Abstract
CD30 ligand (CD30L, CD153), a member of the tumor necrosis factor (TNF) superfamily, and its receptor CD30 are important for differentiation and activation of CD4+ T helper type 17 (Th17) cells. In this report, we demonstrate that the interleukin 17A (IL-17A)-producing γδ T cells normally developed in the fetal thymus, whereas Vγ1−Vγ4− γδ T cells expressed Vγ6/Vδ1 gene transcript selectively decreased in mucosa-associated tissues in naive CD30KO or CD30LKO mice. Moreover, CD30 and CD30L were expressed preferentially by Vγ1−Vγ4− γδ T cells in naive mice. The bacteria clearance was attenuated by the impaired response of the IL-17A-producing γδ T cells and decreased infiltration of neutrophils in CD30KO or CD30LKO mice. In vivo administration of agonistic anti-CD30 monoclonal antibody restored the ability of protection against Listeria monocytogenes by enhancing Vγ1−Vγ4− γδ T cells producing IL-17A not only in wild-type but also CD30LKO mice. Taken together, it appears that CD30L/CD30 signaling plays an important role in the maintenance and activation of IL-17A-producing γδ T cells presumably bearing Vγ6 in the mucosa-associated tissues of mice.
Similar content being viewed by others
INTRODUCTION
The γδ T cells are the first lymphocytes to develop in the thymus. The γδ T cells expressing Vγ5, Vγ6, and Vγ4 (T cell receptor (TCR) nomenclature by Dr S. Tonegawa)1 sequentially develop in this order in the fetal thymus from approximately embryonic day 12 (ED12) and migrate into mucosal epithelia such as skin, intestine, uterus, and lung as tissue-associated cells.1, 2, 3 Unlike conventional T cells, which are exported from the thymus as naive cells and acquire effector functions upon antigen encounter in the periphery, many murine γδ T cells are functionally committed into effector cells producing cytokines within the fetal thymus and are disproportionately distributed in mucosal epithelia.1, 4, 5, 6, 7, 8 We have previously reported that freshly isolated γδ T cells from fetal thymus produce interleukin 17A (IL-17A) in response to phorbol myristate acetate (PMA) and ionomycin stimulation and the number of such IL-17A-producing γδ T cells in the thymus peaks at the perinatal period and gradually decreases with age.9 In the periphery, Vγ6+ IL-17A-producing γδ T cells were predominantly detected in the peritoneal cavity, lamina propria of the gut, and female reproductive organs (reproductive intraepithelial lymphocytes).9 In marked contrast, no Vγ5+ γδ T cells in the skin (skin intraepithelial lymphocytes) or Vγ7+ γδ T cells in the intestinal intraepithelial lymphocytes produced IL-17A. Thus, there is a difference in the frequency of IL-17A-producing cells in γδ T cells depending on the TCR V repertoire and anatomical location in the periphery.
Several members of the tumor necrosis factor (TNF) receptor superfamily (TNFRSF) and TNF superfamily (TNFSF) have been shown to regulate the fate of T cells, including αβ T cells and γδ T cells.10, 11, 12 Members of the TNFR superfamily such as Fas and TNFRI, which contain a death domain in the cytoplasmic tail, are responsible for activation-induced T-cell death, whereas those having no death domain such as CD40 and CD70 play a key role in effective T-cell immunity.10, 11, 12 CD30 ligand (CD30L, CD153) is a 40-kDa type II membrane-associated glycoprotein belonging to the TNFSF10, 11 and is expressed on activated and memory CD4+ T helper (Th) cells. It should be noted that there are several lines of evidence for expression in macrophages, dendritic cells, B cells, and unique CD4+CD3−CD11c− accessory cells, which are the adult equivalent of inducer cells for the development of lymph node and Peyer’s patch in ontogeny.13, 14, 15 CD30, the receptor for CD30L, and belonging to the TNFRSF, was reported to be preferentially expressed by activated/memory Th cells but not by resting B or T cells.10, 11 CD30L/CD30 signaling is thought to be involved in Th2 cell responses and Th2-associated diseases.16, 17, 18 However, a number of recent studies suggest that CD30L/CD30 signaling is also linked to Th1 cell responses and Th1-associated diseases.19, 20, 21 Furthermore, there are several lines of evidence in which naturally occurring regulatory T cells suppressed allograft rejection mediated by memory CD4+ T cells via a CD30-dependent mechanism.22, 23 We recently found that CD30L/CD30 signaling executed by T/T cell interaction plays a critical role in Th1 and Th17 cell differentiation in vitro and in vivo.24, 25, 26 Thus, CD30L/CD30 signaling might not be directly linked to a physiological step for the differentiation of a specific Th cell subset but might be important for amplification and/or activation of effector and/or memory Th cells.
We show here that naturally occurring IL-17A-producing γδ T cells normally develop in the fetal thymus in CD30LKO or CD30KO mice, whereas Vγ1−Vγ4− γδ T cells expressing Vγ6/Vδ1 gene transcript are selectively reduced in the mucosa-associated tissues of adult CD30LKO or CD30KO mice. The numbers of bacteria were significantly larger in the peritoneal cavity at the early stage of Listeria monocytogenes infection accompanied by reduced infiltration of neutrophils and γδ T cells producing IL-17A. Taken together, it appears that CD30L/CD30 signaling plays an important role in the maintenance and activation of a subset of naturally occurring IL-17A-producing γδ T cells in mucosa-associated tissues.
RESULTS
CD30L/CD30 signaling is not required for intrathymic differentiation of γδ T cells
Recently, it has been shown that γδ T cells could be generated and differentiated within the fetal thymus into unconventional IL-17A- or interferon-γ (IFN-γ)-producing effector γδ T cells, which are considered as naturally occurring γδ T cells.6, 7, 8, 9 Therefore, we first examined the role of CD30L/CD30 signaling in intrathymic differentiation of naturally occurring γδ T cells, using CD30LKO or CD30KO mice. Thymocytes were isolated from the fetal thymus of CD30LKO, CD30KO, or wild-type (WT) mice on ED19. As shown in Supplementary Figure S1a and b online, no difference was detected in the cell number of fetal thymocytes, αβ+CD3hi cells, or γδ+CD3hi cells among CD30KO, CD30LKO, and WT mice. The Vγ repertoire of γδ T cells in the fetal thymus at ED19 was assessed by staining with anti-Vγ1, Vγ4, or Vγ5 monoclonal antibodies (mAbs). Consistent with previous reports,1, 2, 27, 28 the γδ T cells in the fetal thymus mainly consisted of Vγ4+, Vγ5+, and Vγ4−Vγ5− γδ T-cell subsets. No differences were found in the frequency and absolute number of Vγ1+, Vγ4+, Vγ5+, or Vγ1−Vγ4−Vγ5− γδ T-cell subsets in the fetal thymus of CD30KO or CD30LKO mice and WT mice (Supplementary Figure S1c online). Upon stimulation with PMA/inonomycin, intracellular cytokine fluorescence-activated cell sorting (FACS) analysis revealed no differences in the proportion of naturally occurring γδ T cells capable of producing IFN-γ or IL-17A in the fetal thymus between WT mice and CD30KO or CD30LKO mice. There were no differences in the Vγ repertoires of IL-17A- or IFN-γ-producing γδ T-cell subsets in the fetal thymus between CD30KO or CD30LKO mice and WT mice (Supplementary Figure S1d and e online). We also found no difference in the number of γδ T cells producing IL-17A or IFN-γ in the adult thymus (Supplementary Figure S2 online). Therefore, these results suggest that CD30L/CD30 signaling is not necessary for intrathymic differentiation of naturally occurring γδ T cells.
CD30L/CD30 signaling selectively contributes to maintenance of Vγ1−Vγ4− γδ T cells in mucosa-associated tissues of naive mice
It has been reported that γδ T cells are exported from the fetal thymus as effector γδ T cells and maintained in peripheral tissues such as the uterus, intestine, or peritoneal cavity of naive mice.9, 27, 28, 29, 30, 31 Therefore, we investigated the role of CD30L/CD30 signaling in the maintenance of γδ T cells in peripheral tissues. There was no difference in the number of γδ T cells in these peripheral tissues, such as peritoneal exudate cells (PECs), lamina propria lymphocytes (LPLs), uterus, liver, lung, and spleen between CD30KO or CD30LKO mice and WT mice (Figure 1a). The Vγ repertoire analysis of γδ T-cell subsets in these peripheral tissues was examined by a flow cytometer with available mAbs against Vγ1 and Vγ4. It was found that the major γδ T cell subset consisted of Vγ1−Vγ4− subsets in PECs, LPLs, and uterus. Furthermore, the frequency and absolute number of Vγ1−Vγ4− γδ T- cell subsets significantly decreased in only these tissues, but not in spleen, liver, and lung of CD30KO and CD30LKO mice relative to WT mice (Figure 1b and Supplementary Figure S3a online). In contrast, the frequency but not the cell number of the Vγ1+ or Vγ4+ γδ T-cell subset was relatively higher in PECs, LPLs, or uterus of CD30KO or CD30LKO mice than that in WT mice (Figure 1b). To identify the Vγ repertoire of Vγ1−Vγ4− γδ T-cell subsets, we examined the TCR V repertoire of γδ T cells in PECs and LPLs from WT mice by reverse transcriptase–PCR analysis, because mAbs against Vγ6 were not available. As shown in Figure 1c, the Vγ repertoires of γδ T cells in LPLs as well as PECs consist mainly of Vγ1, Vγ2, Vγ4, and Vγ6, but no expression of Vγ5 or Vγ7 was detected, which is consistent with our previous report in which Vγ6 was preferentially expressed by γδ T cells in PECs.32 To further identify the repertoire of Vγ1−Vγ4− subset of γδ T cells, Vγ1−Vγ4− γδ T cells were sorted from PECs and determined by reverse transcriptase–PCR. We found that Vγ1−Vγ4− subset of γδ T cells sorted from PECs expressed Vγ2-, Vγ6-, and Vδ1-specific transcripts (Supplementary Figure S3b online). As Vγ2 transcript is often coexpressed with other Vγ transcripts, and Vδ1 chain is known to pair mainly with Vγ5 or Vγ6,5, 6 this result strongly suggests that Vγ1−Vγ4−γδ T cells expressed Vγ6 chain paired with Vδ1 chain. Next, we found that the mRNA expression levels of Vδ1 and Vγ6 decreased markedly in γδ T cells of PECs from CD30KO or CD30LKO mice when compared with those in WT mice (Figure 1d). Therefore, we speculate that CD30L/CD30 interactions may contribute to the maintenance of Vγ1−Vγ4− γδ T cells in mucosa-associated tissues such as PECs, LPLs, and uterus in naive mice.
CD30 ligand (CD30L)/CD30 signal selectively contributes to maintenance of Vγ1−Vγ4− γδ T cells in peripheral tissues. (a) Absolute number of γδ T cells in spleen, peritoneal exudate cells (PECs), lamina propria lymphocytes (LPLs), liver, lung, and uterus from naive wild-type (WT), CD30KO, or CD30LKO mice. (b) Frequencies and absolute numbers of Vγ1−Vγ4−, Vγ1+, and Vγ4+ subset of γδ T cells in PECs, LPLs, or uterus. Data indicate mean±s.d. (n=8) from a representative in three separate experiments. Statistically significant differences are shown (*P<0.05; **P<0.01; ***P<0.001). The γδ T cells (1–2 × 105) were sorted from PECs and LPLs of naive WT mice (c) or 2–5 × 103 of γδ T cells were isolated from PECs of naive WT, CD30KO, or CD30LKO mice (d) using FACSAria. The T cell receptor (TCR) Vγ or Vδ genes of the γδ T cells were analyzed by reverse transcriptase–PCR (RT–PCR) and agarose gel electrophoresis. The data shown are representative of three independent experiments.
CD30L/CD30 signaling is necessary for maintenance of naturally occurring IL-17A-producing Vγ1−Vγ4− γδ T cells in mucosa-associated tissues
We recently demonstrated that naturally occurring γδ T cells are exported from the thymus as effector γδ T cells and maintained in several peripheral tissues, and that these effector γδ T cells have the ability to produce IL-17A without antigen stimulation.9, 33 We examined the ability of γδ T cells to produce IL-17A or IFN-γ in PECs, LPLs, and uterus of CD30KO or CD30LKO mice after culture for 5 h with or without PMA/ionomycin stimulation. As shown in Figure 2a, except for uterus, ∼13% of γδ T cells in PECs and ∼2% of γδ T cells in LPLs of naive WT mice could produce IL-17A only in the presence of brefeldin A. The frequencies and absolute numbers of IL-17A-producing γδ T cells in PECs and LPLs of naive CD30KO or CD30LKO mice significantly decreased as compared with those in WT mice (Figure 2a). Under PMA/ionomycin stimulation, about 70% of γδ T cells in PECs, 36% of γδ T cells in LPLs, and 85% of γδ T cells in uterus could produce IL-17A in naive WT mice, significantly higher than those in CD30KO or CD30LKO mice (Figure 2a). With no stimulation, IFN-γ-producing γδ T cells were not detected and there were no differences in the frequency and cell number of IFN-γ+ γδ T cells in peripheral tissues under PMA/ionomycin stimulation between WT and CD30KO or CD30LKO mice (Supplementary Figure S4 online). These results suggest that CD30L/CD30 signaling may contribute to the maintenance of naturally occurring IL-17A- but not IFN-γ-producing γδ T cells in the mucosa associated with PECs and LPLs.
CD30 ligand (CD30L)/CD30 signaling is necessary for maintenance of a subset of naturally occurring γδ T cells producing interleukin 17A (IL-17A) in mucosa-associated tissues. Cells were isolated from peritoneal exudate cells (PECs) and lamina propria lymphocytes (LPLs) of naive wild-type (WT), CD30KO, or CD30LKO mice and cultured with or without phorbol myristate acetate (PMA)/ionomycin (P/I) stimulation for 5 h; brefeldin A (BFA) was added for the last 4 h of culture. IL-17A production by γδ T cells was analyzed by intracellular staining. (a) Frequency and absolute number of IL-17A-producing γδ T cells. (b) Repertoire analysis of IL-17A-producing γδ T cells. Data indicate mean±s.d. (n=3–6) from a representative in three separate experiments. Statistically significant differences are shown (*P<0.05; **P<0.01; ***P<0.001).
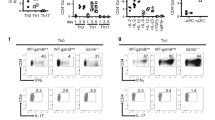
We next analyzed the repertoire of IL-17A-producing γδ T cells in mucosal-associated tissues. As shown in Figure 2b, IL-17A was mainly expressed by Vγ1−Vγ4− presumably bearing Vγ6 γδ T cells in PECs, LPLs, and uterus of naive WT mice in the presence or absence of PMA/ionomycin stimulation, because Vγ1+ γδ T cells only produced IFN-γ but not IL-17A (data not shown). Compared with WT mice, the frequency and cell number of IL-17A+ Vγ1−Vγ4− γδ T cells in PECs and LPLs of naive CD30KO or CD30LKO mice significantly decreased (Figure 2b). There was no significant difference in the percentage of IL-17A+ Vγ4+ γδ T cells in γδ T cells between WT and CD30KO or CD30LKO mice, yet the number of IL-17A+ Vγ4+ γδ T cells was relatively higher only in PECs of CD30KO or CD30LKO mice under PMA/ionomycin stimulation than in WT mice (Figure 2b). These results proved that CD30L/CD30 signaling could specifically expedite the maintenance of the IL-17A-producing Vγ1−Vγ4− γδ T-cell subset in these mucosa-associated tissues such as PECs, LPLs, and uterus of naive mice.
CD30 and CD30L were preferentially expressed by Vγ1−Vγ4− γδ T cells in PECs and LPLs
To demonstrate that CD30L/CD30 interactions are selectively involved in the maintenance of Vγ1−Vγ4− γδ T cells, we first measured whether the mRNA of CD30 or CD30L is expressed in the γδ T cells of PECs and LPLs. It was confirmed that the γδ T cells in PECs and LPLs express both CD30 and CD30L mRNA (Supplementary Figure S5a online). Next, we examined whether CD30 or CD30L is expressed in the surface of γδ T cells. Although the levels of CD30 or CD30L expression were very low (approximately 1.1% of CD30 and 1.7% of CD30L in γδ T cells of freshly isolated PECs and approximately 2% of CD30 and 8% of CD30L in γδ T cells of freshly isolated LPLs), CD30 and CD30L expressions on γδ T cells increased markedly after 24 h of culture without any stimulation (Figure 3). It is significant that CD30 and CD30L were preferentially expressed by Vγ1−Vγ4− γδ T cells, but not Vγ1+ or Vγ4+ γδ T cells in freshly isolated PECs and LPLs (Figure 3). We could not detect CD30 or CD30L expression on γδ T cells from freshly isolated spleen and lymph nodes (Supplementary Figure S5b online). However, TCR stimulation with anti-CD3 mAb induced CD30 or CD30L expression on all Vγ repertoires of γδ T cells investigated in PECs, LPLs, spleen, and lymph nodes (data not shown). It is strongly suggested that CD30L/CD30 interactions contribute to maintenance of Vγ1−Vγ4− bearing Vγ6 γδ T cells that produce IL-17A in mucosa-associated tissues.
CD30 and CD30 ligand (CD30L) were preferentially expressed by Vγ1−Vγ4− γδ T cells in mucosa-associated tissues of naive mice. Cells were isolated from peritoneal exudate cells (PECs) or lamina propria lymphocytes (LPLs) of wild-type (WT), CD30KO (as control for CD30 expression), or CD30LKO mice (as control for CD30L expression), and then CD30 or CD30L expression on subsets of γδ T cells was analyzed by flow cytometry before and/or after culture for 24 h without any stimulation. Data indicate mean (n=3) from a representative in four separate experiments.
Impaired function of γδ T cells in defense against L. monocytogenes infection in CD30KO and CD30LKO mice
IL-17A-producing γδ T cells are known to be activated at an early stage after infection with L. monocytogenes.34, 35 To determine the role of CD30L/CD30 in the function of γδ T cells in the early stage of L. monocytogenes infection, we examined the kinetics of bacterial growth in the PECs after intraperitoneal inoculation with 5 × 105 CFU L. monocytogenes. On days 1, 3, and 5 after infection, the numbers of bacteria in PECs in CD30KO or CD30LKO mice were markedly larger than those in WT mice (Figure 4a). The number of total PECs that increased after infection in WT mice was similar to that of CD30KO or CD30LKO mice (Figure 4b). However, comparing with WT mice, the number of γδ T cells decreased significantly in CD30KO or CD30LKO mice on days 1, 3, and 5 after infection (Figure 4b). On day 5 after infection, the frequency of Vγ1−Vγ4− γδ T cell in PECs of CD30KO or CD30LKO mice was much lower than that in WT mice (Figure 4c). Conversely, the percentage of Vγ1+ or Vγ4+ γδ T cells relatively increased in CD30KO and CD30LKO mice (Figure 4c). A dramatic decrease in the Vγ1−Vγ4− γδ T-cell subset, but not in the Vγ1+ or Vγ4+ γδ T-cell subset, was noticed in CD30KO or CD30LKO mice on days 1, 3, and 5 after infection (Figure 4d).
Impaired ability of protection against Listeria monocytogenes infection in CD30KO or CD30LKO mice. Wild-type (WT), CD30KO, or CD30LKO mice were infected via intraperitoneal injection with viable L. monocytogenes. On days 1, 3, and 5 after L. monocytogenes infection, (a) the bacteria number in peritoneal exudate cells (PECs) was measured and (b) the total cell numbers of PEC and γδ T cells in PECs were counted. (c) Frequency of subsets of γδ T cells in PECs of WT, CD30KO, or CD30LKO mice was analyzed on day 5 after L. monocytogenes infection. (d) Absolute numbers of Vγ1+, Vγ4+, and Vγ1−Vγ4− γδ T cells in PECs of WT, CD30KO, or CD30LKO mice on days as indicated. Data indicate mean±s.d. (n=3–10) from a representative in three separate experiments. Statistically significant differences between WT mice and CD30KO or CD30LKO mice are shown (*P<0.05).
The absolute number of CD11b+Gr-1hiF4/80− neutrophils was lower in PECs of CD30KO or CD30LKO mice than that of WT mice on days 1, 3, and 5 after infection (Figure 5a). Spontaneous cytokine production was measured after infection. The level of IL-17A, but not of IL-23 or IL-12p70, significantly decreased in CD30KO or CD30LKO mice as compared with those in WT mice on days 1, 3, and 5 after infection (Figure 5b). As shown in Figure 5c, γδ T cells could spontaneously produce IL-17A but hardly produce IFN-γ. Furthermore, the frequency and absolute number of γδ T cells producing IL-17A, but not IFN-γ, significantly decreased in CD30KO or CD30LKO mice on days 1, 3, and 5 after infection compared with those in WT mice (Figure 5c and Supplementary Figure S6 online). In accordance with a previous report,35 the Vγ1+ subset of the γδ T cells hardly produced IL-17A, whereas compared with the Vγ4+ subset, the Vγ1−Vγ4− subset of the γδ T cells were the principal source of IL-17A during the early stage of Listeria infection (Figure 5d). Furthermore, the percentage and absolute number of IL-17A producing this subset of γδ T cells decreased significantly in CD30KO or CD30LKO mice on the days indicated as compared with those in WT mice (Figure 5d).
CD30 ligand (CD30L)/CD30 interactions selectively contribute to maintenance of Vγ1−Vγ4− subset of γδ T cells producing interleukin 17A (IL-17A) in peritoneal exudate cells (PECs) during early stage of Listeria monocytogenes infection. (a) Cell number of accumulated neutrophils in PECs after infection. (b) Peritoneal cavity lymphocytes were recovered on day 5 of L. monocytogenes infection and cultured for 24 h without any stimulation in vitro, and then spontaneous cytokine production of IL-23, IL-17A, or IL-12p70 was measured by enzyme-linked immunosorbent assay (ELISA). The frequency and absolute number of (c) IL-17A-producing γδ T cells or (d) Vγ1−Vγ4− γδ T cells in PECs of WT, CD30KO, or CD30LKO mice on days 1 (1d), 3 (3d), and 5 (5d) after L. monocytogenes infection. Data indicate mean±s.d. (n=3) from a representative in three separate experiments. Statistically significant differences between wild-type (WT) mice and CD30KO or CD30LKO mice are shown (*P<0.05, **P<0.01). IFN-γ, interferon-γ P/I, phorbol myristate acetate/ionomycin. (e) CD30LKO mice were treated with 200 μg of agonistic anti-CD30 antibody (Ab) or hamster IgG1 monoclonal antibody (mAb) as control 24 h before 1 × 106 CFU L. monocytogenes infection. On day 3 after infection, bacteria numbers of PECs, the frequency, and cell number of IL-17A-producing Vγ1−Vγ4− γδ T cells were measured. Data indicate mean±s.d. (n=5) from a representative in three separate experiments. Statistically significant differences are shown (*P<0.05).
To determine whether CD30 signaling via CD30L is involved in early protection against L. monocytogenes infection, we next examined the effect of agonistic anti-CD30 mAb on bacterial growth and on γδ T cell producing IL-17A after 1 × 106 CFU of L. monocytogenes infection. As shown in Figure 5e, the Vγ1−Vγ4− subset of the γδ T cells that produced IL-17A increased significantly and bacterial clearance ability was restored by treatment of agonistic anti-CD30 mAb on day 3 after infection not only in WT mice but also in CD30LKO mice. Therefore, these data strongly demonstrate that CD30L/CD30 interactions selectively contribute to maintenance of the Vγ1−Vγ4− subset of γδ T cells, which is the main source of IL-17A during the early stage of protection against L. monocytogenes infection.
DISCUSSION
We show here that CD30L/CD30 signaling plays an important role in maintenance of the naturally occurring IL-17A-producing γδ T cells presumably bearing Vγ6 in the mucosa-associated tissues such as PECs, LPLs, and uterus in naive mice. Furthermore, CD30L/CD30 interactions contributed to IL-17A-producing γδ T cell subset–mediated early protection against L. monocytogenes infection in mice.
There are several possibilities to explain why the maintenance of the Vγ1−Vγ4− subset of naturally occurring γδ T cells producing IL-17A is dependent on CD30L/CD30 signaling in naive mice. The γδ T cell bearing Vγ6 is functionally committed into the effector cell producing IL-17A within the fetal thymus, raising the first possibility that CD30L/CD30 signaling is required for the development of naturally occurring IL-17A-producing γδ T cells in the fetal thymus. However, the γδ T cells producing IL-17A normally developed in the fetal thymus of CD30KO or CD30LKO mice, suggesting that CD30L/CD30 signaling is not essential in the development of γδ T cells in the fetal thymus. Second, we previously reported that gene expression of CCR7 (C-C chemokine receptor type 7), which is preferentially expressed by naive T cells and central memory T cells, was upregulated by CD30 signaling stimulation in the human YT lymphoma cell line and murine T cells.36, 37 Furthermore, expression of CCL21 (chemokine (C-C motif) ligand 21), a ligand for CCR7, was reported to be upregulated by CD30L/CD30 signaling in the absence of lymphotoxin-α.38 Taken together, this suggests that CD30L/CD30 signaling recruits the γδ T-cell subsets from the thymus to the periphery via expression of chemokine receptors on γδ T cells. However, secondary lymphoid tissues such as spleen and lymph node expressed CCL21, recruiting CCR7+ naive T cells or central memory T cells,39, 40 whereas mucosa-associated tissues, such as LPLs and peritoneal cavity, preferentially express CCL20 recruiting CCR6+ effector T cells.41 It is possible that CD30L/CD30 interactions selectively enhance chemokine receptor CCR6 expression in IL-17A-producing Vγ1−Vγ4− bearing Vγ6 γδ T cells in PECs and promote the homing of this subset of γδ T cells producing IL-17A. However, IL-17A-producing Vγ4+ γδ T cells were reported to also express CCR6,42 excluding this possibility. Third, it has been reported that γδ T cells secrete IL-17A in response to IL-23 and IL-1β without TCR activation and without the requirement for antigen-presenting cell (APC).31, 33, 43 Therefore, it is also possible that the γδ T cells may be impaired in response to exogenous IL-23/IL-1β in the absence of CD30L/CD30 signaling. To determine this, we examined the effect of IL-23 or IL-1β on IL-17A-producing γδ T cells from WT, CD30KO, and CD30LKO mice and found that IL-23, but not IL-1β, enhanced IL-17A production by γδ T cells with the same degree, regardless of deficiency of CD30L/CD30 signaling (data not shown). However, the frequency of IL-17A+ γδ T cells remained at significantly lower level in CD30KO or CD30LKO mice than those in WT mice. These results suggest that IL-23 induces IL-17A production by γδ T cells independently of CD30L/CD30 signaling, but does not overcome the defect in CD30L/CD30. Fourth, CD30 has the potency to induce TRAF-2 (TNF receptor-associated factor 2)-mediated nuclear factor-κB activation, can recruit TRAF-1, and can possibly also contribute to increased T-cell survival.44, 45 Thus, it is possible that CD30L/CD30 signaling may promote cell survival of γδ T cells for maintenance in naive mice and increase in infected mice. CD30L and CD30 were reported to be preferentially expressed by activated/memory Th cells but not by resting T cells.10, 11, 13, 14, 15 A number of recent studies suggested that CD30L/CD30 signaling is also linked to memory Th cell responses and their associated diseases, irrespective of the Th subset.16, 17, 18, 19, 20, 21 CD30L/CD30 signaling may also be important for amplification of already differentiated effector or memory T cells including naturally occurring γδ T cells.
An important finding in this study is that only Vγ1−Vγ4− γδ T cells in freshly isolated PECs or LPLs of naive WT mice expressed CD30 and CD30L, and their expressions increased after culture in medium alone. We have recently found the importance of HES1 (hairy and enhancer of split-1) induced by Notch–ligand interaction in the acquisition and maintenance of the IL-17A-producing function of γδ T cells.46 Vγ6+ γδ T cells in PECs express Notch1 and have the ability to produce IL-17A.9, 46 Notch signaling was reported to induce IL-7Rα in T-lineage cells and IL-7 was reported to induce CD30L on innate lymphoid cells.47, 48 Therefore, the Notch/IL-7Ra axis may be involved in the expression of CD30L/CD30 on the γδ T cells. On the other hand, stimulation with anti-CD3 mAb induced CD30 or CD30L on all Vγ repertoires of γδ T cells, not only in PECs or LPLs but also in spleen or lymph nodes (data not shown). These results suggest that Vγ1−Vγ4− γδ T cells may be stimulated upon TCR by recognizing an unknown antigen in the medium or in autoantigens expressed by neighboring γδ T cells. The Vγ6/Vδ1 γδ T cells bear invariant TCRs, even to the nucleotides in the TCR gene junction.2, 49, 50 The canonical sequence is very simple, with no apparent N-region contribution.3 Such a characteristic has led to the hypothesis that this subset of γδ T cells represents a pre-program to recognize a limited set of self-antigens induced by stress or environmental antigens derived from commensal microflora. At present, the specificity of the γδ T cells remains unknown. Therefore, it is of interest to ascertain whether the murine Vγ6 γδ T cells recognize such unique antigens in a manner different from that of other Vγ repertoires of γδ T-cell subsets.
The IL-17A-producing γδ T cells are known to play a protective role at the early stage after L. monocytogenes.35 In this study, we found that the number of IL-17A-producing Vγ1−Vγ4− γδ T cells increased in the peritoneal cavity of WT mice but not in CD30LKO or CD30KO mice at the early stage after intraperitoneal infection with L. monocytogenes. It appears that CD30L/CD30 signaling plays an important role in early protection against L. monocytogenes infection at least by enhancing IL-17A production by γδ T cells. We have previously reported that IL-17A-producing Vγ1−Vγ4− γδ T cells bearing Vγ6 selectively increased after intraperitoneal infection in response to cytokines such as IL-23 in a bystander manner.32 As basal levels of naturally occurring IL-17A-producing Vγ1−Vγ4− γδ T cells were significantly lower in the peritoneal cavity of naive CD30L or CD30KO mice, the number of naturally occurring IL-17A-producing γδ T cells remained at the lower level in the peritoneal cavity of these mice after infection, resulting in an impaired protection against L. monocytogenes infection. It is also possible that de novo synthesis of CD30L and CD30 after infection contributes to activation of IL-17A-producing γδ T cells. Further experiments need to clarify these possibilities.
In conclusion, CD30L/CD30 signaling plays an important role in the maintenance of the naturally occurring IL-17A-producing γδ T cells presumably bearing Vγ6 in the mucosa-associated tissues of naive mice. In addition, CD30L/CD30 interactions contribute to IL-17A-producing Vγ1−Vγ4− γδ T cell–mediated innate immunity against bacterial infection.
METHODS
Mice. Age- and sex-matched C57BL/6 (B6) male mice were purchased from Japan KBT (Shizuoka, Japan). The generation and preliminary characterization of CD30LKO (BALB/c background) mice has been described previously,51 and eight or more generations were backcrossed onto B6 mice. CD30KO (B6.129P2-Tnfrsf8(tm1Mak)/J) mice were purchased from the Jackson Laboratory (Bar Harbor, ME). All mice were maintained under specific pathogen-free conditions and were offered food and water ad libitum. All mice were used at 6–8 weeks of age. This study was approved by the Committee of Ethics on Animal Experiments in the Faculty of Medicine, Kyushu University (Fukuoka, Japan). Experiments were performed under the control of the Guidelines for Animal Experiments.
Microorganisms. L. monocytogenes, strain EGD, was used in all experiments. Bacterial virulence was maintained by serial passages in B6 mice. Fresh isolates were obtained from infected spleens, grown in tryptic soy broth (Nissui Pharmaceutical, Tokyo, Japan), washed repeatedly, resuspended in phosphate-buffered saline, and stored at −70 °C in small aliquots.
Bacterial growth. Mice were inoculated intraperitoneally with viable L. monocytogenes in 0.2 ml of phosphate-buffered saline on day 0 at a sublethal dose of 5 × 105 CFU, which corresponds to one-tenth of the LD50 (lethal dose, 50%) for C57BL/6 mice. On days 1, 3, and 5 after infection, the mice were killed, and PECs were irrigated with 4 ml of ice-cold Hank’s balanced salt solution and harvested after gentle massage. Samples were spread on trypto-soya agar plates, and colonies were counted after incubation for 24 h at 37 °C.
Abs and reagents. Fcγ receptor-blocking mAb (CD16/32; 2.4G2), Alexa Fluor 488, and Alexa Fluor 647 anti-IL-17A (TC11-18H10) were purchased from BD Biosciences (San Diego, CA). Fluorescein isothiocyanate (FITC)–conjugated anti-TCRγδ (GL3), PerCP-eFluor 710 anti-TCRγδ (eBioGL3), phycoerythrin (PE)-conjugated anti-CD30 (mCD30.1), anti-CD153 (RM153), APC-conjugated anti-TCRαβ (GL3), and PerCP-eFluor710 anti-MCH class II (M5/114.15.2) were purchased from e-Bioscience (San Diego, CA). APC-conjugated anti-TCRγδ (GL3), FITC-conjugated and PE-conjugated anti-Vγ1.1 (2.11), and anti-TCR Vγ2 (UC3-10A6) were purchased from BioLegend (San Diego, CA). PE- and FITC-anti-CD3e (145-2C11), FITC-anti-Vγ3 TCR (536), PE-anti-IL-17A (TC11-8H10), and APC-anti-IFN-γ (XMG1.2) were purchased from BD Pharmingen (San Diego, CA). Armenian hamster IgG (eBio299Arm) was purchased from Wako Pure Chemicals (Osaka, Japan). Purified anti-CD3 was obtained from e-Bioscience.
Cell preparation. Single cells were isolated from fetal thymus, adult thymus, splenocytes, PECs, LPLs, liver, lung, and uterus, as previously described.9
Flow cytometric analysis and intracellular cytokine staining. Isolated cells from various tissues of naive mice were incubated with an Fcγ receptor-blocking mAb to prevent nonspecific staining. After washing, cells were stained with various combinations of mAbs. Stained cells were washed twice and resuspended, and propidium iodide (1 μg ml−1) was added before running on a FACSCalibur flow cytometer (BD Biosciences) to detect and exclude dead cells for the analysis of surface staining. For intracellular cytokine staining, cells were stimulated with 25 ng ml−1 of PMA (P-8139, Sigma-Aldrich, Tokyo, Japan) and 1 μg ml−1 of ionomycin (I-0634, Sigma-Aldrich) for 5 h at 37 °C, and 10 μg ml−1 of brefeldin A was added for the last 4 h of culture. In some experiments, cells were co-cultured with IL-23 (20 ng ml−1) and/or IL-1β (20 ng ml−1) for 7 h, and brefeldin A was added at the last 5 h. After culture, cells were harvested and surface stained with various mAbs for 30 min at 4 °C, and then intracellular staining was performed according to the manufacturer’s instructions (BD Biosciences). The data were analyzed by FlowJo software (BD Biosciences).
RNA isolation and reverse transcriptase–PCR analysis. The γδ T cells or Vγ1−Vγ4− γδ T cells were sorted from PECs or LPLs of WT, CD30KO, or CD30LKO mice by FACSAria (BD Biosciences) at >98% purity. Total RNA from sorted cells was purified using the RNeasy Mini Kit (QIAGEN, Germantown, MD), and complementary DNA was synthesized using Superscript II (Invitrogen) according to the manufacturer’s instructions. For the Vγ repertoire analysis, combinations of the following primers were used: forward primers: Vγ 1/2, 5′-ACACAGCTATACATTGGTAC-3′; Vγ2, 5′-CGGCAAAAAACAAATCAACAG-3′; Vγ4, 5′-TGTCCTTGCAACCCCTACCC-3′; Vγ5, 5′-TGTCCTTGCAACCCCTACCC-3′; Vγ6, 5′-GGAATTCAAAAGAAAACATTGTCT-3′; Vγ7, 5′-AAGCTAGAGGGGTCCTCTGC-3′. Vδ1, 5′-ATTCAGAAGGCAACAATGAAAG-3′; Vδ2, 5′-AGTTCCCTGCAGATCCAAGC-3′; Vδ3, 5′-TTCCTGGCTATTGCCTCTGAC-3′; Vδ4, 5′-CCGCTTCTCTGTGAACTTCC-3′; Vδ5, 5′-CAGATCCTTCCAGTTCATCC-3′; Vδ6, 5′-TCAAGTCCATCAGCCTTGTC-3′; Vδ7, 5′-CGCAGAGCTGCAGTGTAACT-3′; and Vδ8, 5′-AAGGAAGATGGACGATTCAC-3′; reverse primers: Cγ, 5′-CTTATGGAGATTTGTTTCAGC-3′; Cδ, 5′-CGAATTCCACAATCTTCT-3′; β-actin, 5′-TAAAACGCAGCTCAGTAACAGTCCG-3′; and 5′-TGGAATCCTGTGGACTCCATGAAAC-3′. PCR was performed on a PCR thermal cycler (Takara, Otsu, Shiga, Japan).
Cytokine enzyme-linked immunosorbent assay. On day 5 after L. monocytogenes infection, PECs from L. monocytogenes–infected mice were harvested and cultured without any stimulation for 24 h at 37 °C in a 96-well-plate. The levels of IL-23, IL-17A, and IL-12p70 in the supernatants were measured by DuoSet ELISA Development System (R&D Systems, Minneapolis, MN) according to the manufacturer’s instructions.
In vivo treatment of mice with agonistic anti-CD30 Abs. Agonistic anti-CD30 mAbs (clone 30.1) were obtained as previously described.26 The mAbs, diluted to 1 mg ml−1 in phosphate-buffered saline, were stored at −80 °C until use. For in vivo activation, 200 μg of agonistic anti-CD30 mAb or isotype control (hamster IgG1; BD Biosciences) was injected intraperitoneally into mice concomitantly with infection.
Statistical analysis. Statistical significance was calculated by Student’s t-test using Prism software (GraphPad Software, La Jolla, CA). Differences with P-values of <0.05 were considered statistically significant.
References
Heilig, J.S. & Tonegawa, S. Diversity of murine gamma genes and expression in fetal and adult T lymphocytes. Nature 322, 836–840 (1986).
Havran, W.L. & Allison, J.P. Developmentally ordered appearance of thymocytes expressing different T-cell antigen receptors. Nature 335, 443–445 (1998).
Lafaille, J.J., DeCloux, A., Bonneville, M., Takagaki, Y. & Tonegawa, S. Junctional sequences of T cell receptor gamma delta genes: implications for gamma delta T cell lineages and for a novel intermediate of V-(D)-J joining. Cell 59, 859–870 (1989).
Ribot, J.C. et al. CD27 is a thymic determinant of the balance between interferon-gamma- and interleukin 17-producing gammadelta T cell subsets. Nat. Immunol. 10, 427–436 (2009).
Hayday, A.C. [gamma][delta] cells: a right time and a right place for a conserved third way of protection. Annu. Rev. Immunol. 18, 975–1026 (2000).
Bonneville, M., O'Brien, R.L. & Born, W.K. Gammadelta T cell effector functions: a blend of innate programming and acquired plasticity. Nat. Rev. Immunol. 10, 467–478 (2010).
Jensen, K.D. et al. Thymic selection determines gammadelta T cell effector fate: antigen-naive cells make interleukin-17 and antigen-experienced cells make interferon gamma. Immunity 29, 90–100 (2008).
Turchinovich, G. & Hayday, A.C. Skint-1 identifies a common molecular mechanism for the development of interferon-gamma-secreting versus interleukin-17-secreting gammadelta T cells. Immunity 35, 59–68 (2011).
Shibata, K., Yamada, H., Nakamura, R., Sun, X., Itsumi, M. & Yoshikai, Y. Identification of CD25+ gamma delta T cells as fetal thymus-derived naturally occurring IL-17 producers. J. Immunol. 181, 5940–5947 (2008).
Croft, M. Co-stimulatory members of the TNFR family: keys to effective T-cell immunity? Nat. Rev. Immunol. 3, 609–620 (2003).
Watts, T.H. TNF/TNFR family members in costimulation of T cell responses. Annu. Rev. Immunol. 23, 23–68 (2005).
Ribot, J.C., debarros, A. & Silva-Santos, B. Searching for “signal 2”: costimulation requirements of γδ T cells. Cell. Mol. Life Sci. 68, 2345–2355 (2011).
Shimozato, O., Taked, K., Ygita, H. & Okumura, K. Expression of CD30 ligand (CD153) on murine activated T cells. Biochem. Biophys. Res. Commun. 256, 519–526 (1999).
Kennedy, M.K., Willis, C.R. & Armitage, R.J. Deciphering CD30 ligand biology and its role in humoral immunity. Immunology 118, 143–152 (2006).
Lane, P.J., Gaspal, F.M. & Kim, M.Y. Two sides of a cellular coin: CD4 (+) CD3- cells regulate memory responses and lymph-node organization. Nat. Rev. Immunol. 5, 655–660 (2005).
Romagnani, S., Del Prete, G., Maggi, E., Chilosi, M., Caligaris-Cappio, F. & Pizzolo, G. CD30 and type 2 T helper (Th2) responses. J. Leukoc. Biol. 57, 726–730 (1995).
Bengtsson, A. The role of CD30 in atopic disease. Allergy 56, 593–603 (2001).
Polte, T., Behrendt, A.K. & Hansen, G. Direct evidence for a critical role of CD30 in the development of allergic asthma. J. Allergy Clin. Immunol. 118, 942–948 (2006).
Flórido, M., Borges, M., Yagita, H. & Appelberg, R. Contribution of CD30/CD153 but not of CD27/CD70, CD134/OX40L, or CD137/4-1BBL to the optimal induction of protective immunity to Mycobacterium avium. J. Leukoc. Biol. 76, 1039–1346 (2004).
Geril, R., Lunardi, C., Viante, F., Bistoni, O., Pizzolo, G. & Pitzalis, C. Role of CD30+ T cells in rheumatoid arthritis: a counter-regulatory paradigm for Th1-driven diseases. Trends Immunol. 22, 72–77 (2001).
Saraiva, M., Smith, P., Fallon, P.G. & Alcami, A. Inhibition of type 1 cytokine-mediated inflammation by a soluble CD30 homologue encoded by ectromelia (mousepox) virus. J. Exp. Med. 196, 829–839 (2002).
Zhang, Z.X., Yang, L., Young, K.J., DuTemple, B. & Zhang, L. Identification of a previously unknown antigen-specific regulatory T cell and its mechanism of suppression. Nat. Med. 6, 782–789 (2000).
Dai, Z. et al. CD4+CD25+ regulatory T cells suppress allograft rejection mediated by memory CD8+ T cells via a CD30-dependent mechanism. J. Clin. Invest. 113, 310–317 (2004).
Tang, C. et al. A novel role of CD30L/CD30 signaling by T-T cell interaction in Th1 response against mycobacterial infection. J. Immunol. 181, 6316–6327 (2008).
Sun, X. et al. A critical role of CD30 ligand/CD30 in controlling inflammatory bowel diseases in mice. Gastroenterology 134, 447–458 (2008).
Sun, X. et al. CD30 ligand/CD30 plays a critical role in Th17 differentiation in mice. J. Immunol. 185, 2222–2230 (2010).
Garman, R.D., Doherty, P.J. & Raulet, D.H. Diversity, rearrangement, and expression of murine T cell gamma genes. Cell 45, 733–742 (1986).
Havran, W.L. & Allison, J.P. Origin of Thy-1+ dendritic epidermal cells of adult mice from fetal thymic precursors. Nature 344, 68–70 (1990).
Asarnow, D.M. et al. Limited diversity of gamma delta antigen receptor genes of Thy-1+ dendritic epidermal cells. Cell 55, 837–847 (1988).
Ito, K. et al. Different gamma delta T-cell receptors are expressed on thymocytes at different stages of development. Proc. Natl. Acad. Sci. USA 86, 631–635 (1989).
Ohga, S., Yoshikai, Y., Takeda, Y., Hiromatsu, K. & Nomoto, K. Sequential appearance of gamma/delta- and alpha/beta-bearing T cells in the peritoneal cavity during an i.p. infection with Listeria monocytogenes. Eur. J. Immunol. 20, 533–538 (1990).
Nakamura, R., Shibata, K., Yamada, H., Shimoda, K., Nakayama, K. & Yoshikai, Y. Tyk2-signaling plays an important role in host defense against Escherichia coli through IL-23-induced IL-17 production by gammadelta T cells. J. Immunol. 181, 2071–2075 (2008).
Shibata, K., Yamada, H., Hara, H., Kishihara, K. & Yoshikai, Y. Resident Vdelta1+ gammadelta T cells control early infiltration of neutrophils after Escherichia coli infection via IL-17 production. J. Immunol. 178, 4466–4472 (2007).
Meeks, K.D., Sieve, A.N., Kolls, J.K., Ghilardi, N. & Berg, R.E. IL-23 is required for protection against systemic infection with Listeria monocytogenes. J. Immunol. 183, 8026–8034 (2009).
Hamada, S. et al. IL-17A produced by gammadelta T cells plays a critical role in innate immunity against listeria monocytogenes infection in the liver. J. Immunol. 181, 3456–3463 (2008).
Muta, H., Boise, L.H., Fang, L. & Podack, E.R. CD30 signals integrate expression of cytotoxic effector molecules, lymphocyte trafficking signals, and signals for proliferation and apoptosis. J. Immunol. 165, 5105–5111 (2000).
Podack, E.R., Strbo, N., Sotosec, V. & Muta., H. CD30: governor of memory T cells? Ann. NY Acad. Sci. 975, 101–113 (2002).
Bekiaris, V. et al. CD30 is required for CCL21 expression and CD4 T cell recruitment in the absence of lymphotoxin signals. J. Immunol. 182, 4771–4775 (2009).
Sallusto, F., Lenig, D., Förster, R., Lipp, M. & Lanzavecchia, A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature 401, 708–712 (1999).
Förster, R. et al. CCR7 coordinates the primary immune response by establishing functional microenvironments in secondary lymphoid organs. Cell 99, 23–33 (1999).
Schutyser, E., Struyf, S. & Van. Damme, J. The CC chemokine CCL20 and its receptor CCR6. Cytokine Growth Factor Rev. 14, 409–426 (2003).
Haas, J.D. et al. Development of interleukin-17-producing γδ T cells is restricted to a functional embryonic wave. Immunity 37, 48–59 (2012).
Sutton, C.E., Lalor, S.J., Sweeney, C.M., Brereton, C.F., Lavelle, E.C. & Mills, K.H. Interleukin-1 and IL-23 induce innate IL-17 production from gammadelta T cells, amplifying Th17 responses and autoimmunity. Immunity 31, 331–341 (2009).
Lee, S.Y. CD30/TNF receptor-associated factor interaction: NF-κB activation and binding specificity. Proc. Natl. Acad. Sci. USA 93, 9699–9703 (1996).
Duckett, C.S., Gedrich, R.W., Gilfillan, M.C. & Thompson., C.B. Induction of nuclear factor κB by the CD30 receptor is mediated by TRAF1 and TRAF2. Mol. Cell. Biol. 17, 1535 (1997).
Shibata, K. et al. Notch-Hes1 pathway is required for the development of IL-17-producing γδ T cells. Blood 118, 586–593 (2011).
Kim, M.Y. et al. OX40 ligand and CD30 ligand are expressed on adult but not neonatal CD4+CD3- inducer cells: evidence that IL-7 signals regulate CD30 ligand but not OX40 ligand expression. J. Immunol. 174, 6686–6691 (2005).
González-García, S. et al. CSL-MAML-dependent Notch1 signaling controls T lineage-specific IL-7R{alpha} gene expression in early human thymopoiesis and leukemia. J. Exp. Med. 206, 779–791 (2009).
Nandi, D. & Allison, J.P. Phenotypic analysis and gamma delta-T cell receptor repertoire of murine T cells associated with the vaginal epithelium. J. Immunol. 147, 1773–1778 (1991).
Heyborne, K. et al. Evidence that murine V gamma 5 and V gamma 6 gamma delta-TCR+ lymphocytes are derived from a common distinct lineage. J. Immunol. 151, 4523–4527 (1993).
Blazar, B.R. et al. CD30/CD30 ligand (CD153) interaction regulates CD4+ T cell-mediated graft-versus-host disease. J. Immunol. 173, 2933–2941 (2004).
Acknowledgements
We thank Mrs K. Akasaki, Akiko Yano, and Miki Kijima for their excellent technical assistance. This work was supported by a grant-in-aid from the Japan Society for the Promotion of Science, and grants from the Japanese Ministry of Education, Science and Culture and Yakult Bioscience Foundation (to Y.Y.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declared no conflict of interest.
Additional information
SUPPLEMENTARY MATERIAL is linked to the online version of the paper
Rights and permissions
About this article
Cite this article
Sun, X., Shibata, K., Yamada, H. et al. CD30L/CD30 is critical for maintenance of IL-17A-producing γδ T cells bearing Vγ6 in mucosa-associated tissues in mice. Mucosal Immunol 6, 1191–1201 (2013). https://doi.org/10.1038/mi.2013.18
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/mi.2013.18
This article is cited by
-
Neuroprotective Effect of miR-483-5p Against Cardiac Arrest-Induced Mitochondrial Dysfunction Mediated Through the TNFSF8/AMPK/JNK Signaling Pathway
Cellular and Molecular Neurobiology (2023)
-
The accumulation of Vγ4 T cells with aging is associated with an increased adaptive Vγ4 T cell response after foodborne Listeria monocytogenes infection of mice
Immunity & Ageing (2022)
-
Differentially expressed long noncoding RNAs in RAW264.7 macrophages during Brucella infection and functional analysis on the bacterial intracellular replication
Scientific Reports (2022)
-
CD30: from basic research to cancer therapy
Immunologic Research (2013)