Abstract
Inappropriate expression of the chemokine CX3CL1 is reportedly known to act on inflammatory conditions in extraocular immune diseases. We studied the expression and effects of CX3CL1 in human patients, cultured human conjunctival cells, and transgenic mice exposed to benzalkonium chloride (BAC), a commonly used preservative in ophthalmic medications despite its proinflammatory properties, to determine whether CX3CL1 is involved in conjunctival inflammation. We report that CX3CL1 expression is increased in the conjunctiva of patients receiving BAC-containing medication, and correlates with clinical inflammation. BAC enhances the production of CX3CL1 in a conjunctival epithelial cell line, through the tumor-necrosis factor-α pathway, which attracts specific leukocyte subsets. In vivo, BAC-induced macrophage infiltration and subsequent inflammation of the conjunctiva is decreased in CX3CR1-deficient mice as compared with CX3CR1+/+ controls. This translational study opens new avenue to investigate ocular surface disorders by focusing on chemokine-related inflammation and immune cell trafficking in the ocular conjunctival mucosa.
Similar content being viewed by others
Introduction
Ocular surface disorders including allergy, dry eye, and eye drop toxicity have in common complex inflammatory–immune processes occurring in the corneal and conjunctival tissue. The conjunctiva is a fragile mucosa constituting the first physical and biological barrier of the eye, which is also exposed to ophthalmic medications. Preservatives are commonly used in a wide range of chronically administered eye drops including anti-allergy drugs, anti-glaucoma medications, as well as tear substitutes.1 However, although providing useful antimicrobial properties, these compounds are known to adversely cause deleterious conjunctival tissue damage and related ocular surface inflammation as previously studied by our team and others.1, 2 Such a toxic side effect has a major health impact in ophthalmology as it is linked with a significant decrease in compliance further leading to treatment failure.3, 4, 5 Several studies have demonstrated that benzalkonium chloride (BAC), the most widely used preservative in ophthalmology, causes irritancy symptoms, treatment discontinuation, and iatrogenic dry eye disease.5, 6, 7, 8 In vitro, BAC induces dose-dependent conjunctival cell apoptosis9, 10, 11, 12, 13 associated with overexpression of cytokines such as tumor-necrosis factor-α (TNF-α) or interleukin (IL)-1β.14 Human and animal studies revealed that exposure to BAC-containing eye drops induced the overexpression of inflammatory markers, such as HLA-DR, ICAM-1, or Fas,15, 16, 17 together with a conjunctival infiltration of inflammatory cells.18, 19, 20 However, the mechanisms leading to this BAC-induced inflammatory cell accumulation at the conjunctival mucosa have not yet been elucidated. More precisely, it has never been determined whether this inflammatory cell infiltration could be attributed to specific interactions between conjunctival epithelial cells and immune cells or only to nonspecific inflammatory processes secondary to tissue and lacrimal toxic damage.
CX3CL1, also called fractalkine, is the sole member of the CX3C-chemokine subfamily and binds to its specific receptor CX3CR1.21, 22 After proteolytic cleavage, this membrane-bound glycoprotein assumes a soluble form that induces migration and activation of CX3CR1-bearing cells such as T cells, natural killers (NKs), monocytes, and monocyte-derived macrophages, as well as dendritic cells.23, 24, 25 Apart from its endothelial expression and its involvement in atherosclerosis,26, 27 CX3CL1 has been detected in epithelial cells from various tissues including bronchial and small intestine mucosae.28, 29, 30, 31 The recent development of transgenic mice, in which a green-fluorescent protein gene was inserted at the CX3CR1 locus, has improved the in vivo study of CX3CR1-mediated cell homing and turnover in several tissues. In the eye, it has not only been notably studied in the retina but also in the cornea in which CX3CR1 signaling could mediate physiological homing of dendritic cells.32, 33, 34, 35, 36
Given both the conjunctival inflammatory changes induced by BAC-containing medications and the role of CX3CL1 in mucosal immune disorders, we hypothesized that CX3CL1 could be involved in ocular surface inflammation. In the present work, the conjunctival epithelial expression of CX3CL1 along with its consequences on immune cell infiltration and related tissue inflammation have been studied in glaucomatous patients treated with preserved eye drops as well as in a conjunctival cell model of toxicity, and in transgenic mice exposed to BAC.
Results
Conjunctival expression of CX3CL1 is increased in patients treated with BAC-containing eye drops and correlates with clinical inflammation
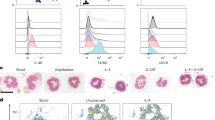
In the present study, we compared the expression of CX3CL1, CX3CR1, and HLA-DR in glaucoma patients receiving BAC-containing ophthalmic medication with that of patients treated with BAC-free medication using conjunctival imprints and flow cytometry (FCM). CX3CL1, CX3CR1, and HLA-DR were detected at relatively low levels in BAC-free control patients (Figure 1a and b). In contrast, CX3CL1 and HLA-DR expressions, but not that of CX3CR1, were significantly increased in the conjunctiva of patients treated with BAC-preserved eye drops. The clinical evaluation of ocular surface inflammation revealed that CX3CL1 conjunctival expression was positively correlated with clinical score of conjunctival damage in BAC-exposed patients ( Table 1 and Figure 1c). Other patient characteristics including clinical tear film instability (tear film break-up time) and treatment features, e.g., the number of daily instillations or the total dose of BAC, were not found to correlate with CX3CL1 level in the conjunctiva.
Conjunctival expression of CX3CL1 is increased in benzalkonium chloride (BAC)-exposed patients and correlates with clinical score of conjunctival damage. Glaucomatous patients treated over the long term with either BAC-containing eye drops (n=20) or BAC-free eye drops (n=20) underwent clinical examination (see Table 1) and conjunctival impression cytology combined with flow cytometry. (a) Conjunctival expression of CX3CL1 was increased in BAC-exposed patients as compared with unexposed patients. CX3CR1 was poorly detected whatever the group. Conjunctival expression of HLA-DR, a known biomarker for ocular surface inflammation, was also increased in BAC-exposed patients. Data are presented as mean±s.e.m. **P<0.01. (b) See representative fluorescence histograms of CX3CL1 and HLA-DR expression in a patient exposed to BAC (shaded area) compared to an unexposed control (solid line). (c) Conjunctival expression of CX3CL1 as quantified by conjunctival imprints and flow cytometry is positively correlated (R2=0.762, P<0.01) with clinical score of conjunctival damage in the patients treated with BAC-containing eye drops.
BAC enhances the expression of CX3CL1 through the TNF-α pathway in a human conjunctival epithelial cell line
To study the mechanisms by which BAC induced the CX3CL1 expression, a human conjunctival epithelial IOBA-NHC cell line was used. CX3CL1 and CX3CR1 were detected at low level by quantitative PCR and immunofluorescence in unstimulated cells (Figure 2a and b). Similar to what was observed in conjunctival imprints from patients, stimulation with BAC enhanced the expression of CX3CL1 but of CX3CR1. BAC significantly enhanced the total expression of CX3CL1 in a dose- and time-dependent manner (Figure 2c–f). Surface membrane expression of CX3CL1 was significantly increased 6 h after stimulation with BAC and total expression was increased 6 and 12 h after, as assessed by FCM (Figure 2e). In correlation, the concentration of soluble CX3CL1 released into the cell supernatant rapidly increased after BAC stimulation as assessed by enzyme-linked immunosorbent assay (ELISA), suggesting a release of membrane-bound CX3CL1 into the extracellular medium (Figure 2f). Influence of the TNF-α–TNF-RI system upon CX3CL1 synthesis was also studied. Recombinant TNF-α alone induced an overexpression of CX3CL1 in conjunctival epithelial cells (Figure 2g). In BAC-exposed cells, incubation with a blocking anti-TNF-RI antibody significantly inhibited BAC-enhanced expression of CX3CL1, suggesting that the TNF-α pathway was involved in the chemokine-inducible synthesis by IOBA-NHC cells (Figure 2g).
The expression and release of CX3CL1 by a human conjunctival epithelial IOBA-NHC cell line is modulated by benzalkonium chloride (BAC) through the tumor-necrosis factor-α (TNF-α) pathway. (a) BAC stimulation increased CX3CL1 expression (12 h after 15-min exposure to 0.01% BAC) whereas CX3CR1 was not detected as assessed by indirect immunofluorescence. Representative images taken from three independent experiments; alexaFluor-488 (green) for CX3CL1 and receptor staining, propidium iodide (red) for nucleus. Original magnification × 200. Bar=50 μm. (b) BAC stimulation increased CX3CL1 mRNA level (3 h after 15-min stimulation with 0.01% BAC) whereas CX3CR1 mRNA was not affected. **P<0.01. (c, d) Dose-dependent effect of BAC stimulation on CX3CL1 total cell expression (12 h after a 15-min stimulation) as assessed by flow cytometry. **P<0.01. See representative gating method in IOBA-NHC cells and representative fluorescence histogram of CX3CL1 expression depending on BAC concentration. (e) Time-dependent effect of 0.01% BAC stimulation (15 min) on CX3CL1 surface membrane expression and total expression as assessed by flow cytometry. **P<0.01 vs. t0. (f) BAC-induced release of CX3CL1 into cell supernatant as assessed by ELISA. **P<0.01 vs. t0. (g) Blockade of TNF-RI using neutralizing anti-body (30-min preincubation) inhibited the BAC-enhanced expression of CX3CL1 (12 h after 15-min exposure to 0.01% BAC) as assessed by flow cytometry. Recombinant TNF-α (10 ng ml−1) was used as positive control. **P<0.01. Data in graphs are presented as mean±s.e.m. from at least three independent experiments.
BAC-stimulated human conjunctival epithelial cells attract leukocytes
The chemotactic effect of CX3CL1 on human primary leukocytes was investigated by performing transmigration assays. Unstimulated conjunctival IOBA-NHC epithelial cells did not show any significant chemotactic properties (Figure 3a). In contrast, BAC-stimulated conjunctival cells attracted specific leukocyte subpopulations including CD45+ CD3+ CD56− lymphocytes, CD45+ CD14+ monocytes, and CD45+ CD3+ CD56+ NK cells as assessed by FCM. Leukocyte preincubation with a blocking anti-CX3CR1 antibody significantly decreased CD45+ CD14+ cell migration and totally inhibited CD45+ CD3+ CD56+ cell migration, whereas CD45+ CD3+ CD56− cells were not significantly affected. Human recombinant CX3CL1 in medium induced a similar effect as BAC-stimulated IOBA-NHC cells on leukocyte migration, which was inhibited by blockade of CX3CR1. In parallel, adherent CD14+ monocyte-derived cells did not migrate toward unstimulated conjunctival epithelial cells but toward BAC-stimulated conjunctival cells (Figure 3b). Such migration was significantly decreased when adherent CD14+ cells are preincubated with the blocking anti-CX3CR1 antibody.
Conjunctival epithelial cells stimulated by benzalkonium chloride (BAC) attract leukocytes through CX3CL1–CX3CR1 interaction. Human primary leukocytes migrated for 3 h toward a human conjunctival epithelial IOBA-NHC cell line stimulated with BAC (0.01% BAC for 15 min, 6 h before the assay). (a) Non-adherent leukocytes were characterized for CD45, CD3, CD14, and CD56 expression using multicolor flow cytometry. Unstimulated conjunctival cells did not show any chemotactic property. BAC-stimulated conjunctival cells attracted leukocytes. Leukocyte preincubation for 30 min with blocking anti-CX3CR1 antibody partially or totally blunted this chemotactic effect. (b) Similar results were found for the migration of CD14+ adherent leukocytes, which migrated toward BAC-stimulated conjunctival cells through CX3CR1 signaling. Recombinant CX3CL1 (10 ng ml−1) in medium was used as positive control. Data in bar graphs are presented as mean±s.e.m. from at least three independent experiments. *P<0.05, **P<0.01 vs. unstimulated conjunctival cells; §§P<0.01 vs. unblocked leukocytes.
BAC-induced macrophage infiltration in the conjunctiva of CX3CR1-deficient mice is decreased as compared with CX3CR1+/+ animals
CX3CR1-deficient mice and their controls were used to confirm our human and in vitro findings. First, the conjunctival expression of CX3CL1 was assessed by ELISA in CX3CR1-deficient mice and CX3CR1+/+ mice after being exposed to BAC. In unexposed eyes, CX3CL1 was not detected in the conjunctiva of both groups. In contrast, CX3CL1 was found in BAC-exposed conjunctiva of both transgenic and control animals (1.145±0.345 ng ml−1 and 0.945±0.295 ng ml−1, respectively, NS), without any difference between both groups.
Second, immune cell infiltration was phenotyped by immunofluorescence (IF) in flat-mounted conjunctiva. The density of resident CX3CR1+ dendritic cells was neither different between CX3CR1-deficient mice and their controls, nor between treated and control eyes (70.5±19.1 cells mm−2 and 50.9±18.1 in unexposed and BAC-exposed eyes of CX3CR1+/+ mice, respectively, nonsignificant (NS); 65.4±10.5 and 47.9±12.9 in CX3CR1-deficient mice, NS). Few CD11b marker-positive cells, CD3+ cells, F4/80+ cells, and very few NKG2D+ cells were detected in the unexposed conjunctiva of both CX3CR1-deficient and CX3CR1+/+ mice (Figure 4a). In contrast, exposure to BAC induced in both groups a strong conjunctival infiltration of CD11b+ cells, CD3+ cells, and F4/80+, but not of NKG2D+ cells (Figure 4a). Interestingly, the density of F4/80+ cells, as mainly assumed as macrophages, was lower in BAC-exposed CX3CR1-deficient mice than in BAC-exposed CX3CR1+/+ mice, suggesting that macrophage migration to the conjunctiva depended upon the CX3CL1–CX3CR1 interaction. For CD11b+ and CD3+ cells, no statistical difference was found between groups.
Macrophage recruitment and cytokine expression pattern are impaired in the conjunctiva of CX3CR1-deficient transgenic mice. Eyes were exposed to 0.05% benzalkonium chloride (BAC)-containing eye drops (15 drops in one hour) 12 h before ex vivo analyzes. (a) Immunophenotyping of cell infiltration in flat-mounted conjunctivae. Low immune cell infiltration was found in the conjunctiva of unexposed eyes of both CX3CR1-deficient and CX3CR1+/+ control animals (n=10 in each group). No significant difference was found between groups. BAC exposure induced a major conjunctival infiltration by immune cells in both groups (n=10 in each group). The conjunctiva of CX3CR1-deficient mice presented a lower density in F4/80+ cells, as mainly assumed as macrophages, compared with CX3CR1+/+ control animals. (b) Cytokine expression profile in the mouse conjunctiva using proteome profiler array. BAC-enhanced level of C5a, tumor-necrosis factor-α (TNF-α), interferon (IFN)-γ, and CXCL11 was reduced in CX3CR1-deficient mice as compared with CX3CR1+/+ control animals (n=10 in each group). In contrast, ICAM-1 and CCL2 level was further increased by BAC in CX3CR1-deficient mice than in controls. Data represent changes in cytokine expression of BAC-exposed conjunctivae relative to unexposed conjunctivae. Data in graph are presented as mean±s.e.m. **P<0.01.
Cytokine profile in the conjunctiva of CX3CR1-deficient mice exposed to BAC is different from that of CX3CR1+/+ animals
To study the cytokine phenotype of infiltrated conjunctivas, cytokine expression pattern in the conjunctiva of transgenic mice and their controls was determined using proteome profiler array. ICAM-1, C5a, TNF-α, IL-1α/ra, interferon (IFN)-γ, CCL2, and CXCL11 were detected in the conjunctiva of both CX3CR1-deficient mice and their controls, whereas G-/GM-/M-CSF, IL-1β/-2/-3/-4/-5/-6/-7/-10/-12/-13/-16/-17/-23/-27, CCL-1/-2/-3/-4/-5/-11/-12/-17, CXCL-9/-10/-12, and MIP-2 were not. In CX3CR1+/+ animals, BAC exposure increased conjunctival expression of ICAM-1, C5a, TNF-α, IFN-γ, IL-1α/ra, CCL2, and CXCL11 as compared with that of unexposed conjunctiva (Figure 4b). BAC-enhanced expression of C5a, TNF-α, INF-γ, and CXCL11 was significantly reduced in CX3CR1-deficient mice as compared to their CX3CR1+/+ controls. On the contrary, BAC-enhanced conjunctival expression of ICAM-1 and of CCL2 was significantly higher in CX3CR1-deficient mice than in control animals (Figure 4b).
Discussion
The chemokine CX3CL1 is involved in the immune cell trafficking by attracting CX3CR1-bearing macrophages and cytotoxic lymphocytes.22, 23, 24, 25, 26 Deregulation of CX3CL1 expression in mucosal or epidermal tissues has been incriminated in several extraocular diseases such as asthma, Sjögren syndrome, and psoriasis.29, 30, 31, 37, 38 Here we originally demonstrated that an epithelial synthesis of CX3CL1 in the conjunctival mucosa is involved in ocular surface inflammation. We also show that BAC, the most widely used preservative in ophthalmic preparations, enhances inflammatory cell migration to the conjunctiva by modulating CX3CL1-mediated epithelial–immune cell interaction.
First, we report a constitutive expression of CX3CL1 in the non-inflammatory conjunctiva of humans as assessed by impression cytology. This basal epithelial expression had been previously reported in the tonsil, gut, small intestine, and skin,29, 30 but not in the ocular surface. Thus, CX3CL1 could be involved in the physiological homing of resident immune cells such as CX3CR1-bearing dendritic cells. On the contrary, CX3CR1 was poorly detected. Consistent with this finding, CX3CR1 has never been detected in any types of epithelial cell or tissue. Conjunctival imprints combined with FCM is a simple, noninvasive ex vivo method recently used to quantify cell markers expressed at the human ocular surface. In the present study, CX3CL1 expression was originally found to be significantly increased in patients receiving BAC-preserved eye drops as compared with patients with BAC-free medication. We had previously identified some biomarkers associated with ocular surface disorders including allergy, dry eye, and preservative-induced inflammation.7, 39 We had previously reported that BAC enhanced the expression of inflammatory markers (HLA-DR, ICAM-1), ILs (IL-8), and both chemokine receptors (CCR4 and CCR5), further underscoring complex inflammatory processes following BAC exposure.16, 17, 39, 40 Here, HLA-DR was also found to be overexpressed in BAC-exposed patients, but no significant correlation was found either between HLA-DR and CX3CL1 expression or between HLA-DR expression and the clinical examination. In contrast, CX3CL1 conjunctival level correlated with conjunctival damage score as assessed by the clinical examination, suggesting that CX3CL1 expression in the conjunctiva closely reflects the clinical severity of the disease. Complementarily, Enriquez-de-Salamanca et al.41 recently reported an increase in CX3CL1 concentration in tears of patients with dry eye, which also correlated with the disease severity, further confirming the role of this chemokine in ocular surface disease. It could have been interesting to quantify the conjunctival density of immune cells, and especially macrophages, to precise whether it correlated with CX3CL1 expression and the disease severity. However, this evaluation would have required conjunctival biopsies to assess the whole conjunctiva immune cell infiltration, which was not ethically feasible in our patients. Hence, CX3CL1 could constitute a new biomarker of inflammation in ocular surface disorders. We thus hypothesized that CX3CL1 epithelial synthesis could have a pivotal role in conjunctival immunity by directly influencing immune cell migration and further inflammatory-mediated cytotoxicity to the fragile ocular surface structures.
In vitro, the human conjunctival epithelial IOBA-NHC cell line was exposed to various concentrations of BAC and assessed for CX3CL1–CX3CR1 expression over time. We had previously developed this toxic cell model to demonstrate that BAC induces apoptosis in a dose-dependent manner.10 Here we report that BAC increases the expression and release of CX3CL1 in a dose- and time-dependent manner. Interestingly, BAC-enhanced expression of CX3CL1 was found to depend upon the TNF-α pathway. A recent in vitro study reported not only an overexpression of TNF-α, but also of IL-1, IL-10, and IL-12, in conjunctival cells exposed to BAC in vitro.14 We suggest that BAC-enhanced expression of TNF-α may upregulate CX3CL1 in an autocrine way by binding with TNF-RI. It is noteworthy that overexpression of CX3CL1 is observed in response to very low concentrations of BAC, i.e., inferior to the minimal concentration leading to significant cell apoptosis.42 Moreover, present BAC concentrations are inferior to what is commonly used in current ophthalmic medications, further underlying a proinflammatory property of this compound even at very low dose.
CX3CL1 assumes both a transmembrane form and a soluble form, presenting the dual functions of an adhesion molecule and a chemoattractant. To define its effects on immune cell attraction to the conjunctiva, chemotaxis migration assay was carried out combining human blood leukocytes and conjunctival IOBA-NHC cells. We demonstrate that BAC exposure gives direct chemotactic properties to conjunctival epithelial cells that significantly attract CD3+, CD14+, and CD56+ leukocytes through the CX3CL1–CX3CR1 interaction. CX3CR1 is known to be expressed in circulating lymphocytes, monocytes, and NKs.21, 22, 23, 24, 25 Herein, CX3CR1 blockade significantly inhibited the migration of CD14+ monocytes–monocyte-derived cells and CD3+ CD56+ NKs, but not that of CD3+ CD56− lymphocytes. Consistent with this, CX3CR1-related immune cell migration is known to depend on the subtype of leukocytes as well as on their expression pattern of chemokine receptors,43 further emphasizing that other chemokine systems are likely to regulate lymphocyte migration.
Transgenic mice have been generated to follow the immune cell turnover in relation with CX3CL1–CX3CR1.39, 40, 43 Given our results from migration assays, we investigated in vivo the inflammatory cell infiltration into the conjunctiva of CX3CR1-deficient mice and their CX3CR1+/+ controls. BAC exposure was found to enhance the conjunctival expression of CX3CL1 in mouse, similarly to what was observed in human. We did not find any difference in the density of CX3CR1+ resident dendritic cells between CX3CR1-deficient mice and their controls. Consistent with this result, a previous study in the mouse retina, iris, and ciliary body reported that dendritic cell homing does not depend on CX3CR1.44 On the contrary, another elegant study by Chinnery et al. found CX3CR1-dependent dendritic cell turnover in the cornea.32 These different findings may stem from differences in structure between the cornea and the other ocular tissues. Similarly, CD45+ cell infiltration was not different in untreated conjunctiva of CX3CR1-deficient mice as compared with that of CX3CR1+/+ animals. In BAC-exposed eyes, we report an accumulation of inflammatory cells at the conjunctiva, as previously observed in patients as well as in murine models.15, 18, 19, 20 Furthermore, we show that BAC enhances in vivo the expression of ICAM-1, C5a, TNF-α, IL-1, and IFN-γ in the conjunctiva. Interestingly, we report that BAC-induced conjunctival infiltration by F4/80+ marker-positive cells, as commonly assume as macrophages, is deeply reduced in the conjunctiva of CX3CR1-deficient animals as compared with CX3CR1+/+ controls. Accordingly, a recent study on an animal model of corneal alkali burn reported that CX3CR1-deficient mice presented a decrease in macrophage infiltration in the cornea compared with wild-type animals, whereas CD11b+ and CD3+ cell infiltration was not affected, similarly to what we observed.45
We report that BAC not only enhances MHC class II and inflammatory cytokine expression in epithelial cells but also induces monocyte-derived cell migration to the conjunctiva via CX3CL1–CX3CR1 interaction. These results suggest that CX3CL1 chemokine axis is also involved in pro-inflammatory processes induced by BAC, whereas CX3CR1+ cells are commonly described in previous studies as “patrolling” monocytes rather than pro-inflammatory ones. Indeed, blood monocytes consist of two principal populations with distinct properties: CX3CR1lo CCR2+ Ly6c+ murine monocytes, which share common features with human CD14+ CD16lo monocytes, are more likely to migrate to inflamed tissue, and CX3CR1hi CCR2− Ly6c− cells, which may correspond to human CD16+ monocytes, could be considered as “patrolling” monocytes.46, 47 However, whether these two monocyte subsets are distinct cells or the same at different stages of maturation is still debated. At least, they are associated with tissue pathology and wound-healing depending on the kind of tissue and the stage/severity of disease. In accordance with our findings, previous studies reported that the recruitment of monocyte-derived macrophages to inflamed tissue is depended, at least in part, on CX3CL1–CX3CR1 interaction. In the vasculature, Combadière et al.26 demonstrated in CX3CR1-deficient mice a 50% decrease in macrophage infiltration and a 59% decrease in plaque formation in a model of atherosclerosis. In the nervous system, the role of CX3CL1–CX3CR1 is debated as it was found to act to neuroprotection by some authors48 or to induce microglial activation and neuroinflammation by others. For instance, Donnelly et al.49 recently reported in a model of spinal cord injury a relative decrease in Ly6c+ iNOS+ MHCII+ CD11c− CD11b+ macrophage infiltration in mice lacking CX3CR1 as compared with wild-type animals, which promoted neuronal recovery.49 Closer to our patients–animal model that is mucosal inflammation at the ocular surface, Kostadinova et al.50 reported a 40% decrease in macrophage infiltration in the mucosa of CX3CR1-deficient mice in an animal model of toxic-mediated acute colitis.
In human patients exposed to BAC, we also reported that conjunctival expression of CX3CL1 by epithelial cells was correlated with ocular surface damage and inflammation as assessed by clinical examination. In experimental stroke, studies demonstrated that a deletion of CX3CL1 or CX3CR1 conferred neuroprotection.51, 52 In toxic-induced inflammatory bowel disease, Kostadinova et al. reported a decrease in the nitrite level of inflamed tissue as well as a decrease in the disease severity in mice lacking CX3CR1 as compared with wild-type animals. Owing to direct apoptotic and oxidative properties of the BAC previously applied onto the conjunctiva, we were not able to assess directly the cytotoxic effects of CX3CR1-related recruitment of F4/80+ cells. Thus, we performed inflammatory marker–cytokine proteome profiling in the mouse conjunctiva to better precise the consequences of this specific immune cell infiltration. We report a significant decrease in complement C5a fragment in the conjunctiva of BAC-exposed CX3CR1-deficient as compared with CX3CR1+/+ controls, which could be linked with a decrease in local inflammatory processes at the ocular surface. We also found in CX3CR1-deficient mice a significant decrease in TNF-α and IFN-γ levels suggesting partial suppression of the TH1 inflammatory cascade and/or a lack of NK infiltration in the conjunctiva of transgenic mice exposed to BAC. Accordingly, previous studies on the intestinal mucosa inflammation demonstrated that CX3CL1 triggered the release of TNF-a, IFN-g, and IL-6, i.e., Th-1 cytokines, whereas Th-2 cytokines were not affected.49, 53 Our team previously studied the conjunctival expression of CCR4 and CCR5 aiming at discriminating the Th1/Th2 processes involved during various ocular surface diseases.39 Interestingly, both CCR4 and CCR5 were found to be increased in patients exposed to BAK, which underlines the complex interactions involved in such a toxic-mediated ocular inflammation. Here we show that CX3CL1–CX3CR1 interaction is involved in macrophage recruitment, conjunctival inflammation, and disease severity in BAC-mediated ocular inflammation, further underscoring that the nature of CX3CR1-related monocyte–macrophage recruitment and activation may depend on tissue- and disease-specific mechanisms.
Clinical surveys as well as experimental studies have previously demonstrated that ophthalmic treatments cause secondary conjunctival inflammation related to the preservative toxicity. Preservatives such as BAC, however, are still commonly used in several ophthalmic medications against allergy, dry eye disease, and glaucoma, adding to ocular surface inflammation and damage. We originally report that a toxic exposure of the conjunctival mucosa modulates epithelial–immune cell interaction through the CX3CL1–CX3CR1 signaling pathway, and directly stimulates immune cell recruitment and related tissue inflammation. These original results suggest that conjunctival expression of CX3CL1 could constitute a novel biomarker for ocular surface inflammation, and has to be further studied in other ocular surface disorders such as allergy or dry eye.
Methods
Patients and conjunctival impression cytology. Twenty glaucomatous patients treated with BAC-preserved medication and 20 patients treated with BAC-free medication underwent clinical ocular examination and impression cytology. Clinical evaluation of the conjunctiva was routinely assessed using the slit-lamp by scoring conjunctival hyperemia (range, 0–5), conjunctival epithelium damage using lissamine green staining54 (van Bijsterveld index, range, 0–9), and tear break up time using fluorescein. Experiments were conducted in the Clinical Investigation Centre for Ocular Surface Pathology (Centre Hospitalier National d’Ophtalmologie des Quinze-Vingts, INSERM-DHOS CIC 503, Paris, France) in accordance with the Declaration of Helsinki, Scotland amendment, 2000. Ethics committee approval was obtained from the Comité de Protection des Personnes (Ile de France V, agreement number 10793) and all the patients gave informed consent before the study. Conjunctival imprints were collected as previously described.55
Human conjunctival epithelial cell line. IOBA-NHC cells, a spontaneously immortalized conjunctival epithelial cell line,56 were grown under standard conditions as previously described.11 Cells from passages 20–30 were used in all experiments. Cells were seeded (80,000 per ml) in six-well culture plates (TPP, Trasadingen, Switzerland) for PCR with reverse transcription, FCM, and ELISA, or grown on 22-mm glass coverslips for IF, fed with medium for 24 h, then stimulated with various concentrations of BAC in PBS for 15 min followed by 12-h culture in medium. TNF-α (50 ng ml−1) was used as a positive control. Blocking anti-TNF-RI antibody (10 μg ml−1, MAB 625, R&D Systems, Minneapolis, MN) was added in both stimulation and recovery mediums to determine the dependence of BAC-induced CX3CL1 synthesis upon TNF-α/TNF-RI. Mouse IgG1 at the same concentration was used as a negative control. For quantitative PCR with reverse transcription, total mRNA was isolated from cultured TCs using the NucleoSpin RNA II extraction kit (Macherey-Nagel, Düren, Germany). For FCM, cells were gently dislodged by 0.05% EDTA, resuspended in PBS with 0.4% paraformaldehyde, and divided into 100,000 cell samples. Cells grown on glass coverslips were dried and fixed in PBS with 4% paraformaldehyde before processing for IF.
Animals. Homozygous CX3CR1gfp/gfp transgenic mouse strains on C57BL/6 background and their CX3CR1+/+ controls were generated as previously described.34, 36 Animals were kept in pathogen-free conditions with food and water available ad libitum and housed in a 12-h light/12-h dark cycle. All the experiments were conducted in accordance with the Association for Research in Vision and Ophthalmology for the Use of Animals in Ophthalmic Research. Acute exposure to BAC was carried out by applying one drop of BAC solution (0.05%, 50 μl 0.9% NaCl) in the right eye and one drop of the vehicle in the left control eye, 15 times at 5-min intervals, consistently with a previously validated toxicological model.57 For CX3CL1 detection in the mouse conjunctiva, 10 mice were euthanized 6 h after BAC exposure and the conjunctiva were carefully removed and immediately conditioned for ELISA. To characterize the immune cell infiltration (20 animals) and related cytokine profile (20 animals), conjunctiva were taken 12 h after the BAC exposure.
Quantitative PCR. Primers for human CX3CL1 (forward: 5′-CATCATGCGGCAAACGCGCA-3′; reverse: 5′-TGCTTCTCGAAGGTGCCGCC-3′) and CX3CR1 (forward: 5′-GGTTCCCTTGGCAGTCCACGC-3′; reverse: 5′-TCCCACCAGGCCAATGGCAA-3′) were designed and purchased from Eurogentec (Angers, France). The mRNA was extracted from IOBA-NHC cells as described above, then reverse-transcribed (CyberGreen, Biorad, Marnes-LA-Coquette, France). Relative quantitation of target genes was calculated according to the comparative Ct method, i.e., normalized to an endogenous control S18 gene and relative to a calibrator after calculating the efficiency coefficient. The results are presented as the relative fold change using the ΔCt method compared with unstimulated control. A negative control was routinely used omitting mRNA from the reaction mixture.
Flow cytometry. Expression of HLA-DR, CX3CL1, and CX3CR1 in human conjunctival impression cytology and epithelial cell line was assessed by FCM using FC 500-CXP flow cytometer (Beckman Coulter, Miami, FL). Samples were incubated for 1 h at room temperature with primary anti-HLA-DR antibody (1/50, clone TAL.1B5, DakoCytomation, Glostrup, Denmark), anti-CX3CL1 (1/200, clone 81506, R&D Systems), or anti-CX3CR1 (1/500, clone 183-2, Imgenex, San Diego, CA) in PBS with 1% bovine serum albumin (BSA) followed by counterstaining with the corresponding fluorescein isothiocyanate-conjugated secondary antibody (DakoCytomation). Isotype-matched antibodies were used as negative controls. Cells were preincubated and immunostained by adding or not 0.5 mg ml−1 saponin to determine total or surface expression respectively. For each specimen, at least 1,000 cells per antibody were analyzed. The results were expressed as the increase in percentage of positive cells compared with that of unspecific isotypic control.
Enzyme-linked immunosorbent assay. The release of CX3CL1 in cell supernatants of human IOBA-NHC cells was measured by an ELISA kit (DY365, R&D Systems). The 96-well microtiter plates were coated overnight with capture monoclonal antibody then nonspecific binding was blocked for 2 h with 1% BSA in PBS. Duplicate samples (100 μl) of cell supernatants or serial dilutions of recombinant CX3CL1 standards were incubated for 2 h, incubated with detection monoclonal antibody for 2 h, followed by 20-min incubation with horseradish peroxidase-conjugated streptavidin. Reaction product was detected using color reagent. Optical density was read (450–570 nm, TECAN, Mannedorf, Switzerland) and averaged from ten measurements per well. Sensitivity was 615 pg ml−1.
CX3CL1 expression in the mouse conjunctiva was measured in tissue lysates by an ELISA kit (DY472, R&D Systems) as described above. Conjunctivae were sonicated on ice during 5 s in lysis buffer (20 mm Tris-HCl, 10% glycerol, 2 mm EDTA, 137 nM NaCl) with a protease inhibitor cocktail (10 μg ml−1 aprotinin, 10 μg ml−1 leupeptin, and 10 μg ml−1 pepstatin), then centrifugated (10,000 g at 4°C) during 5 min. Sensitivity was 781 pg ml−1.
Immunofluorescence. Expression of CX3CL1 and CX3CR1 was assessed by IF in conjunctival cell line. Cells were blocked for nonspecific binding with normal goat serum in PBS/0.1% Triton for 30 min, incubated at room temperature for 2 h with either anti-CX3CL1 (1/200, clone 25088, Abcam, Cambridge, MA) or anti-CX3CR1 (1/200, clone 8021, Abcam) primary antibody, then counterstained with secondary Alexa Fluor 488 antibody (Invitrogen, Carlsbad, CA) for 1 h. Isotype-matched antibodies were substituted for primary as negative controls. Cells were mounted in aqueous mounting medium with propidium iodide to be further analyzed using light epifluorescence microscopy.
Characterization of inflammatory cell infiltration in the mouse conjunctiva was assessed by IF. Conjunctival flat mounts were incubated in 0.1% triton-X in PBS with 1% BSA for 30 min, incubated overnight at 4°C with primary antibodies—anti-CD3 (clone 17-A2), anti-CD11b (cloneM1/70), anti-NKG2D (clone191004), and anti-EMR1/-F4/80 (clone521204)—purchased from R&D Systems, followed by counterstaining with the corresponding secondary Alexa Fluor 555 antibody (Invitrogen) in PBS with 10% mouse serum for 2 h. Specimens were flat mounted in aqueous mounting medium with 46-diamidino-2-phenyl indole and analyzed using a light epifluorescence microscope. Infiltrated cells in the superficial conjunctiva were counted in five 0.15-mm2 fields (magnification × 200) per specimen and the results were reported as number of cells per mm2.
Proteome profiling. Cytokine expression in the mouse conjunctiva was determined by a proteome profiler array (mouse cytokine array panel A, ARY006, R&D Systems) in tissue lysates obtained as described above. Each membrane was incubated overnight at 4°C with each sample mixed with the detection antibody cocktail, revealed with 30-min incubation with streptavidin–horseradish peroxidase followed by 5-min incubation with chemiluminescent reagent, then exposed to X-ray film in cassette during 10 min. Films were scanned and array image files were analyzed using ImageJ software (NIH, Bethesda, MD). Cytokine detection is expressed as the average signal intensity of duplicate spots subtracted from signal background and normalized to total protein concentration.
Chemotactic migration assay.. Primary blood leukocytes were isolated from heparinized venous blood (CTS Saint-Antoine, Paris, France) by one-step centrifugation on a Ficoll separating solution (Eurobio, Courta-boeuf, France) and cultured for 24 h in Dulbecco's modified Eagle's medium with BSA. Non-adherent leukocytes were resuspended at 1,000,000 cells ml−1, whereas adherent leukocytes were harvested with 0.5% EDTA, and resuspended at 200,000 cells ml−1 for separate assays. Chemotaxis was assayed in a six-well chemotaxis chamber through 5- and 8-μm pore-sized filters (Costar, Cambridge, MA). One milliliter of cell suspension of leukocytes was preincubated or not with blocking anti-CX3CR1 antibody at saturating concentration (30 min, 15 μg ml−1, Torrey Pines Biolaboratories, Secaucus, NJ) and was loaded onto the filter. Normal rabbit IgG from R&D Systems was used as negative control. The lower chamber contained medium only, unstimulated conjunctival epithelial cells, or conjunctival cells previously stimulated with 0.01% BAC (15-min stimulation). Recombinant CX3CL1 (10 ng ml−1) in free medium was used as positive control. The plates were incubated for 3 h. Nonadherent blood cells that migrated to the lower chamber were counted and characterized for their expression of CD45, CD3, CD14, and CD56 by FCM using direct multicolor immunostaining (Cyto-Stat6607073, 6603909, Beckman Coulter). In the migration assays for adherent leukocytes, cells that migrated to the bottom surface of the filter were stained with primary anti-CD14, counterstained with Alexa Fluor 488 secondary antibody, and counted in ten fields ( × 200) per filter. The results are expressed as chemotactic index, i.e., the ratio of migrated cells normalized to the control.
Statistical analysis. All data are reported as means±s.e.m. and represent at least three independent experiments. Data were controlled for normal distribution and the analysis was performed with NCSS software (NCSS, Kaysville, UT) using the t-test or the analysis of variance test for comparisons, and the Pearson's coefficient for correlations. A value of P<0.05 was considered significant.
References
Baudouin, C., Labbé, A., Liang, H., Pauly, A. & Brignole-Baudouin, F. Preservatives in eyedrops: the good, the bad and the ugly. Prog. Retin. Eye. Res. 29, 312–434 (2010).
Baudouin, C. Detrimental effect of preservatives in eyedrops: implication for the treatment of glaucoma. Acta. Ophthalmol. 86, 716–726 (2008).
Chawla, A., McGalliard, J.N. & Batterbury, M. Use of eye drops in glaucoma: how can we help to reduce non-compliance? 85, 464 (2007).
Costaglioa, C., Del Prete, A., Incorvaia, C., Fusco, R., Parmeggiani, F. & Di Giovanni, A. Ocular surface changes induced by topical application of latanoprost and timolol: a short-term study in glaucomatous patients with and without allergic conjunctivitis. Graefe's Arch. Clin. Exp. Ophthalmol. 239, 809–814 (2001).
Pisella, P.-J., Pouliquen, P. & Baudouin, C. Prevalence of ocular symptoms and signs with preserved and preservative free glaucoma medication. Br. J. Ophthalmol. 86, 418–423 (2002).
Bielory, L. Allergic and immunologic disorders of the eye. Part II: ocular allergy. J. Allergy Clin. Immunol. 106, 1019–1032 (2000).
Baudouin, C. Allergic reaction to topical eyedrops. Curr. Opin. Allergy Clin. Immunol. 5, 459–463 (2005).
Jaenen, N., Baudouin, C., Pouliquen, P., Manni, G., Figueiredo, A. & Zeyen, T. Ocular symptoms and signs with preserved and preservative-free glaucoma medications. Eur. J. Ophthalmol. 17, 341–349 (2007).
Denoyer, A. et al. Very-high-frequency ultrasound corneal imaging as a new tool for early diagnosis of ocular surface toxicity in rabbits treated with a preserved glaucoma drug. Ophthalmic Res. 40, 298–308 (2008).
De Saint Jean, M., Brignole, F., Bringuier, A.F., Bauchet, A., Feldmann, G. & Baudouin, C. Effects of benzalkonium chloride on growth and survival of Chang conjunctival cells. Invest. Ophthalmol. Vis. Sci. 40, 619–630 (1999).
Debbasch, C., Brignole, F., Pisella, P.-J., Warnet, J.M., Rat, P. & Baudouin, C. Quaternary ammoniums and other preservatives’ contribution in oxidative stress and apoptosis on Chang conjunctival cells. Invest. Ophthalmol. Vis. Sci. 42, 642–652 (2001).
Dogan, A.S., Orhan, M., Söylemezoglu, F., Irkec, M. & Bozkurt, B. Effects of topical antiglaucoma drugs on apoptosis rates of conjunctival epithelial cells in glaucoma patients. Clin. Exp. Ophthalmol. 32, 62–66 (2004).
Pisella, P-J. et al. Conjunctival proinflammatory and proapoptotic effects of latanoprost and preserved and unpreserved timolol: an ex vivo and in vivo study. Invest. Ophthalmol. Vis. Sci. 45, 1360–1368 (2004).
Epstein, S.P., Chen, D. & Asbell, P.A. Evaluation of biomarkers of inflammation in response to benzalkonium chloride on corneal and conjunctival epithelial cells. J. Ocul. Pharmacol. Ther. 25, 415–424 (2009).
Baudouin, C. et al. Ocular surface inflammatory changes induced by topical antiglaucoma drugs. Ophthalmology 106, 556–563 (1999).
Baudouin, C., Hamard, P., Liang, H., Creuzot-Garcher, C., Bensoussan, L. & Brignole, F. Conjunctival epithelial cell expression of interleukins and inflammatory markers in glaucoma patients treated over the long term. Ophthalmology 111, 2186–2192 (2004).
Brignole, F., De Saint-Jean, M., Goldschild, M., Becquet, F., Goguel, A. & Baudouin, C. Expression of Fas-Fas ligand antigens and apoptotic marker APO2.7 by the human conjunctival epithelium: positive correlation with class II HLA DR expression in inflammatory ocular surface disorders. Exp. Eye Res. 67, 687–697 (1998).
Becquet, F., Goldschild, M., Moldovan, M.S., Ettaiche, M., Gastaud, P. & Baudouin, C. Histopathological effects of topical ophthalmic preservatives on rat corneoconjunctival surface. Curr. Eye Res. 17, 419–425 (1998).
Noecker, R.J., Herrygers, L.A. & Anwaruddin, R. Corneal and conjunctival changes caused by commonly used glaucoma medications. Cornea 23, 490–496 (2004).
Sherwood, M.B., Grierson, I., Millar, L. & Hitchings, R.A. Long-term morphologic effects of antiglaucoma drugs on the conjunctiva and Tenon's capsule in glaucomatous patients. Ophthalmology 96, 327–335 (1989).
Bazan, J.F. et al. A new class of membrane-bound chemokine with a CX3C motif. Nature 385, 640–644 (1997).
Imai, T. et al. Identification and molecular characterization of fractalkine receptor CX3CR1, which mediates both leukocyte migration and adhesion. Cell 91, 521–530 (1997).
Chapman, G.A. et al. The role of fractalkine in the recruitment of monocytes to the endothelium. Eur. J. Pharmacol. 392, 189–195 (2000).
Garton, K.J. et al. Tumor necrosis factor-alpha converting enzyme (ADAM17) mediates the cleavage and shedding of fractalkine (CX3CL1). J. Biol. Chem. 276, 37993–38001 (2001).
Goda, S. et al. CX3C-chemokine fractalkine-enhanced adhesion of THP-1 cells to endothelial cells through integrin-dependent and –independent mechanisms. J. Immunol. 164, 4313–4320 (2000).
Combadière, C. et al. Decreased atherosclerotic lesion formation in CX3CR1/apolipoprotein E double knockout mice. Circulation 107, 1009–1016 (2003).
Imaizumi, T., Yoshida, H. & Satoh, K. Regulation of CX3CL1/fractalkine expression in endothelial cells. J. Atheroscler. Thromb. 11, 15–21 (2004).
Lucas, A.D. et al. The transmembrane form of the CX3CL1 chemokine fractalkine is expressed predominantly by epithelial cells in vivo. Am. J. Pathol. 158, 855–866 (2001).
Muehlhoefer, A. et al. Fractalkine is an epithelial and endothelial cell-derived chemoattractant for intraepithelial lymphocytes in the small intestinal mucosa. J. Immunol. 164, 3368–3376 (2000).
Raychaudhuri, S.P., Jiang, W.Y. & Farber, E.M. Cellular localization of fractalkine at sites of inflammation: antigen-presenting cells in psoriasis express high levels of fractalkine. Br. J. Dermatol. 144, 1105–1113 (2001).
Rimaniol, A.-C. et al. The CX3C chemokine fractalkine in allergic asthma and rhinitis. J. Allergy Clin. Immunol. 112, 1139–1146 (2003).
Chinnery, H.R. et al. Turnover of bone marrow-derived cells in the irradiated mouse cornea. Immunology 125, 541–548 (2008).
Chinnery, H.R., Ruitenberg, M.J., Plant, G.W., Pearlman, E., Jung, S. & McMenamin, P.G. The chemokine receptor CX3CR1 mediates homing of MHC class II-positive cells to the normal mouse corneal epithelium. Invest. Ophthalmol. Vis. Sci. 48, 1568–1574 (2007).
Jung, S. et al. Analysis of fractalkine receptor CX3CR1 function by targeted deletion and green fluorescent protein reporter gene insertion. Mol. Cell. Biol. 20, 4106–4114 (2000).
Kezic, J. & McMenamin, P.G. Differential turnover rates of monocyte-derived cells in varied ocular tissue microenvironments. J. Leukol. Biol. 84, 721–729 (2008).
Combadière, C. et al. CX3CR1-dependent subretinal microglia cell accumulation is associated with cardinal features of age-related macular degeneration. J. Clin. Invest. 117, 2920–2928 (2007).
Ruth, J.H. et al. Fractalkine, a novel chemokine in rheumatoid arthritis and in rat adjuvant-induced arthritis. Arthritis Rheum. 44, 1568–1581 (2001).
Wildenberg, M.E., van Helden-Meeuwsen, C.G., Drexhage, H.A. & Versnel, M.A. Altered fractalkine cleavage potentially promotes local inflammation in NOD salivary gland. Arthritis Res. Ther. 10, R69 (2008).
Baudouin, C. et al. CCR4 and CCR5 expression in conjunctival specimens as differential markers of Th1/Th2 in ocular surface disorders. J. Allergy Clin. Immunol. 116, 614–619 (2005).
Baudouin, C. et al. The ocular surface of glaucoma patients treated over the long term expresses inflammatory markers related to both T-helper 1 and T-helper 2 pathways. Ophthalmology 115, 109–115 (2008).
Enriquez-de-Salamanca, A. et al. Tear cytokine and chemokine analysis and clinical correaltions in evaporative-type dry eye disease. Mol. Vis. 16, 862–873 (2010).
Brasnu, E., Brignole-Baudouin, F., Riancho, L., Warnet, J.M. & Baudouin, C. Comparative study on the cytotoxic effects of benzalkonium chloride on the Wong-Kilbourne derivative of Chang conjunctival and IOBA-NHC cell lines. Mol. Vis. 14, 394–402 (2008).
Ancuta, P. et al. Fractalkine preferentially mediates arrest and migration of CD16+ monocytes. J. Exp. Med. 197, 1701–1707 (2003).
Kezic, J., Xu, H., Chinnery, H.R., Murphy, C.C. & McMenamin, P.G. Retinal microglia and uveal tract dendritic cells and macrophages are not CX3CR1 dependent in their recruitment and distribution in the young mouse eye. Invest. Ophthalmol. Vis. Sci. 49, 1599–1608 (2008).
Lu, P. et al. Protective roles of the fractalkine/CX3CL1-CX3CR1 interactions in alkali-induced corneal neovascularization through enhanced antiangiogenic factor expression. J. Immunol. 180, 4283–4291 (2008).
Geissmann, F., Jung, S. & Littman, D.R. Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity 19, 71–82 (2003).
Robbins, C.S. & Swirski, F.K. The multiple roles of monocyte subsets in steady state and inflammation. Cell. Mol. Life Sci. 67, 2685–2693 (2010).
Cardona, A.E. et al. Control of microglial neurotoxicity by the fractalkine receptor. Nat. Neurosci. 9, 917–924 (2006).
Donnelly, D.J. et al. Deficient CX3CR1 signaling promotes recovery after mouse spinal cord injury by limiting the recruitment and activation of Ly6Clo/iNOS+ macrophages. J. Neurosci. 31, 9910–9922 (2011).
Kostadinova, F.I. et al. Crucial involvement of the CX3CL1-CX3CR1 axis in dextran sulfate sdium-mediated acute colitis in mice. J. Leukol. Biol. 88, 133–143 (2010).
Soriano, S.G. et al. (2002) Mice deficient in fractalkine are less susceptible to cerebral ischemia-reperfusion injury. J. Neuroimmunol. 125, 59–65 (2002).
Dénes, A., Ferenczi, S., Halasz, J., Kornyei, Z. & Kovacs, K.J. Role of CX3CR1 (fractalkine receptor) in brain damage and inflammation induced by focal cerebral ischemia in mouse. J. Cereb. Blood Flow Metab. 28, 1707–1721 (2008).
Mizutani, N. et al. Dose-dependent differential regulation of cytokine secretion from macrophages by fractalkine. J. Immunol. 179, 7478–87 (2007).
Klaassen-Broekema, N., Mackor, A.J. & van Bijsterveld, O.P. The diagnostic power of the tests for tear gland related keratoconjunctivitis sicca. Neth. J. Med. 40, 113–116 (1992).
Baudouin, C., Brignole, F., Becquet, F., Pisella, P-J. & Goguel, A. Flow cytometry in impression cytology specimens: a new method for evaluation of conjunctival inflammation. Invest. Ophthalmol. Vis. Sci. 38, 1458–1464 (1997).
Diebold, Y. et al. Characterization of a spontaneously immortalized cell line (IOBA-NHC) from normal human conjunctiva. Invest. Ophthalmol. Vis. Sci. 44, 4263–4274 (2003).
Liang, H. et al. Reduction of quaternary emulsion ammonium-induced ocular surface toxicity by emulsions: an in vivo study in rabbits. Mol. Vis. 14, 204–216 (2008).
Acknowledgements
This work was supported by INSERM grant to AD, DG, FB, WR, and CB, and by MESR grant to JF and IC. We thank Michel Pâques and Manuel Simonutti (INSERM U968, Paris, FR) for providing us with transgenic mice. We also thank Christophe Combadière (INSERM U543, Paris, FR) for his helpful advices.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declared no conflict of interest.
Rights and permissions
About this article
Cite this article
Denoyer, A., Godefroy, D., Célérier, I. et al. CX3CL1 expression in the conjunctiva is involved in immune cell trafficking during toxic ocular surface inflammation. Mucosal Immunol 5, 702–711 (2012). https://doi.org/10.1038/mi.2012.43
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/mi.2012.43
This article is cited by
-
Cathepsin S activation contributes to elevated CX3CL1 (fractalkine) levels in tears of a Sjögren’s syndrome murine model
Scientific Reports (2020)
-
Overexpression of fractalkine and its histopathological characteristics in primary pterygium
Graefe's Archive for Clinical and Experimental Ophthalmology (2019)