Abstract
Crohn’s disease and ulcerative colitis, the two major forms of inflammatory bowel diseases (IBDs), are characterized by high levels of IL-22 production. Rodent studies revealed that this cytokine is protective during colitis but whether this is true in IBDs is unclear. We show here that levels of the soluble inhibitor of IL-22, interleukin 22-binding protein (IL-22BP), are significantly enhanced during IBDs owing to increased numbers of IL-22BP-producing eosinophils, that we unexpectedly identify as the most abundant source of IL-22BP protein in human gut. In addition, using IL-22BP-deficient rats, we confirm that endogenous IL-22BP is effective at blocking protective actions of IL-22 during acute colitis. In conclusion, our study provides new important insights regarding the biology of IL-22 and IL-22BP in the gut and indicates that protective actions of IL-22 are likely to be suboptimal in IBDs thus making IL-22BP a new relevant therapeutic target.
Similar content being viewed by others
Introduction
Etiology of inflammatory bowel diseases (IBDs) is still not fully understood but it is now widely acknowledged that they result from inappropriate and/or deregulated immune responses to the commensal gut microbiota, in genetically predisposed individuals and under the influence of environmental lifestyle factors.1 Both Crohn’s disease (CD) and ulcerative colitis (UC) are characterized by intestinal inflammation and epithelial injury and are mediated by shared and distinct inflammatory pathways involving cytokines.1
IL-22 is an IL-10 cytokine family member2 strongly expressed in both CD and UC.3, 4, 5, 6 IL-22 is induced by various environmental and endogenous signals,2 such as IL-23. Importantly, the identification of IL23R as a susceptibility gene by several genome-wide association studies suggests an important role for this pathway in IBDs.5 In IBDs, IL-22 is mostly produced by CD4+ T lymphocytes7 and group 3 innate lymphoid cells (ILC3).8 IL-22 binds to a heterodimeric membrane receptor (IL-22R) whose expression is restricted to epithelial cells,2 making IL-22 an important mediator between immune and epithelial systems. Mouse models showed a crucial role for IL-22 in the restoration of intestinal homeostasis during acute inflammatory colitis.6, 9, 10, 11, 12, 13 IL-22 protective actions include first, a reinforcement of epithelium barrier function through inducing antimicrobial peptides (AMPs) expression in epithelial cells,12 mucus production by goblet cells6 and restitution of epithelial tight junctions;4 second, an enhancement of epithelial wound healing through IL-22-induced survival and proliferation of epithelial cells.10 However, exacerbated or uncontrolled actions of IL-22 also sustain pathological conditions, as illustrated in psoriasis and colorectal cancer.2 IL-22 is thought to be controlled by a soluble, specific, and potent inhibitor called IL-22-binding protein (IL-22BP).14, 15
In a colorectal cancer model, IL-22BP was shown to prevent long-lasting proliferative actions of IL-22 on malignant epithelial cells.16 In rodent gut, the source of IL-22BP appeared to be a subset of immature conventional dendritic cells (cDCs), namely CD103+ CD11b+ DCs.17 In addition, we showed that retinoic acid is a potent inducer of its expression in human DCs.17 However, how IL-22BP is regulated during IBDs has never been assessed and whether its endogenous production impairs IL-22 actions during colitis is unclear. Given the strength and specificity of the IL-22BP-mediated control on IL-22 actions, addressing these issues is important for understanding the relevance of elevated levels of IL-22 observed in IBDs.
In this study, we show that eosinophils are the most important source of IL-22BP in human healthy gut and contribute to an overproduction of IL-22BP in the inflamed mucosa of IBDs patients. Using IL-22BP-deficient rats, we provide strong evidences that these findings are of pathophysiological relevance as we demonstrate for the first-time that endogenous IL-22BP inhibits the protective actions of IL-22 during experimental colitis.
Results
IL-22BP production is enhanced during inflammation in Crohn’s disease and ulcerative colitis
IL-22 expression is known to be strongly induced in the inflamed colonic mucosa of both CD and UC patients,3, 4, 5, 6 but its role in human IBDs is still not well understood. Moreover, expression of IL-22BP, which specifically binds to- and potently inhibits IL-22, has never been assessed. We thus analyzed the expression of the complete IL-22/IL-22R/IL-22BP axis in paired biopsies from sites with endoscopically inflamed and uninflamed mucosa of colonic CD and UC patients (Table 1 for clinical data). Quantitative real-time PCR (RT-qPCR) analyses confirmed a strong expression of IL-22 in the inflamed mucosa of both IBDs, whereas almost no detection was observed in uninvolved mucosa or in controls (Figure 1a). No noticeable variation of the expression of IL-22R1, the specific chain of the IL-22 membrane receptor, was detected across all groups (Figure 1b). On the contrary, we observed a significant upregulation of IL-22BP mRNA expression in both IBDs (Figure 1c). Concordantly, much more IL-22BP-producing cells were observed in the inflamed mucosa of both CD and UC patients than in controls (Figure 1d,e). Interestingly, RT-qPCR analyses on a small group of non-IBD colitis patients revealed a downregulation of IL-22BP expression in the inflamed mucosa as compared to controls, suggesting that IL-22BP induction observed in IBDs does not extend to all forms of colitis (Supplementary Figure S1 online). Taken together, our results indicate that the strong induction of IL-22 in IBDs is associated with an overproduction of IL-22BP, its specific soluble inhibitor. Increased IL-22BP production in IBDs was rather unexpected as: 1. Previous works reported a downregulation of IL-22BP in mouse models of dextran sulfate sodium (DSS)-induced acute colitis;6, 16 2. We reported that cDCs are the major source of IL-22BP in rodent gut during steady state and that maturation induces dramatic downregulation of IL-22BP expression in rodent and human DCs.17 Our data from IBDs patients thus suggest that different and/or additional cellular sources of IL-22BP might exist in human gut.
IL-22BP production is enhanced during inflammation in Crohn’s disease and ulcerative colitis. Interleukin (IL)-22 (a), IL-22R1 (b) and IL-22BP (c) gene expressions were analyzed by quantitative real-time PCR (RT-qPCR) in colon biopsies from controls (n=16) and paired biopsies of inflamed (Inf) and uninflamed (He) areas of CD (n=14) and UC (n=14) patients. IL-22BP gene expression was normalized to hypoxanthine-guanine phosphoribosyltransferase (HPRT) by the 2−ΔΔCt method of relative quantification. (d) IL-22BP immunohistochemistry of sections from the same inflamed areas of CD (n=10) and UC (n=11) patients, and from controls (n=5). Representative IL-22BP+ cells are designated by black arrows. (e) IL-22BP+ cells were scored in a blinded manner from 10 HPF. Each symbol represents a single patient. ***P<0.001; **P<0.01; *P<0.05; HPF, high power field; HPRT, hypoxanthine-guanine phosphoribosyltransferase; IL, interleukin; NS, non-significant; RT-qPCR, quantitative real-time PCR.
Eosinophils are the major IL-22BP-producers in human gut
The unexpected overexpression of IL-22BP observed in IBDs prompted us to fully characterize IL-22BP cellular sources in human gut. We first assessed IL-22BP mRNA expression in healthy colonic mucosa cells obtained from patients undergoing colectomy for colon cancer. Colon cells were separated into epithelial cells (EpCAM+) and lamina propria (LP) leukocytes, which were then FACS sorted (Figure 2a). Monocyte-derived dendritic cells were used as a positive control for IL-22BP mRNA expression.16, 17 Very low, if any, IL-22BP expression was detected in epithelial cells, lymphocytes, CD11clow/neg cells and neutrophils (Figure 2b). In contrast, high levels were found in the cDC+macrophage fraction. We previously showed in rodent that IL-22BP expression was restricted to the CD103+ CD11b+ subset of cDCs17 and these findings were recently extended to human gut mononuclear phagocytes by another group.18 Thus the high expression of IL-22BP by gut cDCs found in rodents also holds true in human. However, RT-qPCR analyses unexpectedly revealed that, in addition to cDCs, substantial levels of IL-22BP mRNA are also expressed in eosinophils (Figure 2b). High purity of sorted cells ruled out possible contaminations by cDCs (Supplementary Figure S2A). This was further corroborated by a more detailed phenotypic analysis of eosinophils (Supplementary Figure S2B). Of note, no SIGLEC-8 expression was detected in cDCs and macrophages (data not shown). May-Grünwald Giemsa staining of sorted cells also confirmed the typical morphology of eosinophils with bright purplish-red granules and bi-lobed nuclei (Figure 2c).19 Both cDCs and eosinophils preferentially expressed isoform two of IL-22BP mRNA (Figure 2d), that is the only one binding to and inhibiting IL-22.20 Of note, some expression of the long isoform 1 was also detected in both populations.
Analysis of IL-22BP mRNA in isolated human normal colonic mucosa cells. (a) Gating and sorting strategies used to isolate colonic lamina propria cell populations. (b) IL-22BP gene expression was analyzed by RT-qPCR. Bars represent the mean±s.e.m. ratio of IL-22BP gene to HPRT expression as determined by the 2−ΔΔCt method of relative quantification (n=3). (c) May-Grünwald Giemsa staining of sorted eosinophils (d) IL-22BP gene expression was analyzed by RT-PCR using primers allowing amplification of whole IL22RA2 mRNA. The lower band corresponds to isoform 2 (expected length 678 bp). Data are representative of two independent experiments. DCs, dendritic cells; Ep cells, epithelial cells; HPRT, hypoxanthine-guanine phosphoribosyltransferase; IL, interleukin; MΦ, macrophages; RT-qPCR, quantitative real-time PCR.
We then characterized IL-22BP protein expression in human gut. We showed for the first time that IL-22BP protein is constitutively expressed in the ileal and colonic mucosa (Figure 3a). Specificity of the staining was confirmed by isotype control mAb and pre-incubation of the anti-IL-22BP mAb with recombinant IL-22BP protein (Supplementary Figure S3), as well as by western blot (data not shown). In both the ileum and the colon, IL-22BP+ cells are scattered throughout the LP (Figure 3a). IL-22BP staining located in the cytoplasm which is also concordant with the secreted nature of this protein. Most of IL-22BP+ cells displayed a bi-lobed nucleus, a distinctive feature of eosinophils (Figure 3b). The large majority of IL-22BP+ cells was indeed CD13+ CD11c+ CD117− HLA-DR− (Supplementary Figure S4A–D) and expressed the major basic protein (MBP) which is specifically localized in secondary granules of eosinophils (Figure 3c).19 These results were confirmed with a second anti-IL-22BP mAb (Supplementary Figure S5 and data not shown). Additional stainings showed that IL-22BP+ cells were also CD45+ CD11b+ CD16− CD14− CD3− CD4− CD8− Tryptase− and NKp46− (data no shown). Some rare IL-22BP+ HLA-DR+ DC-SIGN+ (CD209) cells likely corresponding to cDCs were also detected (Supplementary Figure S4E and data not shown). Taken together, these results indicate that eosinophils, that represent between 10 and 30% of gut LP leukocytes (Figure 2a),19 and therefore largely outnumber cDCs, likely constitute the major source of IL-22BP in human gut mucosa at steady state.
Immunofluorescence analysis of IL-22BP in normal human gut. (a) Formalin-fixed frozen sections of normal human ileum (left) and colon (right) were stained with anti-IL-22BP mAb (red) and DAPI (blue). Original magnification × 200. Data are representative of at least four independent experiments on different donors. (b) Higher magnification of IL-22BP+ cells in the ileum (upper) and colon (lower). (c) Double-immunofluorescence stainings of human ileum (upper) and colon (lower) with anti-MBP mAb (green), anti-IL-22BP mAb (red) and DAPI (blue). Original magnification × 200. Data are representative of at least three independent experiments on different donors. IL, interleukin; MBP, major basic protein.
As eosinophils numbers are known to be increased in UC and CD lesions,21 we hypothesized that the overproduction of IL-22BP observed in these diseases (Figures 1 and 4a) may results from increased numbers of IL-22BP-poducing eosinophils in the inflamed mucosa. Indeed, phenotypic analysis revealed that the large majority of IL-22BP+ cells in CD and UC were also MBP+ (Figure 4b,c) and therefore eosinophils. Again, only rare IL-22BP+ HLA-DR+ cells were detected (Supplementary Figure S6) suggesting that inflammatory DCs do not represent an important source of IL-22BP either.
IL-22BP is produced by eosinophils in IBDs. Formalin-fixed frozen sections of colonic inflamed areas of CD patients (left) and UC (right) were stained with (a) anti-IL-22BP mAb (red) and DAPI or (b,c) anti-IL-22BP mAb (red), anti-MBP mAb (green) and DAPI. Data are representative of at least four independent experiments on different donors. Original magnification × 200 (a,b) and × 630 (c). CD, Crohn’s disease; IBDs, inflammatory bowel diseases; MBP, major basic protein.
Altogether, our results unexpectedly indicate that eosinophils are the major producers of IL-22BP in human gut, in both non-inflammatory and inflammatory conditions, thus explaining the increased expression of IL-22BP detected in IBDs.
IL-22BP-deficient rats are protected during acute DSS colitis
Upregulation of IL-22BP production in IBDs is of pathophysiological relevance as it indicates that despite a strong production of IL-22 in inflamed mucosa, its actions on epithelial cells are unlikely to be fully effective due to impairment by its soluble inhibitory receptor. Thus, even if we observed interspecies differences regarding IL-22BP cellular sources between human and rodents, it remained important to prove that endogenous IL-22BP production could effectively inhibit IL-22 actions in vivo during gut inflammation. A previous report indicated that mouse models are unsuitable to address this question because IL-22BP is strongly downregulated before the induction of IL-22 during the DSS model of acute colitis, and consequently does not impair protective actions of IL-22.16 Induction of colitis with DSS is also possible in the rat22 (Supplementary Figure S7A–E) and interestingly, kinetics analyses revealed that, despite a conserved inverse pattern of IL-22 and IL-22BP expression, strong levels of cDCs-, but not granulocytes-derived, IL-22BP were present at time of IL-22 induction (Figure 5a and b and Supplementary Figure S8). This suggested that studying IL-22BP inhibitory actions during DSS-induced acute colitis may be more relevant in the rat, at least in the early phases. We thus generated IL-22BP-deficient (Il22ra2−/−) rats. In line with a previous report in mice,16 Il22ra2−/− rats were healthy and reproduced normally. Phenotypic analyses did not reveal modifications of major immune cells in the spleen and the gut at steady state (Supplementary Figure S9A). Histological analysis of the different gut segments was normal and colon length was similar to Il22ra2+/+ littermate control animals (Supplementary Figure S9B,C and data not shown). The absence of detectable IL-22BP expression was confirmed in colon and mesenteric lymph nodes (Supplementary Figure S9D,E).
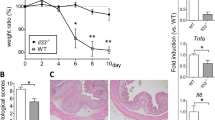
IL-22BP-deficient rats are protected during acute DSS colitis. (a,b) Colitis was induced by 5.5% DSS ad libitum in the drinking water for 7 days. DSS was then switched to regular water for 13 more days. Rats were sacrificed at indicated time points for IL-22 (a) and IL-22BP (b) gene expression analysis by RT-qPCR. Bars represent the mean±s.e.m. ratio of IL-22 or IL-22BP genes to HPRT expression as determined by the 2−ΔΔCt method of relative quantification. Pooled data from two independent experiments (day 0, n=8; days 4, 8, 12 and 20, n=6). (c) Il22ra2+/+ and Il22ra2−/− were given 5.5% DSS ad libitum in the drinking water for 7 days. DSS was then switched to regular water for 3 more days and rats were sacrificed at day 10.Weight and clinical score were assessed every day during colitis. Each symbol represents mean of % weight change or clinical score±s.e.m.. Pooled data from six independent experiments (Il22ra2+/+, n=29; Il22ra2−/−, n=23). (d) Histology score was assessed at day 10 in a blinded manner by a trained pathologist. (e) Representative HES staining of Il22ra2+/+ (left) and Il22ra2−/− (right) rats colon at day 10. (f) Colon length measured at day 10. For d–f, data are representative of at least two independent experiments. Each symbol corresponds to one rat. **P<0.01; ***P<0.001. DSS, dextran sulfate sodium; HPRT, hypoxanthine-guanine phosphoribosyltransferase; IL, interleukin; RT-qPCR, quantitative real-time PCR.
Consistent with the hypothesis that protective actions of IL-22 during DSS colitis6, 9, 10, 11, 12 could be unleashed in the absence of IL-22BP, Il22ra2−/− rats presented a much more attenuated form of colitis as compared with Il22ra2+/+ controls. Indeed, whereas Il22ra2+/+ animals started to lose weight at day 5, Il22ra2−/− only presented a small reduction of weight gain rates (Figure 5c). In addition, disease activity index (DAI) were similar in both groups during the first 3 days of colitis, but started to be significantly more severe in Il22ra2+/+ at day 4, coinciding with the peak of IL-22 expression (Figure 5a,c). DAI in both groups peaked at day 7 but Il22ra2−/− rats rapidly recovered and were almost completely cured at day 10 (Figure 5c). Accordingly, histopathological analyses revealed severe signs of colitis in Il22ra2+/+ rats at day 10, with important inflammatory infiltrates, large epithelial ulcerations, crypt dysplasia, and loss of goblet cells, whereas only mild to no signs were present in Il22ra2−/− animals (Figure 5d,e). Finally, colons were significantly longer in the Il22ra2−/− group (Figure 5f). Altogether these results indicate that, despite a similar induction in both groups during the first 3 days, Il22ra2−/− rats rapidly control the colitis and present an accelerated recovery phase as compared with Il22ra2+/+ animals. Slowing down of disease progression in Il22ra2−/− rats coincided with strong IL-22 induction at day 4 (Figure 5a), whereas for Il22ra2+/+ it started at day 8, coinciding with the removal of DSS and the downregulation of IL-22BP expression (Figure 5b). This suggested that the lack of inhibitory control by IL-22BP during early phases of the colitis allowed more rapidly efficient protective actions of IL-22, leading to attenuated colitis and faster recovery in IL-22BP-deficient rats. Importantly, because IL-22 plays major homeostatic functions in the gut that contribute to shape the microbiota and may influence DSS colitis susceptibility, as illustrated by the colitis prone microbiota that develops in Il22−/− mice,23 it also remained possible that protection of IL-22BP-deficient rats actually relied on a different microbiota composition that would be protective against DSS colitis. To exclude this possibility, we performed co-housing experiments that did not reveal any difference in the course of DSS colitis in between relative co-housed and separately housed rats (Supplementary Figure S10).
IL-22BP deficiency is associated with a more efficient colonic epithelial barrier function during acute colitis
IL-22 protective actions during acute colitis include increased production of mucus and restoration of goblet cells,6 enhanced AMPs production,12 and epithelial cells proliferation.10 IL-22 expression induction was similar in the colon of Il22ra2+/+ and Il22ra2−/− rats during DSS colitis (Figure 6a) but we hypothesized that protection of Il22ra2−/− rats should be associated with an enhancement of known IL-22 protective effects in the colon because of the lack of negative control exerted by IL-22BP. Consistently, mRNA levels of the two IL-22-inducible AMPs LCN2 and beta-defensin 2 (BD2) were significantly higher in Il22ra2−/− vs. Il22ra2+/+ rats during acute colitis (Figure 6b,c). More MUC2 expression (Figure 6d) and complete restoration of goblet cells and mucus production were also selectively observed in Il22ra2−/− animals (Figure 6e). Finally, epithelial cells proliferation was increased in IL-22BP-deficient rats (Figure 6f). Increased actions of IL-22 were not related to higher levels of IL-22R expression by epithelial cell in Il22ra2−/− rats during the course of the colitis as kinetics analyses did not reveal significant variation of Il22ra1 gene expression (data not shown). These properties of IL-22 are important in preventing constitutive bacterial translocations from ileum Peyer’s patches.24 Similarly, bacterial containment may also be part of the beneficial role of IL-22 during acute colitis. Concordantly, we found significantly less bacterial growth in the liver of Il22ra2−/− rats during DSS-induced colitis (Figure 6g), accounting for less bacterial translocations. Taken together, these results indicate that the blockade of IL-22 by IL-22BP is effective during acute colitis and impairs the protective actions of the cytokine on the colonic epithelial barrier.
IL-22BP deficiency is associated with a more efficient barrier function of the colon of during acute colitis. (a) Il22ra2+/+ and Il22ra2−/− were given 5.5% DSS ad libitum in the drinking water for 7 days. DSS was then switched to regular water. Rats were sacrificed at indicated time points for IL-22 gene expression analysis by RT-qPCR. (b) Lipocalin-2, (c) rat beta-defensin-2 and (d) Muc2 gene expressions were analyzed by RT-qPCR at day 6 of colitis. Bars represent mean±s.e.m. of indicated gene expression relative to HPRT expression as determined by the 2−ΔΔCt method of relative quantification. Pooled data from two independent experiments (Il22ra2+/+ n=9; Il22ra2−/− n=10). (e) Representative Alcian blue staining of Il22ra2+/+ (left) and Il22ra2−/− (right) rats colon at day 10 of colitis. Quantification of goblet cells at day 10. Bars represent mean±s.e.m. of goblet cells per field from two different fields at a magnification of x100. Pooled data from two independent experiments (Il22ra2+/+ n=8; Il22ra2−/− n=8). (f) Representative Ki-67 staining of Il22ra2+/+ (left) and Il22ra2−/− (right) rats colon at day 6. (g) Bars represent mean±s.e.m. of colony-forming units (CFU) present in homogenates from the liver at day 6. Pooled data from two independent experiments (Il22ra2+/+ n=9; Il22ra2−/− n=9). ***P<0.001; **P<0.01; *P<0.05; DSS, dextran sulfate sodium; HPRT, hypoxanthine-guanine phosphoribosyltransferase; IL, interleukin; NS: non-significant; RT-qPCR, quantitative real-time PCR.
Discussion
With this study we provide new insights regarding the biology of IL-22 and its soluble inhibitor IL-22BP. We show that the constitutive production of IL-22BP in colon is significantly increased in the inflamed mucosa of IBDs patients owing to eosinophils production that we unexpectedly identified as the major source of the protein in human gut. We also evidence the pathophysiological relevance of these findings with a first-time demonstration that endogenous IL-22BP inhibits actions of IL-22 during colitis.
High levels of IL-22 expression in IBDs have been first described 10 years ago.3 Even if IL-22 production was initially ascribed to T cells,3, 7, 25, 26 recent evidence suggest that the major source of the cytokine may actually be ILC3, in response to microbiota-induced production of IL-23 and IL-1β by a subset of CD14+ CX3CR1+ mononuclear phagocytes27 expanded in the gut of IBDs patients.28 In vitro experiments indicated that the known protective effects of ILC3-derived IL-22 observed during mouse colitis at inducing AMPs and mucus synthesis as well as tissue repair6, 9, 10, 11, 12, 13 are also effective on human gut epithelial cells.4, 29 However, whether these beneficial actions fully take place in human IBDs is now challenged by our finding that high levels of IL-22BP levels are also present in lesions of both CD and UC patients because of major recruitment of eosinophils,21 that we identify as the major source of IL-22BP in human gut.
The finding that eosinophils, which account for 10–30% of total gut LP leukocytes19 at steady state (Figure 2a) and consequently largely outnumber cDCs, represent the most abundant source of IL-22BP in human gut mucosa was unexpected as we and others did not previously identified them as IL-22BP-producers in rodents,16, 17 although analysis of Flt3−/− and Flt3l−/− mice suggested that other sources of IL-22BP than cDCs could exist.17 The reasons for these differences are unclear and would deserve furthers studies. It is possible that rodent gut eosinophils only express preformed IL-22BP protein without mRNA expression as already described for MBP.30 However, none of the currently available antibodies to rodent IL-22BP allowed us to convincingly identify IL-22BP protein in vivo. Because human eosinophils do not express IL-22BP mRNA or protein in the blood,17 its expression is likely influenced by specific factors in the gut environment, similarly to what we described previously for cDCs.17 Consequently, another hypothesis would be that housing facilities, which markedly influence gut microbiota composition and mucosal immune system functions,31 as illustrated by segmented filamentous bacteria-dependent Th17 cells differentiation,32, 33 may not promote expression of IL-22BP by eosinophils in rodent gut. Diet components also strongly shape the mucosal immune system. Interestingly, Belkaid and colleagues recently identified retinoic acid, the active metabolite of vitamin A, as a critical factor for IL-22-producing ILC3 development in the gut34 and we previously showed that it also potently induces IL-22BP expression in cDCs.17 Retinoic acid modulates eosinophils functions in the gut35 but cultures of human blood or rat colon eosinophils in a retinoic acid-containing medium did not induce IL-22BP expression, nor did the culture with important molecules for gastrointestinal eosinophils biology (CCL11, IL-5, IL-3, and GM-CSF) or TLRs ligands (data no shown). Therefore, the molecular signals inducing IL-22BP expression in human gut lamina propria eosinophils remain to be identified. Also, interspecies differences between rodent and human eosinophils exist36 and thus cannot be ruled out regarding IL-22BP expression. Finally, even if the DSS model mimics some important pathogenic aspects of human IBDs like epithelial barrier defects and microbiota dependency for instance, the lack of adaptive immunity involvement illustrates well it cannot fully recapitulate all pathophysiological mechanisms in IBDs. It is thus also possible that differences in IL-22BP levels between human IBDs and DSS colitis account for specific signals that would not be reproduced in this particular animal model.
Beyond their role in anti-helminthes defenses, eosinophils are now thought to exert important homeostatic functions in the gut and participate in the regulation of intestinal barrier function through interaction with different components of the mucosal immune system.19 A recent report showed that constitutive production of TGF-β by eosinophils is critical for IgA class switching and retinoic acid production by CD103+ cDCs.37 Because accumulating evidences indicate that the constitutive production of IL-22 by ILC3 in the gut is important to control and shape the constitution of the microbiota,23, 24, 38 it is possible that in addition to the previously identified cDCs, eosinophils also regulate the threshold of IL-22 actions on epithelial cells through IL-22BP production, thus participating to the dynamic equilibrium established between the host immune system and the commensal flora.39 One can speculate that reason for this additional level of control assumed by human gut eosinophils might be linked to a higher degree of fluctuation in gut IL-22 production in a wild environment as opposed to controlled animal housing facilities. Moreover, given the protumorigenic roles of IL-22 in supporting malignant epithelial cells proliferation,16, 40 strengthening the control of IL-22 might be beneficial in long-lived species like humans as compared with rodents.
In addition to these emerging functions in gut homeostasis, IL-22 is also a crucial mediator of mucosal healing during acute colitis. Most of the knowledge on IL-22 actions during acute colitis comes from mouse studies. A body of evidence has now accumulated to suggest that IL-22 is exerting important protective actions to promote colonic epithelial barrier reinforcement and regeneration, and thus to suppress colitis. First, DSS and transfer models of colitis are always associated with more severe intestinal inflammation and tissue damage in IL-22-deficient mice.9, 10 Second, blocking IL-22 with neutralizing antibodies in immunocompetent mice delays the recovery phase in the DSS model.6 Third, administering exogenous IL-226 or enhancing its endogenous production11, 13 markedly dampens the severity of colitis, even in the TNBS model in which IL-22 is not induced.11 In the DSS model, mouse studies mostly suggested that IL-22 was important for the recovery phase of the colitis because of the observed delay following anti-IL-22 blockade and lack of difference during early phase.6 Protective actions were thus linked to IL-22-dependent epithelium regeneration through the induction of numerous wound-healing-associated genes and the restoration of goblet cells.6, 10 In spite of this amount of evidence supporting IL-22-protective actions, two studies suggest that in some conditions, IL-22 may actually drive acute colitis.41, 42 In the first one, IL-22 secretion by transferred memory CD45RBlo CD25− CD4+ T cells into Rag1−/− mice induced aberrant proliferation of colon epithelial cells responsible for a mild-to-moderate colitis with mucosal hyperplasia and thickening but no ulceration.41 Preservation of epithelial barrier function in this model probably explains why sources of IL-22 are restricted to T cells whereas in IBDs and other colitis models mentioned above, IL-22 mostly originates from ILC3 in response to IL-23-derived mononuclear phagocytes stimulated by luminal MAMPs after epithelial injury.27 In the second one, the authors showed that injection of anti-CD40 antibodies into Rag1−/− mice induced a colitis that could be slightly dampened but not prevented by IL-22 neutralization.42 Hydrodynamic delivery of an IL-22-encoding plasmid in Il23r−/− Rag1−/− did not induce colitis unless injection of anti-CD40 antibody, further supporting a role for other important pathogenic mechanisms in this model, including IL-23-dependent secretion of IFNγ by NKp46+ RORγt− ex-LTi cells.43 Thus, even if these two studies used highly selected models, they emphasize that actions of IL-22 strongly depend on its cellular origin and microenvironment factors and underline the importance to clearly decipher regulatory mechanisms of the IL-22/IL-22R axis, including IL-22BP.
Concordant with an inhibitory role for IL-22BP on IL-22 actions, we found an accelerated regeneration phase in Il22ra2−/− rats, leading to an almost complete recovery at day 10 (Figure 5c). In addition, we observed early significant clinical differences between Il22ra2−/− and Il22ra2+/+ rats (Figure 5c) associated with higher AMPs production and epithelial cells proliferation during acute colitis, suggesting that endogenous IL-22BP may also impair early protective actions of IL-22. In agreement with this, low to no weight loss was reported when excess of IL-22 levels was induced by administering recombinant IL-22 from day 0, probably because of an overcoming of IL-22BP inhibitory actions.13 The IL-22-dependent induction of AMPs in epithelial cells is important for early host defenses during infectious colitis as it allows bacterial containment apart from the epithelium.44 We found here that lack of IL-22BP correlated with enhanced epithelial-specific AMPs expression during acute colitis and less bacterial translocation likely contributing to reduced systemic effects. Our results demonstrating that endogenous IL-22BP can block IL-22 during acute colitis are contrasting with a previous work using IL-22BP-deficient mice.16 Indeed, in their study, Huber et al., concluded that an early downregulation of IL-22BP expression during DSS colitis occurred before the induction of IL-22 and thus explained the lack of inhibitory effects of IL-22BP. Differences in IL-22 and IL-22BP kinetics that we observed here are likely to explain why IL-22BP exerts inhibitory actions on IL-22 in our study. Reasons for these differences may rely on interspecies differences as further suggested by the fact that Huber et al. did not detect significant constitutive IL-22BP expression in the small intestine in mouse whereas this is the case in rats17 and in human.14 Also, while environmental factors and housing facilities may influence IL-22BP expression in the gut, as suggested by a recent report that did not observe any downregulation during murine acute DSS colitis,45 in our hands, kinetics of IL-22BP was similar to that described by Flavell and coll. in mice from the same genetic background (data not shown). Whatever the reasons, our study prove for the first time that when present in the inflammatory environment, as it the case in human IBDs (Figure 1), endogenous IL-22BP inhibits the protective actions of IL-22 on epithelial cells during acute colitis.
To conclude, our work strongly suggests that overproduction of IL-22BP by colonic eosinophils in the inflamed mucosa of IBDs patients is an important pathophysiological finding as IL-22BP inhibits the protective actions of IL-22 during colitis. Also, even if more characterization on the role of IL-22 in human IBDs should be provided first, our study offers new perspectives regarding potential prospects of therapeutic modulation and indicates that targeting IL-22BP could be a relevant strategy. Enhancing IL-22 protective actions through transiently blocking IL-22BP during early phases of IBDs flares would allow limiting the extent and the duration of gut inflammation that leads to destructive damage. Moreover, Huber et al, proposed that early actions of IL-22 during colitis are important to avoid a delayed colonic repair and increased intestinal inflammation that promotes tumor development.16 Targeting the natural inhibitor of IL-22 would probably constitute a safer approach than IL-22 administration as already suggested11 because it would limit unwanted long-lasting effects of IL-22, that can also lead to malignant epithelial cell proliferation,16, 40 or systemic dissemination and adverse effects such as psoriasis-like skin lesions.46
Methods
Patients. Paired colonic biopsies of involved and uninvolved mucosal areas were obtained from 14 active CD and 14 active UC patients undergoing endoscopy (Table 1 for clinical data). Healthy controls included colonic mucosal biopsy samples from 16 patients screened for dysplasia or colorectal cancer. Biopsies were part of an existing biobank approved by the French Ministry of Education and Research (agreement number DC-2011-1399). For identification of IL-22BP cellular sources, specimens were obtained from patients undergoing resection for colorectal cancer. Normal ileal or colonic tissue samples were taken 10 cm downstream to the tumor. The tissue fragments were processed according to the French guidelines for research on human tissues. Informed patient consent was obtained according to the French bioethics law. Ethical approval was obtained from the local ethics committee.
Animals. Il22ra2−/− and Il22ra2+/+ control littermates rats were generated on the Sprague–Dawley background using zinc-finger nucleases (Sigma-Aldrich, St Louis, MO) at our local Rats Transgenesis Platform facility IBISA-CNRS.47 Zinc-finger nucleases-targeted sequences in the exon 3 of Il22ra2 gene (5′-ACCCATACGAGCCATactatgGGAGGGTGATGATGGCCTG-3′; binding sites are underlined). Pronuclear injections of in vitro-transcribed mRNA-encoding zinc-finger nucleases were performed as previously described.47 Mutations in newborn founders (6 out of 28 founders) were detected by PCR using the following primers: 5′-AGCACGCTGGAAACACTGG-3′, forward; 5′-ATAGCATCAAGCCAGAGAGCAT-3′ reverse. One of the founders that presented a 36 bp deletion of the following sequence: 5′-ACTATGGAGGGTGATGATGGCCTGGGCTGGAAGCT-3′ and a C-insertion instead, leading to a frameshift and a premature stop-codon by the fifth nucleotide in 5′ after the deletion was selected. It was mated with a wild-type partner and heterozygous mutated animals were obtained. Mating between them allowed generation of homozygous Il22ra2−/− rats. Absence of IL-22BP mRNA expression was confirmed by RT-PCR. All animal experiments were performed in accordance with the European Union Guidelines. All animal studies were conducted according to the guidelines of the French Agriculture Ministry. These studies were approved by the Veterinary Departmental Services committee (# E.44011).
DSS-induced colitis. Eight to 10 weeks old Il22ra2+/+ or Il22ra2−/− male rats were given 5.5% DSS (molecular weight: 40,000 g mol−1) (TdB consultancy, Uppsala, Sweden) ad libitum in the drinking water for 7 days. DSS was then switched to regular water until sacrifice. Body mass was monitored every day and a loss of 20% from initial weight was a criteria of sacrifice. Clinical scoring48 was also performed every day. At time of sacrifice, colon lengths were measured and samples were taken for histopathological and RT-qPCR analyses. Colonic sections were stained with hematoxylin and eosin or Alcian blue. Histopathological scoring48 was performed in a blinded manner by a trained pathologist.
Measurement of colony-forming units in liver. Livers were sterilely removed at sacrifice and mechanically homogenized in sterile PBS. Colony-forming units were determined via serial dilutions on LB agar. Colonies were counted after 2 days of culture at 37 °C.
Human colonic lamina propria cells isolation. The mucosa was carefully stripped from the underlying compartment as previously described.49 Epithelial cells were separated by 30 min incubation in a Ca2+ and Mg2+ free solution of EDTA 30 mM under slow rotation (100 r.p.m.). Epithelial cells were then filtered through 100-μm pore size cell strainers and transferred into TRIzol (Invitrogen, Carlsbad, CA) for RT-qPCR analysis. The mucosa was washed in PBS, cut into 5-mm pieces and digested 1 h in 10 ml of collagenase D at 2 mg/ml (Roche Diagnostics, Meylan, France) and DNAse I at 100 μg/ml (Sigma-Aldrich), under 300 r.p.m. rotation and vortexing every 10 min. Cell suspension was then filtered twice through 100-μm and a 40-μm pore size cell strainers successively (BD Biosciences, Le Pont-de-Claix, France) and processed for FACS analysis and/or cell sorting.
Flow cytometry analysis. Colon cells were stained as for cell sorting and phenotyping of eosinophils was performed by switching HLA-DR-FITC for one of the following antibodies: CD14 (clone M5E2/FITC), CD16 (clone 3G8/FITC), CD25 (clone M-A251/FITC), CD23 (clone M-L233/FITC), CD123 (clone 7G3/FITC) all from BD Biosciences; CD117 (clone 104D2D1/PC5.5) and CD11b (clone Bear1/FITC) both from Beckman Coulter (Villepinte, France). Cells were analyzed on a BD FACSCanto II flow cytometer (BD Biosciences). Data were analyzed using FlowJo software (Treestar, Ashland, OR).
Cell sorting of colonic lamina propria cells. Human colon lamina propria cells were stained with antibodies specific for CD45 (clone HI30/V500), CD3 (clone SK7/PECy7), CD19 (clone SJ25C1 /PECy7), CD11c (clone S-HCL-3 /APC), HLA-DR (clone G46-6/FITC) (BD Biosciences) and SIGLEC-8 (clone 7C9/PE) (BioLegend, San Diego, CA). Dead cells were excluded by gating on 4',6-diamidino-2-phenylindole (DAPI)-negative cells. Cell sorting was performed on a BD FACS Aria Cell sorter (BD Biosciences) using the gating strategies defined in the results section.
Real-time quantitative PCR. Total RNA was isolated using TRIzol reagent (Invitrogen) according to manufacturers’ instructions. Reverse transcription was performed using Murine Moloney Leukemia Virus Reverse Transcriptase (Invitrogen) following manufacturer’s instructions. Gene expression was assessed with the TaqMan Fast Advanced Master Mix 2 × reagent (Applied Biosystems, Foster City, CA). Primers and probes were purchased from Applied Biosystems (see Supplementary Table S1 for complete list). Real-time PCR was performed using the StepOne Plus system (Applied Biosystems). For both human and rat, relative expression was normalized to hypoxanthine-guanine phosphoribosyltransferase and calculated using the 2−ΔΔCt method. Results were expressed in arbitrary units.
Whole IL-22BP cDNA amplification. In order to identify IL-22BP isoforms, whole-cDNA amplification was performed (forward, 5′-GGCTTCCTCATCAGTTTCTTCC-3′; reverse 5′-TTCCACACATCTCTCTTCACTTCTC-3′ for human IL-22BP).17 Amplification was performed using HERCULASE II Fusion Enzyme (Agilent, Santa Clara, CA).
Immunohistochemistry. Immunohistochemistry was performed on 5 μm formalin-fixed, paraffin-embedded sections using an indirect immunoperoxidase method. For rat colonic samples sections, were stained with a rabbit anti-Ki-67 pAb (Merck-Millipore, Darmstadt, Germany). For human colonic biopsies, sections were stained with a mouse IgG1 monoclonal anti-IL-22BP antibody (clone 214518; R&D Systems Europe, Lille, France). The immunological reaction was visualized with the Envision detection system (Dako, Les Ulis, France) and 3,3-diaminobenzidine tetrahydrochloride as a chromogen. Slides were counterstained with Mayer's Hematoxylin solution. Signal intensity was scored on a scale from 0 to 3 in a blinded manner by a trained pathologist.
Immunofluorescent stainings. Formalin-fixed frozen sections were saturated 30 min with a solution of PBS/BSA1%/Serum10%. For single staining, mouse monoclonal IgG1anti-IL-22BP (clone 214518, R&D or clone AH22BP9.1, homemade) labeled with the Alexa Fluor 568 Antibody Labeling Kit following manufacturer’s instruction (Invitrogen) was incubated 2 h at room temperature. After washing, DAPI was incubated 15 min. Slides were mounted with ProLong Gold antifade (Invitrogen). For double immunostainings, a second purified antibody was mixed with the anti-IL-22BP antibody except for mouse antibodies of the same isotype which were incubated before the anti-IL-22BP mAb and revealed before a second step of saturation with mouse serum 10%. Purified antibody was revealed with adapted secondary antibodies labeled with Alexa488 (Invitrogen). Images were obtained with A1 R Si Confocal microscope (Nikon, Champigny sur Marne, France).
Statistical analysis. Statistical analysis was performed with GraphPad Prism Software (GraphPad Software, San Diego, CA). Means comparisons of unpaired samples were performed using the Mann–Whitney U-test or the Kruskal–Wallis test with Dunn’s post-test. The Wilcoxon signed-rank test was used for paired samples. P-values <0.05 were considered statistically significant.
References
Kaser, A., Zeissig, S. & Blumberg, R.S. Inflammatory bowel disease. Annu. Rev. Immunol. 28, 573–621 (2010).
Sabat, R., Ouyang, W. & Wolk, K. Therapeutic opportunities of the IL-22-IL-22R1 system. Nat. Rev. Drug. Discov. 13, 21–38 (2013).
Andoh, A. et al. Interleukin-22, a member of the IL-10 subfamily, induces inflammatory responses in colonic subepithelial myofibroblasts. Gastroenterology 129, 969–984 (2005).
Brand, S. et al. IL-22 is increased in active Crohn’s disease and promotes proinflammatory gene expression and intestinal epithelial cell migration. Am. J. Physiol. Gastrointest. Liver Physiol. 290, G827–G838 (2006).
Schmechel, S. et al. Linking genetic susceptibility to Crohn’s disease with Th17 cell function: IL-22 serum levels are increased in Crohn’s disease and correlate with disease activity and IL23R genotype status. Inflamm. Bowel. Dis. 14, 204–212 (2008).
Sugimoto, K. et al. IL-22 ameliorates intestinal inflammation in a mouse model of ulcerative colitis. J Clin Invest 118, 534–544 (2008).
Pariente, B. et al. Activation of the receptor NKG2D leads to production of Th17 cytokines in CD4+ T cells of patients with Crohn’s disease. Gastroenterology 141, 217–226 (2011).
Geremia, A. et al. IL-23-responsive innate lymphoid cells are increased in inflammatory bowel disease. J. Exp. Med. 208, 1127–1133 (2011).
Zenewicz, L.A. et al. Innate and adaptive interleukin-22 protects mice from inflammatory bowel disease. Immunity 29, 947–957 (2008).
Pickert, G. et al. STAT3 links IL-22 signaling in intestinal epithelial cells to mucosal wound healing. J. Exp. Med. 206, 1465–1472 (2009).
Monteleone, I. et al. Aryl hydrocarbon receptor-induced signals up-regulate IL-22 production and inhibit inflammation in the gastrointestinal tract. Gastroenterology 141, 237–248 (2011).
Zindl, C.L. et al. IL-22-producing neutrophils contribute to antimicrobial defense and restitution of colonic epithelial integrity during colitis. Proc. Natl Acad. Sci. USA 110, 12768–12773 (2013).
Mielke, L.A. et al. Retinoic acid expression associates with enhanced IL-22 production by γδ T cells and innate lymphoid cells and attenuation of intestinal inflammation. J. Exp. Med. 210, 1117–1124 (2013).
Xu, W. et al. A soluble class II cytokine receptor, IL-22RA2, is a naturally occurring IL-22 antagonist. Proc. Natl Acad. Sci. USA 98, 9511–9516 (2001).
Logsdon, N.J., Jones, B.C., Josephson, K., Cook, J. & Walter, M.R. Comparison of interleukin-22 and interleukin-10 soluble receptor complexes. J. Interferon Cytokine Res. 22, 1099–1112 (2002).
Huber, S. et al. IL-22BP is regulated by the inflammasome and modulates tumorigenesis in the intestine. Nature 491, 259–263 (2012).
Martin, J.C.J. et al. Interleukin-22 binding protein (IL-22BP) is constitutively expressed by a subset of conventional dendritic cells and is strongly induced by retinoic acid. Mucosal Immunol. 7, 101–113 (2014).
Watchmaker, P.B. et al. Comparative transcriptional and functional profiling defines conserved programs of intestinal DC differentiation in humans and mice. Nat. Immunol. 15, 98–108 (2014).
Jung, Y. & Rothenberg, M.E. Roles and regulation of gastrointestinal eosinophils in immunity and disease. J. Immunol. 193, 999–1005 (2014).
Dumoutier, L., Lejeune, D., Colau, D. & Renauld, J.C. Cloning and characterization of IL-22 binding protein, a natural antagonist of IL-10-related T cell-derived inducible factor/IL-22. J. Immunol. 166, 7090–7095 (2001).
Hogan, S.P., Waddell, A. & Fulkerson, P.C. Eosinophils in infection and intestinal immunity. Curr. Opin. Gastroenterol. 29, 7–14 (2013).
Gaudio, E. et al. Dextran sulfate sodium (DSS) colitis in rats: clinical, structural, and ultrastructural aspects. Dig. Dis Sci. 44, 1458–1475 (1999).
Zenewicz, L.A. et al. IL-22 deficiency alters colonic microbiota to be transmissible and colitogenic. J. Immunol. 190, 2306–2312 (2013).
Sonnenberg, G.F. et al. Innate lymphoid cells promote anatomical containment of lymphoid-resident commensal bacteria. Science 336, 1321–1325 (2012).
Kleinschek, M.A. et al. Circulating and gut-resident human Th17 cells express CD161 and promote intestinal inflammation. J. Exp. Med. 206, 525–534 (2009).
Ramesh, R. et al. Pro-inflammatory human Th17 cells selectively express P-glycoprotein and are refractory to glucocorticoids. J. Exp. Med. 211, 89–104 (2014).
Longman, R.S. et al. CX3CR1+ mononuclear phagocytes support colitis-associated innate lymphoid cell production of IL-22. J. Exp. Med. 211, 1571–1583 (2014).
Kamada, N. et al. Unique CD14 intestinal macrophages contribute to the pathogenesis of Crohn disease via IL-23/IFN-gamma axis. J. Clin. Invest. 118, 2269–2280 (2008).
Begue, B. et al. Defective IL10 signaling defining a subgroup of patients with inflammatory bowel disease. Am. J. Gastroenterol. 106, 1544–1555 (2011).
Nakajima, T. et al. Gene expression screening of human mast cells and eosinophils using high-density oligonucleotide probe arrays: abundant expression of major basic protein in mast cells. Blood 98, 1127–1134 (2001).
Macpherson, A.J. & McCoy, K.D. Standardised animal models of host microbial mutualism. Mucosal Immunol. 8, 476–486 (2014).
Ivanov, I.I. et al. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell 139, 485–498 (2009).
Gaboriau-Routhiau, V. et al. The key role of segmented filamentous bacteria in the coordinated maturation of gut helper T cell responses. Immunity 31, 677–689 (2009).
Spencer, S.P. et al. Adaptation of innate lymphoid cells to a micronutrient deficiency promotes type 2 barrier immunity. Science 343, 432–437 (2014).
Ueki, S. et al. Retinoic acids up-regulate functional eosinophil-driving receptor CCR3. Allergy 68, 953–956 (2013).
Lee, J.J. et al. Human versus mouse eosinophils: ‘that which we call an eosinophil, by any other name would stain as red’. J. Allergy Clin. Immunol. 130, 572–584 (2012).
Chu, V.T. et al. Eosinophils promote generation and maintenance of immunoglobulin-A-expressing plasma cells and contribute to gut immune homeostasis. Immunity 40, 582–593 (2014).
Goto, Y. et al. Segmented filamentous bacteria antigens presented by intestinal dendritic cells drive mucosal Th17 cell differentiation. Immunity 40, 594–607 (2014).
Kamada, N., Seo, S.-U., Chen, G.Y. & Núñez, G. Role of the gut microbiota in immunity and inflammatory disease. Nat. Rev. Immunol. 13, 321–335 (2013).
Kryczek, I. et al. IL-22(+)CD4(+) T cells promote colorectal cancer stemness via STAT3 transcription factor activation and induction of the methyltransferase DOT1L. Immunity 40, 772–784 (2014).
Kamanaka, M. et al. Memory/effector (CD45RB(lo)) CD4 T cells are controlled directly by IL-10 and cause IL-22-dependent intestinal pathology. J. Exp. Med. 208, 1027–1040 (2011).
Eken, A., Singh, A.K., Treuting, P.M. & Oukka, M. IL-23R+ innate lymphoid cells induce colitis via interleukin-22-dependent mechanism. Mucosal Immunol. 7, 143–154 (2014).
Vonarbourg, C. et al. Regulated expression of nuclear receptor RORγt confers distinct functional fates to NK cell receptor-expressing RORγt(+) innate lymphocytes. Immunity 33, 736–751 (2010).
Zheng, Y. et al. Interleukin-22 mediates early host defense against attaching and effacing bacterial pathogens. Nat. Med. 14, 282–289 (2008).
Moriwaki, K. et al. The Necroptosis Adaptor RIPK3 Promotes Injury-Induced Cytokine Expression and Tissue Repair. Immunity 41, 567–578 (2014).
Zelante, T. et al. Tryptophan catabolites from microbiota engage aryl hydrocarbon receptor and balance mucosal reactivity via interleukin-22. Immunity 39, 372–385 (2013).
Geurts, A.M. et al. Knockout rats via embryo microinjection of zinc-finger nucleases. Science 325, 433 (2009).
Maxwell, J.R., Brown, W.A., Smith, C.L., Byrne, F.R. & Viney, J.L. Methods of inducing inflammatory bowel disease in mice. Curr. Protoc. Pharmacol. Chapter 5, Unit 5.58 (2009).
Jarry, A. et al. Mucosal IL-10 and TGF-beta play crucial roles in preventing LPS-driven, IFN-gamma-mediated epithelial damage in human colon explants. J. Clin. Invest. 118, 1132–1142 (2008).
Acknowledgements
We thank Professor DL Baeten (Academic Medical Center, Amsterdam, The Netherlands) for critically reviewing the manuscript. This work was realized in the context of the IHU-Cesti project which received French government financial support managed by the National Research Agency via the “Investment Into The Future” program ANR-10-IBHU-005. The IHU-Cesti project is also supported by Nantes Métropole and the Pays de la Loire Region. This work was also supported by a grant from CHU Nantes (Appel d’offre interne 2013 # RC14_0042) to JM. JM also received support from CHU de Nantes through “Année Supplémentaire d’Internat”. GB was supported by the Région Pays de la Loire through the IMBIO-DC network. AA was supported by a French-tunisian UTIQUE grant from the 2015 Hubert Curien program.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declared no conflict of interest.
Additional information
Author contributions
JM and RJ conceived the project, designed experiments, interpreted the data, and wrote the paper; JM, GB, MH, and AJ designed and performed experiments, interpreted the data; CB performed histological analyses; PH performed confocal microscopy analyses; SM, RT, and IA generated Il22ra2−/− rats; AA, CJ, FH, and AB performed experiments; BL and AB handled the biobank and selected patients; JCR provided reagents.
SUPPLEMENTARY MATERIAL is linked to the online version of the paper
Supplementary information
Rights and permissions
About this article
Cite this article
Martin, J., Bériou, G., Heslan, M. et al. IL-22BP is produced by eosinophils in human gut and blocks IL-22 protective actions during colitis. Mucosal Immunol 9, 539–549 (2016). https://doi.org/10.1038/mi.2015.83
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/mi.2015.83
This article is cited by
-
Population pharmacokinetics and pharmacodynamics of efmarodocokin alfa (IL-22Fc)
Journal of Pharmacokinetics and Pharmacodynamics (2024)
-
Role of IL-22 in intestinal microenvironment and potential targeted therapy through diet
Immunologic Research (2023)
-
TNF hampers intestinal tissue repair in colitis by restricting IL-22 bioavailability
Mucosal Immunology (2022)
-
The good and the bad about separation anxiety: roles of IL-22 and IL-22BP in liver pathologies
Seminars in Immunopathology (2021)
-
Interleukin-22-mediated host glycosylation prevents Clostridioides difficile infection by modulating the metabolic activity of the gut microbiota
Nature Medicine (2020)