Abstract
Antimicrobial peptides are secreted by the intestinal epithelium to defend from microbial threats. The role of human β defensin-1 (hBD-1) is notable because its gene (beta-defensin 1 (DEFB1)) is constitutively expressed and its antimicrobial activity is potentiated in the low-oxygen environment that characterizes the intestinal mucosa. Hypoxia-inducible factor (HIF) is stabilized even in healthy intestinal mucosa, and we identified that epithelial HIF-1α maintains expression of murine defensins. Extension to a human model revealed that basal HIF-1α is critical for the constitutive expression of hBD-1. Chromatin immunoprecipitation identified HIF-1α binding to a hypoxia response element in the DEFB1 promoter whose importance was confirmed by site-directed mutagenesis. We used 94 human intestinal samples to identify a strong expression correlation between DEFB1 and the canonical HIF-1α target GLUT1. These findings indicate that basal HIF-1α is critical for constitutive expression of enteric DEFB1 and support targeting epithelial HIF for restoration and maintenance of intestinal integrity.
Similar content being viewed by others
Introduction
The intestinal epithelium lines the entire gastrointestinal tract, covering a surface area of approximately 300 m2 in the adult human and forming an essential barrier to the outside world. This single layer of epithelium must also defend this expanded interface from invasion by luminal microbiota. The anatomy of the intestine provides a fascinating metabolic profile. For example, it is well documented that a steep oxygen gradient exists from the anaerobic lumen of the intestine across the epithelium into the highly vascularized sub-epithelium. From this perspective, it is perhaps not surprising that the epithelium has evolved a number of features to cope with this metabolic setting. In fact, studies comparing functional responses between epithelial cells from different tissues have revealed that intestinal epithelial cells seem to be uniquely resistant to hypoxia and that an extremely low level of oxygenation within the normal intestinal epithelial barrier (so-called “physiological hypoxia”) may be a regulatory adaptation mechanism to the steep oxygen gradient.1 As a result, the intestinal epithelium has evolved coping mechanisms that include basal regulation of hypoxia-inducible factor (HIF), which is central for aspects of physiology and immunity. For example, iron absorption is dependent on multiple HIF-2α target genes.2 Likewise, epithelial HIF-1α regulates the multidrug resistance gene,3 mucin-3,4 and the intestinal trefoil factor gene5 important in maintenance of epithelial barrier function.
Antimicrobial peptides constitute a substantial part of the mucosal barrier. β-Defensins are the dominant class of antimicrobial peptide secreted by the epithelium. The four well-characterized human β defensins (hBD-1–4, encoded by beta-defensin 1 (DEFB1), DEFB4, DEFB103, and DEFB104) are small (30–47 amino acid), cationic, cysteine-rich peptides that possess broad antimicrobial activity.6, 7 In the gut, hBD-1 has two characteristics that confer prominence. First, its antimicrobial activity is potentiated under reducing conditions that exist in the hypoxic gut lumen, whereas other antimicrobial peptides, such as hBD-3, are diminished in the reduced state.8 Second, expression of hBD-1 is constitutive, whereas other defensins are expressed in response to microbial and inflammatory stimuli.9, 10, 11 Given these properties, it is not surprising that defective expression of hBD-1 is associated with mucosal diseases, such as inflammatory bowel disease,12, 13, 14 candidia carriage,15 periodontitis,16 and dental carries.17 Despite the homeostatic role of HIF in maintaining other aspects of the gut barrier, defensin expression has not previously been linked to HIF signaling.
In the present study, we report that HIF-1α selectively regulates basal murine and human defensin expression. This work identified and characterized a critical role for basal HIF-1α in DEFB1 expression that is dependent on a hypoxia response element (HRE) consensus sequence in the DEFB1 promoter. Because species-specific expression of DEFB1 precludes in vivo models, we used human intestinal samples to correlate the expression between DEFB1 and a canonical HIF-1α target gene. These findings are the first to link HIF to defensin expression and illuminate a mechanism for constitutive DEFB1 expression that provide rationale for targeting epithelial HIF for therapeutic treatment of mucosal disease.
Results
Basal regulation of defensins by HIF in murine colonic epithelia
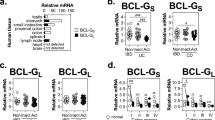
Initially, we confirmed previous observations of “physiological hypoxia” in the murine colon.1 To visualize regions of low pO2 in the colonic mucosa in healthy mice (C57BL/6), we utilized HypoxyProbe-1, a pimonidazole HCl adduct-forming hypoxia marker that enables fluorescent antibody–mediated visualization of tissues experiencing pO2⩽10 mm Hg. As shown in Figure 1a, and consistent with previous observations,18 we observed prominent localization of the pimonidazole adducts in the brush-border epithelial cells that line the colonic mucosa, with graded decreases toward the base of the crypt. From such observations, we sought to determine how basal HIF signaling influences the transcriptional profile of the intestinal epithelium. To do this, we performed gene expression arrays using epithelial-rich mucosal scrapings from mice with a targeted intestinal epithelial Hif1a knock out. This analysis revealed a cluster of 11 defensin-related genes (murine β defensin-6, various defensin cryptdins, and cryptdin-related sequences) that exhibited reduced expression with epithelial Hif1a knock out (Figure 1b).
Physiological hypoxia and basal hypoxia-inducible factor (HIF) maintain mouse and human defensin expression. (a) Pimonidazole HCl forms adducts in tissue experiencing tissue pO2⩽10 mm Hg and enabled immunohistochemical visualization (red) of “physiological hypoxia” in brush-border epithelia lining the colon of healthy C57BL/6J mice. Bar=100 μM. (b) This physiological hypoxia maintains expression of murine defensin genes as evidenced by reduced expression in epithelial scrapings from conditional Hif1a−/−mice vs. control. (c) Human intestinal epithelial cell lysate (Caco-2) contained detectable HIF-1α, even in normoxic conditions, which was increased by overnight incubation in hypoxia. (d) A screen of defensin expression in Caco-2 cells with lentiviral ARNT (HIF-1β) knock down (KD) revealed a role for basal HIF in beta-defensin 1 (DEFB1) expression. Panel b performed with three animals per group. Panel d represents five independent experiments. Error bars represent s.e.m. *P<0.05, **P<0.01, ***P<0.001 as determined by one-way analysis of variance (b) and paired Student’s t-test (d). Sh, short hairpin.
Selective regulation of human DEFB1 by HIF-1α in human intestinal epithelia
Similar to “physiological hypoxia” in vivo, basal HIF-1α is detectable in Caco-2 cells cultured in normoxic conditions (Figure 1c). To determine whether basal HIF contributes to human defensin expression, we generated Caco-2 intestinal epithelial cells with lentiviral knock down of HIF-1β (encoded by ARNT), which serves as the binding partner of HIF-1α and HIF-2α required for the transcriptionally active heterodimer. We then used real-time PCR to screen expression of hBDs in these cells. Although some targets were unchanged (DEFB103 and DEFB104) or undetectable (DEFB4), ARNT knock down significantly reduced expression of human DEFB1 (Figure 1d).
To determine the specificity of DEFB1 regulation by HIF, we used lentiviral knockdown to target either HIF-1α or HIF-2α in Caco-2 as previously published19 and T84 intestinal epithelial cell lines with confirmation of knock down shown in Figure 2a. This analysis revealed that DEFB1 is regulated selectively by HIF-1α in both cell lines (Figure 2b,c). Interestingly, exposure to 1% O2 for 2, 4, or 8 h did not influence DEFB1 expression in wild-type or knockdown cells, suggesting that such regulation is basally controlled. Co-transfection of a plasmid encoding stabilized HIF-1α or HIF-2α, despite marked stimulation of a HRE-containing promoter control (Figure 2d), did not increase DEFB1 promoter activity (Figure 2e).
Hypoxia-inducible factor-1α (HIF-1α) is critical for beta-defensin 1 (DEFB1) expression. Lentiviral HIF-1α and HIF-2α knock down (KD) of Caco-2 (previously validated) and T84 cells (knock down shown in (a)) reveal that loss of HIF-1α, but not HIF-2α, reduced DEFB1 expression in (b) Caco-2 and (c) T84 intestinal epithelial cells. Exposure to hypoxia (1% O2) did not induce DEFB1. (d) Co-transfection of HIF-1α or HIF-2α plasmids containing mutated oxygen-dependent degradation domains with a HRE (hypoxia response element) luciferase reporter plasmid demonstrates the ability of oxygen-stable HIF to stimulate promoter activity. However, (e) HIF plasmid co-transfection did not stimulate DEFB1 promoter activity, possibly due to basal HIF saturation. (f) Ascorbate supplementation (1 mM, 24 h) did not influence cytoplasmic localization of NF-κB (p65). Shown here are three independent samples with (+) and without (−) ascorbate. Regardless, supplementation with the prolyl-hydroxylase cofactor ascorbate (Asc; 1 mM for 24 h) reduced (g) cytoplasmic and nuclear HIF-1α and (h) DEFB1 expression consistent with the model that basal HIF saturates the DEFB1 promoter. All figures (a–h) represent the mean of at least three independent experiments. Error bars represent s.e.m. *P<0.05, ***P<0.0001 as determined by one-way analysis of variance comparing HIF-1α knockdown to baseline short hairpin (Sh) control (b, c) and paired Student’s t-test (c, d). NF, nuclear factor.
To define whether basal HIF-1α activity accounted for the maintenance of DEFB1 expression, we reduced basal activity of HIF and examined DEFB1 expression. To reduce baseline HIF activity, we supplemented cell media with ascorbate (1 mM), a cofactor for the oxygen-dependent hydroxylation of HIF by prolyl-hydroxylase enzymes that initiate degradation.20 Culture of wild-type Caco-2 cells in supplemented media for 24 h had no effect on nuclear factor-κB p65, indicating a degree of specificity for ascorbate-dependent pathways (Figure 2f). Supplementation reduced cytoplasmic and nuclear HIF-1α by ∼25% (Figure 2g) and decreased DEFB1 expression >40% (Figure 2h), suggesting at least some role for basal HIF-1α in DEFB1 expression.
Influence of HIF-1α on hBD-1 protein expression
We next used immunofluorescence staining to determine whether lentiviral-mediated HIF-1α knock down influenced hBD-1 protein expression. As shown in Figure 3a, hBD-1 was expressed diffusely in the cytoplasm of both Caco-2 and T84 cells. Prominent decreases in hBD-1 were noted in epithelial cells lacking HIF-1α relative to either short hairpin controls or epithelial cells lacking HIF-2α (Figure 3a). Likewise, examination of hBD-1 secreted into cell supernatants by enzyme-linked immunosorbent assay revealed a 48±6% decrease in hBD-1 secreted by HIF-1α knockdown cells relative to short hairpin control and HIF-2α knockdown cells (P<0.01, Figure 3b).
Impact of hypoxia-inducible factor-1α (HIF1a) knock down (KD) on functional human β defensin-1 (hBD-1) protein. (a) HIF-1α knock down reduced hBD-1 staining in both Caco-2 and T84 intestinal epithelial cell lines compared with short hairpin (Sh) control or HIF-2α knockdown. (b) HIF-1α knock down also decreased hBD-1 secreted into cell supernatant as measured by enzyme-linked immunosorbent assay. (c) A bacterial killing assay, using Escherichia coli Nissle, which is sensitive to oxidized recombinant hBD-1 and resistant to hyposmotic stress from dilution of salt that inactivates hBD-1, revealed a relevance of a 50% reduction in recombinant hBD-1 on bacterial viability. All figures (a–c) are representative (a) or the mean (b, c) of at least three independent experiments. Error bars represent s.e.m. **P<0.01 as determined by one-way analysis of variance (b). CFU, colony-forming unit; IgG, immunoglobulin G.
To define the functional relevance of a 50% decrease in hBD-1 on bacterial survival, we developed a killing assay with Escherichia coli Nissle 1917. This bacterium was selected because it is sensitive to oxidized recombinant hBD-1 and it is resistant to low osmolarity to which it was subject during experimental incubation with salt-sensitive hBD-1.21 As shown in Figure 3c, increasing concentrations of recombinant hBD-1 (rhBD-1; range 0–8 μg ml−1) revealed marked increase in E. coli killing by rhBD-1 at concentrations >2 μg ml−1. These results indicate that at such concentrations, a 50% decrease in hBD-1 expression could increase the E. coli burden by several orders of magnitude (Figure 3c).
Molecular mechanism of regulation of DEFB1 by HIF-1α
Analysis of the proximal 1.5 kb of the DEFB1 promoter revealed a single HRE consensus sequence at position −463 relative to the transcription start site. We used chromatin immunoprecipitation with Caco-2 cells cultured in normoxic conditions to evaluate binding of HIF-1α to this HRE. As shown n Figure 4a, PCR amplification with HRE-spanning primers demonstrated a 7.8±1.4-fold enrichment over immunoglobulin G (IgG) control following precipitation of sheared DNA with anti-HIF-1α. To experimentally demonstrate the relevance of this HRE, we mutated three bases of the core HRE sequence (depicted in Figure 4b) in a DEFB1 luciferase reporter plasmid. We transfected the wild-type and HRE mutant plasmid into Caco-2 cells and observed a 43±13% decrease in luciferase activity in the mutant plasmid (Figure 4b). Taken together, these studies demonstrate that direct HIF-1α binding to the DEFB1 HRE contributes to the maintenance of DEFB1 expression in intestinal epithelial cells.
Hypoxia-inducible factor-1α (HIF-1α) binds and regulates the beta-defensin 1 (DEFB1) promoter. (a) Chromatin immunoprecipitation with anti-HIF-1α showed DEFB1 promoter enrichment using hypoxia response element (HRE) spanning primers. (b) Mutation of this HRE (MUT) prevented HIF binding and reduced promoter activity compared with wild-type (WT) promoter after transfection into Caco-2 cells. Both figures (a, b) represent the mean of three independent experiments. Error bars represent s.e.m. *P<0.05 as determined by paired Student’s t-test. IgG, immunoglobulin G.
Correlation of DEFB1 expression with canonical HIF-1α target gene in human tissue
There are several challenges to studying DEFB1 regulation by HIF-1α in vivo. First, model organisms, including mice, do not express hBD-1. Amino-acid sequence homology is only 53% comparing mouse with hBD-1, and there is also dissimilarity in promoter sequences.22, 23 Second, because HIF-1α is primarily regulated through post-translational, oxygen-dependent degradation, changes in RNA expression do not reflect cellular protein. Therefore, to test the hypothesis that DEFB1 is a HIF-1α target in human tissue, we used real-time PCR to correlate expression of DEFB1 with a canonical HIF-1α target gene, GLUT1.24 Human intestinal samples were obtained from the TissueScan quantitative PCR gene expression arrays containing cDNA from normal and inflamed human intestinal tissues obtained during surgical resection and biopsy. PCR amplification of both DEFB1 and GLUT1 was detected in 94 of 95 unique patient samples on the arrays. Correlation analysis revealed a significant association between DEFB1 and GLUT 1 expression in these 94 samples (P<0.0001, Figure 5a). DEFB1 and GLUT1 expression are reported to be greater in the large intestine than in the small intestine.25, 26 This raises the possibility that combining samples from both the locations might confound the correlation. Therefore, we correlated samples from the small intestine and the large intestine independently and found that the correlation was not influenced by this distinction (P<0.0001, Figure 5a). We preformed similar analyses with samples from patients with no disease, Crohn’s disease, and ulcerative colitis separately and found a positive correlation independent of disease status (P<0.0001, Figure 5b). Finally, to ensure that the association between DEFB1 and GLUT1 was not an artifact based on varying epithelial content of the samples, we analyzed expression as a function of mucosal fraction. Pathologist reports that accompanied 47 of these samples provide information on the percentage of mucosa, submucosa, and muscle of each specimen. No correlation was found between the percentage of mucosa and DEFB1 or GLUT1, indicating that variation in sample composition cannot explain the correlation of these genes (Figure 5c). Taken together, such findings strongly implicate a common mechanism of regulation for DEFB1 and GLUT1 in the samples tested here. Finally, immunofluorescence staining of hBD-1 in human colonic biopsies revealed positive staining within the epithelium, with greatest intensity in the epithelium adjacent to the lumen and less intensity within the crypt (Figure 5d). This staining pattern mirrors the low pO2 microenvironment of the colon in vivo as revealed by oxygen-sensitive pimonidazole staining (Figure 1a).
Beta-defensin 1 (DEFB1) correlates with a canonical hypoxia-inducible factor-1α (HIF-1α) target in human samples. (a) DEFB1 expression correlates with GLUT1, a canonical HIF-1α target gene, in 94 human samples from the large and small intestine. This correlation was not abolished by analyzing samples from the small and large intestine independently. Likewise, (b) analysis of samples from patients with no disease, Crohn’s disease, or ulcerative colitis revealed persistent correlation. In all, 47 of 94 samples are linked with data from pathology reports regarding sample composition (percentage of mucosa vs. submucosa vs. muscularis). (c) Mucosal fraction (epithelial rich) did not correlate with expression. This indicates that variation in sample composition does not explain the DEFB1-GLUT1 correlation. For each sample, expression of DEFB1 and GLUT1 was normalized to actin (ACTB) by calculating ΔCT (actin CT minus target CT). Analyses were done using the Pearson product-moment correlation test. (d) Immunofluorescence staining for human β defensin-1 in human colonic biopsies demonstrated epithelial localization with greatest intensity in cells adjacent to the lumen and attenuated intensity in cells within the crypt.
Discussion
The functional anatomy of the intestinal mucosa provides a particularly interesting oxygenation profile, wherein even under physiological conditions, the intestinal mucosa experiences profound fluctuations in blood flow, oxygenation, and metabolism.27 Studies comparing functional responses between epithelial cells from different tissues, for example, have revealed that intestinal epithelial cells appear to be uniquely resistant to even severe hypoxia and that low pO2 within the normal intestinal epithelial barrier may be a regulatory adaptation mechanism to the steep oxygen gradient.5 Thus, both the absorptive and barrier properties of the intestinal epithelium are regulated by the availability of O2 in both health and disease.27 Here, we describe a prominent physiological role for low pO2 in the basal regulation of DEFB1 by HIF-1α.
Oxygen-sensitive staining of the mouse colon enabled us to visualize a distinct layer of tissue subjected to low pO2 affecting the colonic epithelium (Figure 1a). This finding confirms previous work18 and underlies our interest in the role of baseline hypoxia and resulting HIF on epithelial immunity. Utilizing an established mouse model wherein intestinal epithelial cells lacked Hif1a expression,18 our expression array identified a provocative cluster of 11 defensin-related genes that were significantly abrogated with loss of epithelial HIF-1α expression. Characterization of these animals have found them more susceptible to experimental colitis, likely related to multiple defects in barrier function.18 The pleiotropic role of intestinal HIF-1α has made it difficult to identify the particular contribution of HIF-regulated defensins in normal physiological function. It is notable that the reduced expression of murine defensin-related genes in mice lacking intestinal epithelial HIF-1α was identified in healthy animals in the absence of hypoxia-inducing insult. This underscores the homeostatic role for basal HIF-1α signaling in the intestinal epithelium. This observation in murine tissues led us to investigate the contribution of HIF to human defensin expression in intestinal epithelia.
Classically, HIF is induced in hypoxic conditions, yet we detected basal HIF-1α in intestinal epithelial cells cultured in normoxic conditions (Figure 1c). Therefore, we sought to determine the role of basal and hypoxia-stabilized HIF on defensin expression in this model. Initial studies indicated that human defensin expression was not changed by exposing cultured epithelia to hypoxia, yet targeted knock down of HIF-1α and HIF-1β significantly decreased DEFB1 mRNA expression. This seemingly paradoxical finding, namely that exposure to even severe hypoxia (1% O2) did not change DEFB1 expression despite the role of HIF-1α in maintenance of DEFB1 expression, is not without precedent. For example, one study analyzed expression array data sets and identified genes differentially expressed in hypoxia that also contained at least one HRE within the proximal promoter. Of the top 200 predicted HIF target genes, only 81 responded to hypoxia in at least 3 of the 6 cell types evaluated.28 This finding provides evidence that a categorical response to hypoxia is not characteristic of all HIF target genes. It is possible that counter-regulatory mechanisms in hypoxia might explain the absence of hypoxia induction of DEFB1. However, the well-characterized mechanism, in which hypoxia-induced microRNA-155 directly suppresses HIF-1α transcript in Caco-2 cells, is unlikely to explain our results because such effects occur after prolonged hypoxia.29 The duration of hypoxia exposure in our experiments (8 h), while sufficient to alter expression of HIF-target genes, obviate concern that microRNA-155 counter-regulation affected our results. To test the hypothesis that the transcriptional DEFB1 response was saturated by basal HIF-1α, we supplemented media with ascorbate to enhance HIF-1α degradation. This maneuver has precedent as an approach to reduce basal HIF.30 As a cofactor for prolyl-hydroxylase enzymes that initiate the oxygen-dependent degradation of HIFα,20 ascorbate supplementation decreased both cytoplasmic and nuclear HIF-1α and DEFB1. Additional evidence for HIF-mediated regulation was provided by chromatin immunoprecipitation assay and functional promoter site-directed mutagenesis of the classic HIF HRE. In conjunction with results demonstrating no response to hypoxia, this finding strongly supports our hypothesis that basal HIF-1α saturates the DEFB1 transcriptional response through classic HIF-mediated molecular mechanisms. Additionally, we found that co-transfection plasmid encoding stabilized HIF-1α or HIF-2α increase activity of a control (HRE-containing promoter reporter) but not the DEFB1 promoter reporter. This enabled us to selectively increase transcriptionally active HIF-1α and HIF-2α independent of hypoxia. These results argue that counter-regulation of HIF in hypoxia is unlikely to explain the absence of DEFB1 induction by hypoxia in our experiments. The inability of oxygen-stable HIF-1α to induce DEFB1 promoter activity is consistent with our conclusion that basal HIF-1α saturates the DEFB1 promoter in these intestinal epithelial cell lines.
Because murine experiments were not tenable for reasons given previously, we sought to examine the relationship between DEFB1 and HIF-1α in human tissue samples. We used GLUT1, a canonical HIF-1α target gene,24 as a proxy because HIF-1α is primarily regulated by post-translational oxygen-dependent degradation; therefore HIF-1α transcript does not reflect the levels of HIF-1α protein. Results from 94 unique tissue samples from various locations in the lower gastrointestinal tract indicated a significant correlation between DEFB1 and GLUT1, consistent with in vitro findings that HIF-1α is critical for DEFB1 expression. Because both DEFB1 and GLUT1 expression are reported to be higher in the large intestine than in the small intestine,25, 26 we analyzed samples from each location separately (Figure 5a) as well as by disease status (Figure 5b), yet these factors did not abolish the correlation nor was the association an artifact of variation in the mucosal content in each sample (Figure 5c). The information on the fraction of mucosa, submucosa, and muscularis was available for 47 of our samples, and this data was preferred over determining expression of epithelial markers, such as EpCAM, that may be influenced by inflammation.31 Furthermore, intensity of immunofluorescence staining of hBD-1 in human colonic biopsies (Figure 5d) correlated with regions of hypoxic epithelium identified by oxygen-sensitive staining in the mouse colon. Taken together, these observational analyses support our hypothesis that intestinal HIF-1α is critical for DEFB1 expression in vivo.
These correlations also imply that, in the human intestine, HIF-1α signaling is within a dynamic range where DEFB1 expression could be influenced. If so, this adds to growing interest in targeting epithelial HIF as a therapeutic target for restoration and maintenance of barrier integrity.32, 33, 34 Moreover, from the perspective of pathophysiology, transcriptional regulation by HIF-1α may represent a mechanism whereby DEFB1 could be induced when a tissue normally exposed to an oxygen-rich environment becomes hypoxic. One potential organ is the lung, where DEFB1 was recently found to be inversely associated with functional measures in chronic obstructive pulmonary disease.35 It is tempting to speculate that upregulation of DEFB1 in the diseased lung is mediated by hypoxia and HIF-1α, but this remains to be determined in future studies.
In summary, we show, for the first time, that basal HIF-1α is critical for the constitutive expression of DEFB1 in intestinal epithelial cells and that DEFB1 correlates with a canonical HIF-1α target in the human intestine. These findings exemplify a unique epithelial response to physiological hypoxia, support therapeutic targeting of epithelial HIF, and provide the basis for investigating the DEFB1–HIF-1α relationship in other tissues.
Methods
Animals. HypoxyProbe-1 was used to visualize tissue hypoxia according to the manufacturer’s instructions (Hypoxyprobe, Burlington, MA) in healthy 8-week-old C57BL/6J mice. Intestinal epithelial HIF-1α null mice were generated using Cre-lox technology (Fabpl-Cre and Hif1aloxP/loxP mice) on a C57BL/6-129/SvJ background as previously described.18 RNA was isolated from colonic mucosal scrapings (three animals per group), reverse transcribed, and hybridized with the PGA mouse v1.1 chip. Experiments were approved by the Institutional Animal Care and Use Committee at the University of Colorado Denver in compliance with National Institutes of Health guidelines for use of live animals.
Cell culture. Caco-2 (ATCC# HTB-37) and T84 (ATCC# CCL-248) human intestinal epithelial cells were obtained from ATCC (Manassas, VA) and maintained in 95% air with 5% CO2 at 37 °C according to ATCC’s instructions. Hypoxia (1% O2) exposure was performed using a humidified O2 control glove box at 37 °C (Coy Labs, Grass Lake, MI) by displacement with 5% CO2, 95% N2. Ascorbate supplementation was done with L-ascorbic acid 2-phosphate sesquimagnesium salt hydrate (Sigma-Aldrich, St Louis, MO), a comparatively stable form that accumulates as ascorbate intracellularly.36 Lentiviral particles encoding a panel of short hairpin RNAs directed against human HIF-1β, HIF-1α or HIF-2α (MISSION TRC, Functional Genomics, University of Colorado, Boulder, CO) were used to transduce Caco-2 cells using standard protocols as described previously.37
Transcriptional analysis. TRIzol (Invitrogen, Grand Island, NY) was used to isolate RNA from cultured cells. iScript cDNA Synthesis Kit (Bio-Rad Laboratories, Hercules, CA) and SYBR Green (Applied Biosystems, Warrington, UK) were used for real-time PCR analysis using the following primer sequences: β-actin (sense 5′-CCTGGCACCCAGCACAAT, antisense 5′-GCCGATCCACACGGAGTACT), DEFB1 (sense 5′-ATACTTCAAAAGCAATTTTCCTTTAT, antisense 5′-TTGTCTGAGATGGCCTCAGGTGGTAAC), GLUT1 (sense 5′-CTTTGTGGCCTTCTTTGAAGT, antisense 5′-CCACACAGTTGCTCCACAT).
Protein analysis. Secreted hBD-1 was measured using an enzyme-linked immunosorbent assay kit capable of relative quantification of hBD-1 (Adipo Bioscience, Santa Clara, CA). HIF-1α was measured after nuclear and cytoplasmic extraction using the NE-PER kit (Thermo Scientific, Rockford, IL) using the Meso Scale Discovery Total HIF-1 α Kit (Gaithersburg, MD). Sample protein was quantified for normalization using the Pierce BCA Protein Assay Kit (Thermo Scientific). Western blotting performed as previously described38 with whole-cell lysate after overnight hypoxia/normoxia using mouse anti-human HIF-1α antibody 610959 (BD Biosciences, San Jose, CA).
Immunofluorescence. Cells were grown to confluence on coverslips, fixed with ice-cold 100% ethanol, permeabilized with 0.2% Triton X 100 in phosphate-buffered saline, blocked with 5% normal goat serum and 1% bovine serum albumin in phosphate-buffered saline. Primary antibodies were rabbit polyclonal IgG anti-hBD-1 (AbD Serotec, Raleigh, NC) and mouse monoclonal IgG anti-human ZO-1 (Invitrogen) both diluted to 1:100 in blocking solution. Slides were washed three times in phosphate-buffered saline, and secondary antibodies (Alexa Fluor 555 goat anti-mouse IgG and Alexa Fluor 488 goat anti-rabbit IgG (Invitrogen)) were used in a 1:500 dilution in blocking solution before mounting with ProLong Gold antifade (Invitrogen). The rabbit polyclonal IgG anti-hBD-1 (AbD Serotec) and Alexa Fluor 488 goat anti-rabbit IgG (Invitrogen) were used at the same concentrations for staining human colonic biopsy tissue.
Bacterial killing assay. E. coli Nissle 1917 (kind gift of Paul Cohen) was grown in Luria Broth. Cultures in log-phase growth were diluted 1000 × in deionized water to reduce salt, which inhibits the antimicrobial activity of hBD-1.21 Bacteria were incubated for 1 h at 37 °C with recombinant hBD-1 (ProSpec, East Brunswick, NJ) at a final concentration of 8, 4, 2, 1, 0.5, and 0.25 μg ml−1. In all, 10 μl samples were plated in serial dilutions on agar in triplicate, grown at 37 °C for until colonies were visible for enumeration of colony-forming units.
Promoter experiments. Chromatin-immunoprecipitation was performed with Caco-2 cells maintained in normoxic conditions as previously described.39 Rabbit polyclonal anti-HIF-1α antibody NB-100-134 (Novus Biologicals, Littleton, CO) was used for immunoprecipitation. Amplification with both conventional and real-time PCR was done using HRE-spanning primers (sense 5′-TTCTGAGGAGTGCCCTTTGG, antisense 5′-CGGCTCTAAGCTGGTGTTGG). DEFB1 promoter luciferase reporter plasmid (SwitchGear Genomics, Menlo Park, CA) was used with the QuickChange Lightning site-directed mutagenesis kit (Agilent Technologies, Santa Clara, CA) with the following primers: sense 5′-TTTCTGAGGAGTGCCCTTTG and antisense 5′-TAAGCTGGTGTTGGCCTCTT. Plasmid was transfected into subconfluent wild-type Caco-2 cells (six-well plate; 1 μg per well) using Fugene HD transfection reagent (Promega, Madison, WI) and luciferase activity determined at 48 h. Experiments using co-transfection of stabilized HIF-1α and HIF-2α (containing a mutated oxygen-dependent degradation domain) were performed with 200 ng per well ΔODD HIF-1α or HIF-2α plasmid and 1 ugper well DEFB1 promoter luciferase reporter plasmid or HRE-containing luciferase reporter plasmid as control (kind gift of Dan Theodorescu).40
Human tissue. De-identified human intestinal tissue cDNA was obtained using the TissueScan Crohn’s/Colitis I and II arrays (OriGene Technologies, Rockville, MD). Of 95 cDNA samples, 94 amplified with reverse transcriptase–PCR corresponding to 45 female and 49 males, age range 19–89 years. Distribution of diagnoses was 12 normal, 35 Crohn’s, and 47 colitis. Analysis of samples by classification of no disease, Crohn’s disease, and ulcerative colitis included 90 total samples because 4 samples from patients with “colitis” were not specified as Crohn’s or ulcerative colitis. Complete patient/sample characteristics are accessible from the company website (www.origene.com). Immunofluorescence staining of hBD-1 as described above was performed on human colon biopsy tissue obtained under University of Colorado Denver approved protocols.
Statistical analysis. GraphPad Prism 5 (GraphPad Software, La Jolla, CA) was used to create figures and perform statistical analyses, including one-way analysis of variance with Tukey’s post-hoc test, paired Student’s t-test, and Pearson product-moment correlation coefficient.
References
Colgan, S.P. & Taylor, C.T. Hypoxia: an alarm signal during intestinal inflammation. Nat. Rev. Gastroenterol. Hepatol. 7, 281–287 (2010).
Mastrogiannaki, M., Matak, P., Keith, B., Simon, M.C., Vaulont, S. & Peyssonnaux, C. HIF-2alpha, but not HIF-1alpha, promotes iron absorption in mice. J. Clin. Invest. 119, 1159–1166 (2009).
Comerford, K.M., Wallace, T.J., Karhausen, J., Louis, N.A., Montalto, M.C. & Colgan, S.P. Hypoxia-inducible factor-1-dependent regulation of the multidrug resistance (MDR1) gene. Cancer Res. 62, 3387–3394 (2002).
Louis, N.A., Hamilton, K.E., Canny, G., Shekels, L.L., Ho, S.B. & Colgan, S.P. Selective induction of mucin-3 by hypoxia in intestinal epithelia. J. Cell. Biochem. 99, 1616–1627 (2006).
Furuta, G.T. et al. Hypoxia-inducible factor 1-dependent induction of intestinal trefoil factor protects barrier function during hypoxia. J. Exp. Med. 193, 1027–1034 (2001).
Pazgier, M., Hoover, D.M., Yang, D., Lu, W. & Lubkowski, J. Human beta-defensins. Cell. Mol. Life Sci. 63, 1294–1313 (2006).
Ganz, T. Defensins: antimicrobial peptides of innate immunity. Nat. Rev. Immunol. 3, 710–720 (2003).
Schroeder, B.O. et al. Reduction of disulphide bonds unmasks potent antimicrobial activity of human beta-defensin 1. Nature 469, 419–423 (2011).
Harder, J., Bartels, J., Christophers, E. & Schroder, J.M. Isolation and characterization of human beta -defensin-3, a novel human inducible peptide antibiotic. J. Biol. Chem. 276, 5707–5713 (2001).
O'Neil, D.A. et al. Expression and regulation of the human beta-defensins hBD-1 and hBD-2 in intestinal epithelium. J. Immunol. 163, 6718–6724 (1999).
Zhao, C., Wang, I. & Lehrer, R.I. Widespread expression of beta-defensin hBD-1 in human secretory glands and epithelial cells. FEBS Lett. 396, 319–322 (1996).
Peyrin-Biroulet, L. et al. Peroxisome proliferator-activated receptor gamma activation is required for maintenance of innate antimicrobial immunity in the colon. Proc. Natl. Acad. Sci. USA 107, 8772–8777 (2010).
Kocsis, A.K. et al. Association of beta-defensin 1 single nucleotide polymorphisms with Crohn's disease. Scand. J. Gastroenterol. 43, 299–307 (2008).
Wehkamp, J. et al. Inducible and constitutive beta-defensins are differentially expressed in Crohn's disease and ulcerative colitis. Inflamm. Bowel Dis. 9, 215–223 (2003).
Jurevic, R.J., Bai, M., Chadwick, R.B., White, T.C. & Dale, B.A. Single-nucleotide polymorphisms (SNPs) in human beta-defensin 1: high-throughput SNP assays and association with Candida carriage in type I diabetics and nondiabetic controls. J. Clin. Microbiol. 41, 90–96 (2003).
Schaefer, A.S. et al. A 3' UTR transition within DEFB1 is associated with chronic and aggressive periodontitis. Genes Immun. 11, 45–54 (2010).
Ozturk, A., Famili, P. & Vieira, A.R. The antimicrobial peptide DEFB1 is associated with caries. J. Dent. Res. 89, 631–636 (2010).
Karhausen, J., Furuta, G.T., Tomaszewski, J.E., Johnson, R.S., Colgan, S.P. & Haase, V.H. Epithelial hypoxia-inducible factor-1 is protective in murine experimental colitis. J. Clin. Invest. 114, 1098–1106 (2004).
Keely, S. et al. Hypoxia-inducible factor-dependent regulation of platelet-activating factor receptor as a route for gram-positive bacterial translocation across epithelia. Mol. Biol. Cell 21, 538–546 (2010).
Flashman, E., Davies, S.L., Yeoh, K.K. & Schofield, C.J. Investigating the dependence of the hypoxia-inducible factor hydroxylases (factor inhibiting HIF and prolyl hydroxylase domain 2) on ascorbate and other reducing agents. Biochem. J. 427, 135–142 (2010).
Goldman, M.J., Anderson, G.M., Stolzenberg, E.D., Kari, U.P., Zasloff, M. & Wilson, J.M. Human beta-defensin-1 is a salt-sensitive antibiotic in lung that is inactivated in cystic fibrosis. Cell 88, 553–560 (1997).
Morrison, G.M. et al. Mouse beta defensin-1 is a functional homolog of human beta defensin-1. Mamm. Genome 9, 453–457 (1998).
Huttner, K.M., Kozak, C.A. & Bevins, C.L. The mouse genome encodes a single homolog of the antimicrobial peptide human beta-defensin 1. FEBS Lett. 413, 45–49 (1997).
Wood, S.M. et al. Selection and analysis of a mutant cell line defective in the hypoxia- inducible factor-1 alpha-subunit (HIF-1alpha). Characterization of hif- 1alpha-dependent and -independent hypoxia-inducible gene expression. J. Biol. Chem. 273, 8360–8368 (1998).
Yoshikawa, T., Inoue, R., Matsumoto, M., Yajima, T., Ushida, K. & Iwanaga, T. Comparative expression of hexose transporters (SGLT1, GLUT1, GLUT2 and GLUT5) throughout the mouse gastrointestinal tract. Histochem. Cell Biol. 135, 183–194 (2011).
Malik, A.N. & Al-Kafaji, G. Glucose regulation of beta-defensin-1 mRNA in human renal cells. Biochem. Biophys. Res. Commun. 353, 318–323 (2007).
Taylor, C.T. & Colgan, S.P. Hypoxia and gastrointestinal disease. J. Mol. Med. (Berl) 85, 1295–1300 (2007).
Benita, Y., Kikuchi, H., Smith, A.D., Zhang, M.Q., Chung, D.C. & Xavier, R.J. An integrative genomics approach identifies hypoxia inducible factor-1 (HIF-1)-target genes that form the core response to hypoxia. Nucleic Acids Res. 37, 4587–4602 (2009).
Bruning, U. et al. MicroRNA-155 promotes resolution of hypoxia-inducible factor 1alpha activity during prolonged hypoxia. Mol. Cell. Biol. 31, 4087–4096 (2011).
Nytko, K.J., Maeda, N., Schlafli, P., Spielmann, P., Wenger, R.H. & Stiehl, D.P. Vitamin C is dispensable for oxygen sensing in vivo. Blood 117, 5485–5493 (2011).
Gires, O. et al. Tumor necrosis factor alpha negatively regulates the expression of the carcinoma-associated antigen epithelial cell adhesion molecule. Cancer 92, 620–628 (2001).
Cummins, E.P. et al. The hydroxylase inhibitor dimethyloxalylglycine is protective in a murine model of colitis. Gastroenterology 134, 156–165 (2008).
Hindryckx, P. et al. Hydroxylase inhibition abrogates TNF-alpha-induced intestinal epithelial damage by hypoxia-inducible factor-1-dependent repression of FADD. J. Immunol. 185, 6306–6316 (2010).
Robinson, A., Keely, S., Karhausen, J., Gerich, M.E., Furuta, G.T. & Colgan, S.P. Mucosal protection by hypoxia-inducible factor prolyl hydroxylase inhibition. Gastroenterology 134, 145–155 (2008).
Andresen, E., Gunther, G., Bullwinkel, J., Lange, C. & Heine, H. Increased expression of beta-defensin 1 (DEFB1) in chronic obstructive pulmonary disease. PLoS One 6, e21898 (2011).
Vislisel, J.M., Schafer, F.Q. & Buettner, G.R. A simple and sensitive assay for ascorbate using a plate reader. Anal. Biochem. 365, 31–39 (2007).
Keely, S. et al. Hypoxia-inducible factor-dependent regulation of platelet-activating factor receptor as a route for gram-positive bacterial translocation across epithelia. Mol. Biol. Cell 21, 538–546 (2009).
MacManus, C.F., Campbell, E.L., Keely, S., Burgess, A., Kominsky, D.J. & Colgan, S.P. Anti-inflammatory actions of adrenomedullin through fine tuning of HIF stabilization. FASEB J. 25, 1856–1864 (2011).
Rosenberger, P., Khoury, J., Kong, T., Weissmuller, T., Robinson, A.M. & Colgan, S.P. Identification of vasodilator-stimulated phosphoprotein (VASP) as an HIF-regulated tissue permeability factor during hypoxia. FASEB J. 21, 2613–2621 (2007).
Sheta, E.A., Trout, H., Gildea, J.J., Harding, M.A. & Theodorescu, D. Cell density mediated pericellular hypoxia leads to induction of HIF-1alpha via nitric oxide and Ras/MAP kinase mediated signaling pathways. Oncogene 20, 7624–7634 (2001).
Acknowledgements
This work was supported by National Institutes of Health grants F30DK096709 (C.J.K.), TL1RR025778 (C.J.K.), DK50189 (S.P.C.), HL60569 (S.P.C.), DK095491 (S.P.C.) and by grants from the Crohn’s and Colitis Foundation of America.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declared no conflict of interest.
Rights and permissions
This work is licensed under the Creative Commons Attribution-NonCommercial-No Derivative Works 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/3.0/
About this article
Cite this article
Kelly, C., Glover, L., Campbell, E. et al. Fundamental role for HIF-1α in constitutive expression of human β defensin-1. Mucosal Immunol 6, 1110–1118 (2013). https://doi.org/10.1038/mi.2013.6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/mi.2013.6
This article is cited by
-
Hypoxia: molecular pathophysiological mechanisms in human diseases
Journal of Physiology and Biochemistry (2022)
-
Betulinic acid hydroxamate prevents colonic inflammation and fibrosis in murine models of inflammatory bowel disease
Acta Pharmacologica Sinica (2021)
-
Ability of prebiotic polysaccharides to activate a HIF1α-antimicrobial peptide axis determines liver injury risk in zebrafish
Communications Biology (2019)
-
Proteolytic Degradation of reduced Human Beta Defensin 1 generates a Novel Antibiotic Octapeptide
Scientific Reports (2019)
-
Hydrogen-water ameliorates radiation-induced gastrointestinal toxicity via MyD88’s effects on the gut microbiota
Experimental & Molecular Medicine (2018)