23 Pioneering studies also reported unresponsiveness to ATP43 and the absence of P2X7 receptors on human neutrophils.44 Here, by using a Ply-deficient bacteria and recombinant Ply, we demonstrated that this pore-forming toxin has a critical role in NLRP3 activation on lung neutrophils. Understanding ATP’s contribution to NLRP3 inflammasome activation in our model of infection will require further investigation. Nevertheless, millimolar levels of ATP are only released during active inflammation and therefore it is likely that ATP is only involved in NLRP3 activation once an infection is well-established and major tissue damages occurred.
It has been suggested that during S. pneumoniae infection of the cornea, inflammasome priming is dependent on direct recognition of the pneumococcus’ pathogen-associated molecular patterns.21 In contrast, we observed that a TNF-α-mediated mechanism seems to dominate over direct microbial component recognition in neutrophil priming during the early steps of infection. However, authors studied the in vitro priming of bone marrow neutrophils with heat-killed bacteria and so the mechanisms are likely to differ in the lung in vivo.21 Although indirect activation of the innate immune system during bacterial infection is rather counterintuitive, pathogens have evolved ways of evading recognition by germline-encoded receptors and thus preventing elimination by the host. For instance, S. pneumoniae expresses an extracellular capsule that subverts innate immunity recognition by masking the underlying cell surface structures. In line with our findings, it has been shown that upon in vitro macrophage infection with the encapsulated bacteria Streptococcus pyogenes, NLRP3 inflammasome priming was independent of Toll-like receptor and the P2X7 receptor signaling.45 Moreover, since the anatomy and physical barrier in the lungs preclude a direct contact between bacteria and neutrophils early after infection, the host circumvents this situation by integrating soluble signals (AM-derived TNF-α and bacterial-derived pneumolysin) to mount an efficient neutrophilic NLRP3 inflammasome-dependent protective response. From an evolutionary point of view, the immunological pathway reported here might reflect the perpetual competition between microbes and hosts. Our study revealed a novel mechanism for rapidly priming the NLRP3 inflammasome and initiating a protective response against encapsulated bacterial pathogens prior to their dissemination. The fast-acting mechanism identified here enables the host to stay one step ahead of the pathogen by mounting an effective response in the lung parenchyma at a time when the bacteria are still confined to the alveoli. Nevertheless, it is possible that at later time-points (i.e., when the bacteria have colonized the lung parenchyma) that the engagement of various pattern recognition receptors might be the main route for NLRP3 inflammasome priming.
Although recent data suggest that sterile signals (e.g., TNF-α) induce weaker, more delayed NLRP3 inflammasome priming in vitro than microbial products do,46 our present results demonstrate that TNF-α can rapidly prime the NLRP3 inflammasome in neutrophils following infection. TNF-α is rapidly produced by AMs, resulting in proIL-1β accumulation in neutrophils as early as 6 h post-S. pneumoniae infection. However, we could not exclude that non-hematopoietic cells can participate in TNF-α production such as epithelial cells. The mechanisms underlying this cytokine production were not investigated here but probably rely on direct (opsonic-independent) recognition of pneumococcus cell-wall components by AMs. Secondly, our data reveal that TNF-R1 is fully functional on a large proportion of mouse lung neutrophils. The inflammasome priming step is tightly regulated by the transcription factor NF-κB.34 Given that TNF-R1 intracellular signaling leads to the mobilization/activation of NF-κB,47 our results are fully in line with the latest literature data. Another novel observation was that neutrophils participated indirectly in their own recruitment to the inflamed tissue. This finding agrees with a recent demonstration that IL-1β regulates airway neutrophilia in response to S. pneumoniae serotype 2 (strain D39) infection.31 Further studies will be required to determine whether the neutrophils’ accessory functions are restricted to specialized subsets in particular niches or are a feature of the whole population when induced by a particular inflammatory environment.
From a translational point of view, we found that the mechanisms involved in NLRP3 inflammasome activity in S. pneumoniae-infected mice (i.e., TNF-α and Ply) were also active in human neutrophils. Anti-TNF-α mAbs are highly effective in the treatment of patients with severe inflammatory/autoimmune disorders (such as rheumatoid arthritis, ankylosing spondylitis, and Crohn’s disease). However, these treatments are associated with adverse effects, including elevated susceptibility to infections. Blockade of the pathway highlighted in the present report might be involved in these complications. In line with our hypothesis, volunteers treated with Humira showed a significantly lower airway neutrophilic influx in response to inhaled endotoxin, when compared with a control group.48 In the same vein, a link between TNF-α and IL-17A production by lung γδT cells has recently been identified in the context of exposure to pollutants.49
In conclusion, we found that neutrophils and AMs are important early-stage components in γδT17 cell activation. Neutrophils exert this accessory role through a functional NLRP3 inflammasome. The rapid release of NLRP3-dependent IL-1β by neutrophils relied on a mechanism involving TNF-R1 engagement by AM-dependent TNF-α (signal 1) and bacterial Ply (signal 2). It remains to be seen whether this pathway is clinically relevant in other immune situations or can be used to the design of more refined immune-intervention strategies in this context.
Methods
Mice. Eight- to twelve-week-old male WT C57BL/6J mice were purchased from Janvier (Le Genest-St-Isle, France). C57BL/6J TCRδ-deficient (Tcrd−/−) and IL-17A-deficient (Il17a−/−) mice were bred in house at the Pasteur institute of Lille. C57BL/6J IL-1R1-deficient (Il1r1−/−) and IL-23p19-deficient (Il23p19−/−) mice were bred in house at the University of Orléans (INEM, CNRS UMR 7355, Orléans, France). C57BL/6J Nlrp3−/−, Asc−/−, Casp1/11−/−, and Tnfrsf1a−/− mice were bred in house at Ghent University Campus-VIB. Mice were bred under pathogen-free conditions. All animal work conformed to the French governmental, local (CEEA number 00357.01) and Ghent University animal care and use committee guidelines.
Human samples. Peripheral blood was collected from non-smokers healthy donors. Written informed consent was received from participants prior to inclusion in the study, according to ethics committee on human experimentations.
Reagents and Abs. Polyclonal anti-IL-1β Ab, anti- Ly6G (NIMP-R14) mAb, anti-TNF-α mAb (XT3.11), and their respective isotype controls were purchased from either Bio X Cell (West Lebanon, NH) or R&D systems (Lille, France). Monoclonal antibodies against mouse CD45 (APCCy7- or FITC- or AF700- or PeCy7- or PE-conjugated), CD3 (Pacific Blue- or PerCPCy5.5-conjugated), TCRδ (PerCpCy5.5-conjugated), TCRβ (FITC- or APC-conjugated), NK1.1 (PeCy7- or FITC-conjugated), Ly6G (APC-Cy7- or FITC-conjugated), CD11b (PerCPCy5.5- or FITC-conjugated), Siglec F (Pe-CF594- or BV421-conjugated), CD11c (APC- or FITC-conjugated), MHCII (AF700- or FITC-conjugated), CD4 (PerCPCy5.5-conjugated), CD127 (PeCy7-conjugated), CD90.2 (AF700-conjugated), Vγ1 (APC-conjugated), Vγ4 (PeCy7-conjugated), F4/80 (PeCy7-conjugated), TNF-α (PE-conjugated), IL-17A (PE-conjugated), pro-IL-1β (PE-conjugated), and appropriated isotype controls were purchased from BioLegend, BD Pharmingen and eBioscience (San Diego, CA). Phosphate-buffered saline (PBS)-57 glycolipid-loaded and unloaded control CD1d tetramers (APC- or PE-conjugated) were from the National Institute of Allergy and Infectious Diseases Tetramer Facility (Emory University, Atlanta, GA). The mAb for Vγ6Vδ1 TCR detection (clone 17D1) was a kind gift from Prof. R. Tigelaar (Yale University, New Haven, CT). Propidium iodide was purchased from BD Pharmingen. Gating strategies used in this study are presented in Table 1. Recombinant mouse and human TNF-α were purchased from eBioscience. Mouse IL-23 and IL-1β were from Peprotech (Neuilly-sur-Seine, France). MCC950 was purchased from Sigma-Aldrich (Saint Quentin Fallavier, France). Clodronate and control liposomes are from ClodLip BV (Amsterdam, The Netherlands). Recombinant pore-forming and non-pore-forming Ply were expressed in E. coli and purified as described.37 Briefly, toxins were passed three times through an EndoTrap endotoxin removal column (Profos, Regensburg, Germany), after which lipopolysaccharide was undetectable using the PyroGene recombinant factor C assay (Lonza; detection limit 0.01 endotoxin units ml−1). Mouse and human enzyme-linked immunosorbent assay kits are from R&D systems (Minneapolis, MN) and eBioscience.
Infection with S. pneumoniae. S. pneumoniae serotype 1 clinical isolate E1586 sequence type ST304 has been described elsewhere.50 In some experiments, parental and ply::cat mutant D39 strains were used. Mice were anesthetized and administered intranasally (i.n.) with 50 μl of PBS containing live bacteria.
Neutrophil and AM depletion. Neutrophils were depleted 24 h prior S.p. infection using an anti-Ly6G mAb (100 μg per mouse intraperitoneally, clone: NIMP-R14). Alveolar macrophages were depleted using clodronate liposomes. Clodronate liposomes and control liposomes were delivered twice by i.n. route 48 and 24 h prior infection (50 μl per mouse).
Measurement of cytokines in the whole lungs. Perfused lungs were collected in liquid nitrogen until processing. An anti-protease solution was prepared by dissolving one tablet of anti-protease (25 ×) in 2 ml of distilled water. T-Per Tissue protein extraction buffer (1 ml) containing 1 × of the anti-protease solution was added onto lungs and homogenized for 1 min. Lung lysates were kept on ice for 20 min then centrifuged at 10,000 rpm for 15 min. The supernatants were collected in sterile eppendorfs and stored at −20 °C until cytokine analysis.
Preparation of pulmonary immune cells. Mice treated with anti-TNF-α (150 μg per mouseintraperitoneally) or isotype controls were infected or not with S. pneumoniae. Lung mononuclear cells were prepared by classical procedures. Briefly, lungs were perfused with PBS 2% fetal calf serum (FCS), excised and finely minced, followed by enzymatic digestion for 20 min at 37 °C in PBS containing 1 mg ml−1 collagenase type VIII (Sigma-Aldrich) and 1 μg ml−1 DNase type I (Roche, Boulogne-Billancourt, France). After wash, lung homogenates were resuspended in a 20% Percoll gradient, and centrifuged at 2,000 rpm without brake at room temperature for 15 min. The pellet was washed in PBS 2% FCS and red blood cells were removed using lysis buffer (Sigma-Aldrich).
Labeling of vascular vs. interstitial lung leukocytes. Anesthetized mice were injected with 2 μg of PeCy7-labelled rat anti-CD45 (clone: 30-F11) mAb via the retro-orbital venous plexus. The mAb was allowed to circulate for 5 min in order to label all leukocytes in the vascular space including circulating and marginated leukocytes.29 After extensive perfusion (to remove circulating leukocytes), lungs were removed and lung leukocytes were prepared as previously described. The cell suspension was then labeled with appropriate mAbs for cell subset detection and PE-labeled anti-CD45 (clone I3/2.3) mAb to exogenously stain all lung leukocytes including marginated and interstitial pulmonary leukocytes. Marginated leukocytes were defined as double positive for CD45-PeCy7 and CD45-PE and interstitial leukocytes were defined as CD45-PeCy7 negative and CD45-PE positive.
Cell sorting and in vitro/ex vivo assays. Lungs were harvested from naïve or infected mice and cell suspensions were prepared as previously described. Cells were sorted using an ARIA cell sorter (BD Biosciences). To purify γδT cells, lung mononuclear cells were labeled with PB-conjugated anti-CD3 antibody, PerCp-Cy5.5-conjugated anti-TCRδ mAb and PeCy7-conjugated anti-CD27 mAb. For neutrophil purification, lung mononuclear cells were labeled with FITC-conjugated anti-Ly6G mAb and PerCp-Cy5.5-conjugated anti-CD11b mAb. After cell surface labeling, cells were sorted using a FACSAria (BD Biosciences). This protocol yielded >98% cell purity as evaluated by FACS. In some experiments, pulmonary neutrophils were enriched using positive selection (anti-Ly6G) on LS column with a MidiMACS Separator (Miltenyi Biotec, Paris, France). Purity after magnetic-activated cell sorting enrichment yielded around 85–90% as evaluated by FACS. For in vitro stimulation assays, neutrophils were cultured for 3 h in complete RPMI 5% FCS media containing recombinant mouse TNF-α, and then pneumolysin was added for another 90 min. Purified CD27− or CD27+ γδT cells were cultured 20 h in complete media containing recombinant IL-23 (1 ng ml−1), and/or recombinant IL-1β (1 ng ml−1), or conditioned media from cultured neutrophils isolated from infected or naive mice. In some cases, anti-IL-1β (5 μg ml) was added to culture.
Intracellular FACS staining and cytospins. Lungs were harvested at different time points and mononuclear cells were prepared as described above. Cells were incubated in RPMI 1640 5% FCS containing Golgi Plug/Golgi Stop (BD Biosciences) for 2 h at 37 °C. Then, cells were washed with appropriate dilutions of the different antibodies for 30 min in PBS 2% FCS. Cells were washed, and fixed using IC Fixation Buffer (eBioscience, CliniSciences, Montrouge, France). Fixed cells were then permeabilized in Permeabilization Buffer (eBioscience), according to the manufacturer’s instructions. Cells were stained with PE-conjugated mAbs against TNF-α, IL-17A, or pro-IL-1β and analyzed on a LSR Fortessa or a Canto II (BD Biosciences). FACS analyses were performed using the FlowJo software (Treestar, OR). A morphology-based differential cell analysis was conducted on cytospin preparations from the broncho-alveolar lavage fluid samples from PBS or S.p.-infected mice and subjected to May-Grünwald Giemsa staining.
Western blotting. Pulmonary neutrophil protein lysates were prepared in 1% NP-40-containing lysis buffer. Proteins were denatured by boiling in Laemmli buffer and subjected to sodium dodecyl sulfate–polyacrylamide gel electrophoresis. Separated proteins were transferred to PVDF membranes and stained using primary antibodies against IL-1β (GeneTex, Irvine, CA), NLRP3 (AdipoGen, Epalinges, Switzerland) and horseradish peroxidase-coupled anti-mouse β-actin (Santa Cruz, Heidelberg, Germany). Subsequently, membranes were exposed to the corresponding secondary antibodies (Jackson ImmunoResearch, Newmarket, UK) and developed with ECL (GE Healthcare, Vélizy-Villacoublay, France).
Generation of D39 mutant strains. Genomic DNA from D39 strain for amplification was prepared by suspending 1 ml of pelleted D39 (OD600 0.4) in 100 μl lysis buffer (0.01% sodium dodecyl sulfate, 0.1% sodium deoxycholate, 0.015 M sodium citrate), incubating the mixture at 37 °C for 30 min, and then diluting the mixture with 100 μl PBS. Plasmid DNA was extracted from harboring E. coli strain with NucleoSpin Plasmid Kit (Macherey-Nagel, Hoerdt, France) following manufacturer’s protocols. Pneumolysin deletion mutant was constructed using sequence overlap extension followed by allelic replacement of native pneumolysin gene with chloramphenicol marker. Upstream flanking region was amplified from genomic D39 DNA using primer set LK162 and LK163 (5′-CTAGCCTTGACAACTAGCCAATC-3′; 5′-CTCACAAAAATCCGAGCTCCACCGCTTCTACCTCCTAATAAGTTCCTGG-3′). Chloramphenicol marker amplified from plasmid pKOC with primer set LK164 and LK 165 (5′-CCAGGAACTTATTAGGAGGTAGAAGCGGTGGAGCTCGGATTTTTGTGAG-3′; 5′-CGCAAGCATTCTCCTCTCCGCTAGGGCGCTGGCAAG-3′). Downstream flanking region was amplified from genomic D39 DNA using primer set LK166 and LK167 (5′-CTTGCCAGCGCCCTAGCGGAGAGGAGAATGCTTGCG-3′; 5′-TGCAAATAGAAAGTTTCAGCC-3′). The overlapping regions of fragments are indicated by underlined and bolded sections. All fragments were amplified using DreamTaq polymerase (ThermoFisher Scientific; Waltham, MA, USA) with 1 min extension and a 55 °C annealing temperature for 34 cycles. Fragments joined using this standard PCR reaction except with a 3 min extension using terminal primers LK162 and LK167. Pneumococcal transformation was performed by addition of 100 ng ml−1 of synthetic CSP-1 to 1 ml of D39 grown to OD600 0.12 followed by incubation at 37 °C for 12 min. Approximately 200 ng of DNA added to 100 μl of activated cells followed by incubation at 30 °C for 20 min. Cells diluted 10 times in C+Y media and incubated for 1.5 h at 37 °C before plating on Columbia agar supplemented with 4% defibrinated sheep blood and 4.5 μg ml−1 chloramphenicol. Positive colonies were screened and verified by sequencing.
Isolation and culture of human cells. To obtain total blood leukocytes, blood samples collected on heparanized tubes were treated with Buffer EL (Qiagen, Hilden, Germany) according to manufacturer’s instructions to remove erythrocytes. Neutrophils have been isolated as previously described.51 This procedure routinely yielded >85% cell purity as assessed by FACS and May-Grünwald Giemsa stain (Sigma-Aldrich). Cells (4 × 105 per well) were cultured for 3 h in complete RPMI 5% FCS media containing recombinant human TNF-α (100 ng ml−1), and then pneumolysin (500 ng ml−1) was added for another 90 min. After culture, cell viability was evaluated by propidium iodide staining.
Statistical analysis. All statistical analysis was performed using GraphPad Prism software. The statistical significance was evaluated using non-parametric Mann-Whitney U or Kruskal-Wallis (followed by a Dunn’s post-test) tests to compare the means of biological replicates in each experimental group. Survival rates after S. pneumoniae challenge were analyzed using a log-rank test. Results with a P-value of less than 0.05 were considered significant. ns: not significant; *P<0.05; **P<0.01; ***P<0.001.
References
Kadioglu, A., Weiser, J.N., Paton, J.C. & Andrew, P.W. The role of Streptococcus pneumoniae virulence factors in host respiratory colonization and disease. Nat. Rev. Microbiol. 6, 288–301 (2008).
van der Poll, T. & Opal, S.M. Pathogenesis, treatment, and prevention of pneumococcal pneumonia. Lancet Lond. Engl. 374, 1543–1556 (2009).
O’Brien, K.L. et al. Burden of disease caused by Streptococcus pneumoniae in children younger than 5 years: global estimates. Lancet Lond. Engl. 374, 893–902 (2009).
Johnson, H.L. et al. Systematic evaluation of serotypes causing invasive pneumococcal disease among children under five: the pneumococcal global serotype project. PLoS Med. 7, pii: e1000348; doi: 10.1371/journal.pmed.1000348 (2010).
Iwakura, Y., Ishigame, H., Saijo, S. & Nakae, S. Functional specialization of interleukin-17 family members. Immunity 34, 149–162 (2011).
Kolls, J.K. & Khader, S.A. The role of Th17 cytokines in primary mucosal immunity. Cytokine Growth Factor Rev. 21, 443–448 (2010).
Cao, J. et al. Activation of IL-27 signalling promotes development of postinfluenza pneumococcal pneumonia. EMBO Mol. Med. 6, 120–140 (2014).
Wilson, R. et al. Protection against Streptococcus pneumoniae lung infection after nasopharyngeal colonization requires both humoral and cellular immune responses. Mucosal. Immunol. 8, 627–639 (2015).
Hayday, A.C. Gammadelta T cells and the lymphoid stress-surveillance response. Immunity 31, 184–196 (2009).
Prinz, I., Silva-Santos, B. & Pennington, D.J. Functional development of γδ T cells. Eur. J. Immunol. 43, 1988–1994 (2013).
Roark, C.L., Simonian, P.L., Fontenot, A.P., Born, W.K. & O’Brien, R. L. gammadelta T cells: an important source of IL-17. Curr. Opin. Immunol. 20, 353–357 (2008).
Sutton, C.E., Mielke, L.A. & Mills, K.H.G. IL-17-producing γδ T cells and innate lymphoid cells. Eur. J. Immunol. 42, 2221–2231 (2012).
Ribot, J.C. et al. CD27 is a thymic determinant of the balance between interferon-gamma- and interleukin 17-producing gammadelta T cell subsets. Nat. Immunol. 10, 427–436 (2009).
Paget, C. et al. CD3bright signals on γδ T cells identify IL-17A-producing Vγ6Vδ1+ T cells. Immunol. Cell Biol. 93, 198–212 (2015).
Kolaczkowska, E. & Kubes, P. Neutrophil recruitment and function in health and inflammation. Nat. Rev. Immunol. 13, 159–175 (2013).
Kopf, M., Schneider, C. & Nobs, S.P. The development and function of lung-resident macrophages and dendritic cells. Nat. Immunol. 16, 36–44 (2015).
Mantovani, A., Cassatella, M.A., Costantini, C. & Jaillon, S. Neutrophils in the activation and regulation of innate and adaptive immunity. Nat. Rev. Immunol. 11, 519–531 (2011).
Hussell, T. & Bell, T.J. Alveolar macrophages: plasticity in a tissue-specific context. Nat. Rev. Immunol. 14, 81–93 (2014).
Nathan, C. Neutrophils and immunity: challenges and opportunities. Nat. Rev. Immunol. 6, 173–182 (2006).
Guarda, G. et al. Differential expression of NLRP3 among hematopoietic cells. J. Immunol. Baltim. Md 1950 186, 2529–2534 (2011).
Karmakar, M. et al. Neutrophil IL-1β processing induced by pneumolysin is mediated by the NLRP3/ASC inflammasome and caspase-1 activation and is dependent on K+ efflux. J. Immunol. Baltim. Md 1950 194, 1763–1775 (2015).
Mankan, A.K., Dau, T., Jenne, D. & Hornung, V. The NLRP3/ASC/Caspase-1 axis regulates IL-1β processing in neutrophils. Eur. J. Immunol. 42, 710–715 (2012).
Bakele, M. et al. Localization and functionality of the inflammasome in neutrophils. J. Biol. Chem. 289, 5320–5329 (2014).
Chow, M.T. et al. Type I NKT-cell-mediated TNF-α is a positive regulator of NLRP3 inflammasome priming. Eur. J. Immunol. 44, 2111–2120 (2014).
Franchi, L., Eigenbrod, T. & Núñez, G. Cutting edge: TNF-alpha mediates sensitization to ATP and silica via the NLRP3 inflammasome in the absence of microbial stimulation. J. Immunol. Baltim. Md 1950 183, 792–796 (2009).
Waters, J.P., Pober, J.S. & Bradley, J.R. Tumour necrosis factor in infectious disease. J. Pathol. 230, 132–147 (2013).
Croft, M. The role of TNF superfamily members in T-cell function and diseases. Nat. Rev. Immunol. 9, 271–285 (2009).
Mizgerd, J.P. Acute lower respiratory tract infection. N. Engl. J. Med. 358, 716–727 (2008).
Barletta, K.E. et al. Leukocyte compartments in the mouse lung: distinguishing between marginated, interstitial, and alveolar cells in response to injury. J. Immunol. Methods 375, 100–110 (2012).
Sutton, C.E. et al. Interleukin-1 and IL-23 induce innate IL-17 production from gammadelta T cells, amplifying Th17 responses and autoimmunity. Immunity 31, 331–341 (2009).
José, R.J. et al. Regulation of neutrophilic inflammation by proteinase-activated receptor 1 during bacterial pulmonary infection. J. Immunol. Baltim. Md 1950 194, 6024–6034 (2015).
Ma, J. et al. Morphine disrupts interleukin-23 (IL-23)/IL-17-mediated pulmonary mucosal host defense against Streptococcus pneumoniae infection. Infect. Immun. 78, 830–837 (2010).
Lamkanfi, M. & Dixit, V.M. Mechanisms and functions of inflammasomes. Cell 157, 1013–1022 (2014).
Gross, O., Thomas, C.J., Guarda, G. & Tschopp, J. The inflammasome: an integrated view. Immunol. Rev. 243, 136–151 (2011).
Karmakar, M., Sun, Y., Hise, A.G., Rietsch, A. & Pearlman, E. Cutting edge: IL-1β processing during Pseudomonas aeruginosa infection is mediated by neutrophil serine proteases and is independent of NLRC4 and caspase-1. J. Immunol. Baltim. Md 1950 189, 4231–4235 (2012).
Schreiber, A. et al. Neutrophil serine proteases promote IL-1β generation and injury in necrotizing crescentic glomerulonephritis. J. Am. Soc. Nephrol. JASN 23, 470–482 (2012).
Coll, R.C. et al. A small-molecule inhibitor of the NLRP3 inflammasome for the treatment of inflammatory diseases. Nat. Med. 21, 248–255 (2015).
McNeela, E.A. et al. Pneumolysin activates the NLRP3 inflammasome and promotes proinflammatory cytokines independently of TLR4. PLoS Pathog. 6, e1001191 (2010).
Itohara, S. et al. Homing of a gamma delta thymocyte subset with homogeneous T-cell receptors to mucosal epithelia. Nature 343, 754–757 (1990).
Shibata, K. et al. Notch-Hes1 pathway is required for the development of IL-17-producing γδ T cells. Blood 118, 586–593 (2011).
Karmakar, M., Katsnelson, M.A., Dubyak, G.R. & Pearlman, E. Neutrophil P2X7 receptors mediate NLRP3 inflammasome-dependent IL-1β secretion in response to ATP. Nat. Commun. 7, 10555 (2016).
Chen, K.W. et al. The murine neutrophil NLRP3 inflammasome is activated by soluble but not particulate or crystalline agonists. Eur. J. Immunol. 46, 1004–1010 (2016).
Martel-Gallegos, G. et al. Human neutrophils do not express purinergic P2X7 receptors. Purinergic Signal. 6, 297–306 (2010).
Vaughan, K.R. et al. Inhibition of neutrophil apoptosis by ATP is mediated by the P2Y11 receptor. J. Immunol. Baltim. Md 1950 179, 8544–8553 (2007).
Harder, J. et al. Activation of the Nlrp3 inflammasome by Streptococcus pyogenes requires streptolysin O and NF-kappa B activation but proceeds independently of TLR signaling and P2X7 receptor. J. Immunol. Baltim. Md 1950 183, 5823–5829 (2009).
Bezbradica, J.S., Coll, R.C. & Schroder, K. Sterile signals generate weaker and delayed macrophage NLRP3 inflammasome responses relative to microbial signals. Cell. Mol. Immunol. (2016).
Wertz, I.E. & Dixit, V.M. Regulation of death receptor signaling by the ubiquitin system. Cell Death Differ. 17, 14–24 (2010).
Michel, O., Dinh, P.H.D., Doyen, V. & Corazza, F. Anti-TNF inhibits the airways neutrophilic inflammation induced by inhaled endotoxin in human. BMC Pharmacol. Toxicol. 15, 60 (2014).
Mathews, J.A. et al. γδ T cells are required for pulmonary IL-17A expression after ozone exposure in mice: role of TNFα. PloS One 9, e97707 (2014).
Muñoz, N. et al. Mucosal administration of flagellin protects mice from Streptococcus pneumoniae lung infection. Infect. Immun. 78, 4226–4233 (2010).
Oh, H., Siano, B. & Diamond, S. Neutrophil isolation protocol. J. Vis. Exp. JoVE 23, pii:745 doi:10.3791/745 (2008).
Acknowledgements
C.P., J.-C.S., and C.F. were supported by INSERM. B.R. and F.T. were supported by CNRS. Work in M.L.’s laboratory is supported by VIB, Ghent University (BOF 01N02313, BOF 01J11113, BOF14/GOA/013), the Fund for Scientific Research-Flanders (grants G030212N and G011315N), and the European Research Council (grant 281600). M.H. was the recipient of a doctoral fellowship from the AZM foundation. E.C.P. was supported by a post-doctoral fellowship from the French Institute of cancer (INCa). Dr Isabelle Wolowczuk (CIIL, Institut Pasteur, Lille) is acknowledged for critical reading of this manuscript. We thank Nathalie Messéant and Bruno Couvreur for mouse husbanding. We also thank Aurélie Maillard, Pauline Chenuet, and the BICeL flow cytometry core facility for technical assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declared no conflict of interest.
Additional information
SUPPLEMENTARY MATERIAL is linked to the online version of the paper
Supplementary information
Rights and permissions
About this article
Cite this article
Hassane, M., Demon, D., Soulard, D. et al. Neutrophilic NLRP3 inflammasome-dependent IL-1β secretion regulates the γδT17 cell response in respiratory bacterial infections. Mucosal Immunol 10, 1056–1068 (2017). https://doi.org/10.1038/mi.2016.113
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/mi.2016.113
This article is cited by
-
IL-17 and IL-17-producing cells in protection versus pathology
Nature Reviews Immunology (2023)
-
Divergent metabolic programmes control two populations of MAIT cells that protect the lung
Nature Cell Biology (2023)
-
The immune system view of the coronavirus SARS-CoV-2
Biology Direct (2020)
-
Interleukin-7 protects against bacterial respiratory infection by promoting IL-17A-producing innate T-cell response
Mucosal Immunology (2020)
-
NAIP/NLRC4 inflammasome activation in MRP8+ cells is sufficient to cause systemic inflammatory disease
Nature Communications (2017)