Abstract
The study of animal immune physiology and animal models of human disease have accelerated many aspects of translational research by allowing direct, definitive investigations. In particular, the use of mice has allowed genetic manipulation, adoptive transfer, immunization, and focused cell and tissue sampling, which would obviously be unthinkable for studies in humans. However, the disease relevance of some animal models may be uncertain and difficulties in interpretation may occur as a consequence of immunological differences between the two species. In this review, we will consider general differences in the structure and development of human and mouse mucosal lymphoid microenvironments and then discuss species differences in mucosal B- and T-cell biology that relate to the current concepts of intestinal immune function.
Similar content being viewed by others
Mouse and Human Mucosal Defense
In evolutionary terms, mice and humans diverged between 65 and 75 million years ago, and over the millennia, have exploited different habitats and differed in their exposure to potential pathogens.1 Therefore, immunological priorities and mechanistic differences are to be expected. The range of differences in the immune system between mice and humans is extensive, and we challenge anyone not to find surprises that are of direct relevance to intestinal inflammation in the excellent general review of this area by Mestas and Hughes.1 For example, whereas Paneth cells located in the crypts of the mouse small intestine secrete >20 α-defensins, only 2 α-defensins are secreted by human Paneth cells. On the other hand, mouse neutrophils do not produce defensins but human neutrophils do.2, 3, 4 It is noteworthy that there is also a difference in the relative frequency of neutrophils in mice and humans. Mouse blood comprises 70–90% lymphocytes and only 10–30% neutrophils. In contrast, human blood contains 30–50% lymphocytes and 50–70% neutrophils.1, 5 Thus, the pathways involved in the evolution of intestinal inflammation are not conserved across these species boundaries. The potential scope for comparisons of factors that relate to intestinal biology is enormous, especially if variability across many species is considered. Therefore, in this review, we will focus on the similarities and differences between B- and T-cell lineages in the intestines of mice and humans.
Comparison of the Structure of Human and Mouse Lymphoid Microenvironments
In terms of anatomy, the gastrointestinal tract is similar in both mice and humans. There are three distinct mucosal lymphoid microenvironments: first, the mucosa-associated lymphoid tissue (MALT); second, the diffuse connective tissue termed “lamina propria (LP)” in the mucosae (stroma in the exocrine glands), which hosts effector cells that secrete immune mediators, such as cytokines and mucosal immunoglobulins; and third, a unique niche between epithelial cells along the length of the gastrointestinal tract that contains intraepithelial lymphocytes (IELs).6, 7 In addition, mesenteric nodes are sites of immunological activity related to the protection of mucosae that are common to both species, although they tend to be relatively small and difficult to identify in fresh unfixed human mesentery.
MALT is an organized lymphoid tissue resembling the basic units of lymph nodes in structure. It is defined by the juxtaposition of organized lymphoid to specialized lymphoepithelium termed the “follicle-associated epithelium (FAE).” In mice and humans, most MALT is located in the gastrointestinal tract (gut-associated lymphoid tissue, GALT) in either clusters (Peyer's patches, PPs) or isolated lymphoid follicles (ILFs) distributed along the length of the large and small intestines. Mice generally have more abundant MALT in the bronchi (bronchus-associated lymphoid tissue) than do humans.8 This has been linked to the fact that mice inhale close to the ground and may take up more antigens than humans through this route. Unlike mice, humans have an appendix that is a rich source of MALT.9
The FAE that forms the barrier between the intestinal lumen and the internal environment of the body is highly conserved. It mediates antigen transport and is the portal for particulate antigen entry. The FAE contains microfold cells, or M cells, which are specialized epithelial cells that sample the lumen and thus expose the associated lymphoid tissues to luminal contents, including particulate material and IgA-coated antigens.10, 11 The frequency of M cells in the FAE depends on species and age, although in general, mice have relatively more M cells than do humans. Similar to other components of the FAE, M cells are present before birth in humans.12, 13 The FAE is disrupted by a B- and T-cell infiltrate, the composition of which is distinct from the IEL population in the epithelia that are not associated with organized lymphoid tissues.9, 14, 15 The B-cell component of the FAE is different between species. In mice, intraepithelial B cells express the IgD immunoglobulin isotype. In contrast, B cells in the human FAE tend not to. The lymphocytic component of the FAE of humans is apparent without antigenic exposure, but the infiltrate increases as the FAE matures in response to microbial colonization in both species.12, 16, 17 The FAE function itself is regulated by Toll-like receptors (TLRs) and associates intimately with a multifunctional dendritic cell component that is central to the development of IgA.18, 19, 20
MALT: Dependence on the Microflora
The microflora in the intestines of mice and humans is broadly similar, being composed predominantly of two bacterial phyla, namely the Firmicutes and the Bacteroidetes.21 The differences between mice and humans in the composition of the microbiota is far greater than those between individuals within a species. Differences are likely to be affected by diet and hygiene, including coprophagy in mice.22 Coprophagy also exposes the small intestinal mucosa to microbe-associated molecular patterns in feces that are normally more restricted to the large bowel in humans.
In mice and in humans, ILFs are found through the intestine, but PPs and ILFs are more abundant in the distal small bowel. The concentration of PP in the ileum is more marked in humans than in mice.23, 24, 25 In mice, PPs and ILFs develop sequentially. Whereas PPs are constitutive and induced to develop before birth by lymphoid tissue inducer cells (LTi),26 ILFs develop after birth in response to the flora through colonization of clusters of LTi-like cells termed “cryptopatches”.27, 28, 29 Nucleotide-binding oligomerization domain 1 is crucially involved in maturation of murine ILFs as the gut becomes colonized, through recognition of bacterial peptidoglycans.30 In humans, PPs, ILFs, and appendix have been described in the fetal intestine as organized structures, identifiable from 22 weeks of gestation.12, 16, 31 Human cryptopatches have not been described, but clusters of cells resembling inducer cells (CD4+, CD3−, CD45+, class II MHC (major compatibility complex)+++) are identifiable from ∼11 weeks of gestation in the absence of exogenous antigen.32, 33 Organized clusters of B and T cells can be observed in the human fetal intestine in groups and as isolated follicles, thus indicating that PPs and ILFs may be constitutive in humans. However, in addition to constitutive GALT, GALT can be acquired in humans in response to bacteria. For example, MALT is acquired in the stomach that normally does not contain lymphoid tissues, in response to infection with the pathogenic gastric bacterium, Helicobacter pylori.34
Origin of IgA Plasma Cells
Profound differences exist in mouse and human B-cell functional capability. Mouse, but not human B cells, express TLR4 that enables them to respond polyclonally and T-cell independently to lipopolysaccharide.35 This may be highly relevant in the gut that is rich in microbe-associated molecular patterns, such as lipopolysaccharide. In addition, marked differences in the consequences of signal transduction through the B-cell receptor are apparent in the different phenotypes of Bruton's tyrosine kinase deficiency in mice and humans. Mutations of Bruton's tyrosine kinase, which is critical for B-cell development and function, cause an almost total B-cell developmental block in humans that manifests as X-linked agammaglobulinemia. In mice, there is a less severe phenotype with impairment of B-cell development and function referred to as X-linked immunodeficiency.36 Therefore, differences in mucosal B-cell function in mice and humans are inevitable.
Germ-free animals and newborn humans have few or no IgA plasma cells in the LP, respectively.37, 38, 39 Colonization, even with a single bacterial species induces IgA plasma cell generation, and the IgA produced includes specificity for the luminal microbiota.40, 41, 42 The origin of the IgA response is still debated, partly because there has been some confusion over the relative importance of the LP and GALT as inductive sites, and how this relates to the involvement of the B-1 B-cell lineage in the IgA response in mice. The so-called “dual origin” of IgA plasma cells initially referred to the origin of IgA plasma cells in mice from either B-1 or B-2 cell lineages.43 Murine B-1 B cells are a self-renewing and self-sustaining B-cell subset associated with innate immunity through their production of polyspecific antibodies. The frequency and subset composition of B-1 B cells vary between mouse strains,44 and the precise contribution of the B-1 B-cell subset to the plasma cell population in mice is not clear, but estimates of up to 50% have been suggested.45 The peritoneal B-1 B-cell population has no direct equivalent in humans, although B cells that express CD5 are present in the human fetal intestine.16, 46 B-1 B cells may originate in the fatty omentum in the peritoneum of mice,47 but again there is no evidence that the adult human omentum contains B cells that might either be precursors of or substitutes for B-1 B cells in the murine peritoneum.48
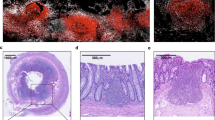
There is a link between the murine spleen and the mucosa in this context. It has been recently shown that in mice, B-1a B cells and mucosal IgA derive from lineage-negative precursors found in the fetal liver that reside in the spleen after birth. Splenic precursors replenish plasma cells producing natural antibodies.49 The human spleen does not contain developing B cells; indeed, the structure and composition of the mouse and human spleen are markedly different.50 There is proportionately more white pulp in the mouse spleen compared with the human spleen, and the vasculature of the marginal zone is different. In mice, the marginal zone forms the external zone of cells surrounding the whole of the white pulp, whereas in humans, it encircles the B-cell zone (Figure 1).
Sections of ileal Peyer's patches from (a and c) mouse and (b and d) humans and spleen from (e) mouse and (f) humans. Sections are stained with hematoxylin and eosin (panels a, b, e, and f) or antibody to IgD (panels c and d). Germinal centers are marked GC. Although the basic histological structure is similar in mouse and human Peyer's patches, the boundary of the germinal center is not precisely defined in mice as it is in humans. The marginal zone of IgD-negative B cells that surrounds the mantle zone in human PP is not apparent in mice. In the mouse spleen, the white pulp is relatively more abundant than the red pulp, and the marginal zone forms the boundary at the periphery of the white pulp. The mouse spleen shares a lineage relationship with the B-1 B-cell precursors of IgA plasma cells, which is not seen in humans. In contrast, in humans, most of the spleen is red pulp. In white pulp, the marginal zone encircles the mantle zone. The human spleen and Peyer's patches have a marginal zone B-cell subset that is morphologically similar and that seems to share a common migratory pathway.
The “dual-origin” concept was built upon by studies claiming that IgA could originate from either the LP or the GALT as inductive sites in mice.51 B-1 B-cell function in the LP was related to the ability of dendritic cells to extend dendritic processes through the intestinal epithelium and sample antigens,52, 53 thus potentially initiating local B-1 B-cell activity in the LP and development of an IgA response. However, recent studies that sampled mouse LP cells while ensuring that no cells from ILFs were present in the isolates, saw no evidence for expression of activation-induced deaminase, which is an enzyme that is crucially required for class-switch recombination and therefore for the development of class-switched IgA responses.24, 54 These data, together with the observation that GALT structures are required for IgA responses,55 suggest that previous data indicating that LP is an inductive site for IgA responses in mice were a consequence of inadvertent sampling of ILFs. Therefore, the question of whether activation-induced deaminase is expressed in the human LP, and whether inductive events and class-switch recombination can occur in the human LP is still debated.56, 57
It is generally accepted that IgA plasma cells can be generated from germinal-center responses in mouse and human GALT. This response is promoted by the IgA class-switch factor transforming growth factor-β (TGFβ), and depends on cognate B-cell–T-cell interaction involving CD40–CD40L interactions.58, 59 However, mice with defects in the CD40–CD40L interaction still have an IgA response, although IgA does not have a somatic mutation in variable region genes.24, 54 This is consistent with potential germinal-center independence and T-cell independence of IgA responses. Similarly, IgA responses can be generated in human B cells without CD40 or TGFβ; class switching to IgA that is independent of T cells can be promoted by the B-cell survival factor APRIL (a proliferation-inducing ligand).60 Activation-induced deaminase can be expressed in mouse and human GALT, as well as inside and outside the germinal center within GALT.24, 61 B-cell division required for class-switch recombination also occurs in mouse and human GALT, both inside and outside germinal centers.24, 61 Therefore, there is evidence that in both mice and humans, the T-cell dependent and T-cell independent routes to class-switch recombination to IgA can occur in GALT.
Most intestinal IgA plasma cells from the mouse and human intestine have somatic mutations in their variable regions genes, although this does not necessarily infer specificity.62 Some of the earliest studies of the somatic hypermutation mechanism exploited this property of GALT germinal-center cells from mice that form without “immunization”.63 However, the frequency of mutation in the plasma cell population seems to be lower in mice. Mice have approximately five mutations per variable region on average, in which the population includes some sequences with no mutations. Human variable region genes from intestinal plasma cells have on average ∼15 mutations.54, 61 This may reflect different properties or dynamics of the GALT germinal-center response in mice, or it may reflect a more complex origin of murine compared with human IgA plasma cells, with contributions from B-1 and B-2 B-cell lineages in mice. The observation that murine peritoneal B-1 B cells may have mutations in IgV gene rearrangements switched to Cα suggests that even this population may not be germinal center or GALT independent.64
There are fundamental differences in the B-cell subsets residing in murine and human GALT. In humans, the germinal center has a clear boundary and is surrounded by a narrow mantle zone of naive B cells. This is surrounded by a zone of cells known as the mucosal marginal zone because of the morphological resemblance of cells in this location to splenic marginal zone B cells, their lack of expression of IgD, and their microanatomical location around the mantle.14 It is highly unlikely that marginal-zone B cells in these locations are directly analogous; e.g., those in the spleen tend to have a low expression of IgD, whereas those in the mucosa tend to lack IgD. In mice, cells of GALT that express IgD do not form such a distinct boundary with the mantle and they extend up to and into the FAE in what seems to be a single continuous population of cells65 (Figure 1).
In both mice and humans, the end point of mucosal B-cell response is the production of plasma cells predominantly of the IgA isotype. Table 1 details the differences in the IgA system in mice and humans. In mice, IgA is much more restricted in its function to the protection of mucosal surfaces than in humans.
Mucosal T-Cell Compartments
T cells within the GALT tend to phenocopy those in active organized lymphoid tissues in the periphery. In contrast, T cells that reside in the LP, and to an even greater extent in the epithelium, display distinct features uniquely characteristic of mucosal T cells, usually exhibiting an effector/memory phenotype.
Intestinal Epithelia: a Niche for Unconventional T Cells
In both mice and humans, the ratio of T cells:enterocytes in the epithelia of the small intestine is in the range of 1:5–10, although this ratio decreases in the colon where enterocytes outnumber T cells 40-fold.66 Despite this similarity, the profiles of T cells that reside in the intestinal epithelium vary dramatically between the two species. Furthermore, the populations of T cells in the gut can alter with age,67 and recent data suggest that the cytokine profile of gut T cells is also heavily influenced by the microflora implying variations in T-cell populations that are not inherent to the species but dependent on the environment.68, 69, 70
The percentage of γδ T cells varies between the mouse and human intestine. γδ T cells are the prototypic “unconventional” T cells, so called because of their lack of classical MHC restriction, their “activated yet resting” phenotype, and their mode of antigen recognition.71 In the mouse, up to 50% of murine IELs in the small intestine express the γδ T-cell receptor (TCR). This is in marked contrast to the periphery where γδ T cells represent only a small fraction of T cells. However, this high frequency of γδ T cells observed in the mouse intestine is not apparent in the human gut, and γδ T cells represent <10% of the IEL population in humans with the exception of diseases such as celiac disease in which a dramatic increase in γδ T cells is observed.72 Nevertheless, in both mice and humans, the clonal repertoire of γδ cells in the gut is restricted with mice mainly expressing the Vγ7 TCR coupled with Vδ4 or Vδ673 and humans expressing Vδ1 and to a lesser extent Vδ3 with a predominance of Vγ8.74, 75 In each case, this repertoire is distinct from that in the periphery of either species and it is believed that, although the specificity of γδ T cells remain ill-defined, these cells may recognize endogenous stress antigens in the gut. Human γδ Vδ1T cells can recognize the polymorphic antigens, MICA/B, induced by stress on the intestinal epithelia, either through their TCR or through their expression of NKG2D.76 However, there is no MICA/B in mice, although other NKG2D ligands are present such as members of the Rae1 and H60 families, which are also usually stress induced. Thus, in both cases, although by recognition of different molecules and possibly by different receptors (e.g., there is no evidence in mice that the γδ TCR can bind directly to Rae1), the γδ T-cell population in the gut may recognize the stressed epithelium.
Evidence from mouse studies also suggests that the gut can harbor large numbers of other unconventional T cells independent of those that express the γδ TCR.77, 78 Thus, in addition to TCRαβ+ cells that express CD4 or CD8αβ, akin to systemic T cells, unconventional TCRαβ+ cells that express the CD8αα homodimer, and “double-negative” TCRαβ+ cells that lack both CD4 and CD8 have been identified, which share many unconventional characteristics with γδ T cells.79 Such cells have also been identified in the human fetal intestine,16, 80, 81 and improving sample procurement and analytical technologies have permitted us and others (E Binda and C Mueller in preparation) to show that, as in mice, the human adult intestine harbors significant numbers of unconventional TCRαβ+ T cells. Indeed, unconventional T cells including γδ, double-negative, and CD8αα have been identified in the intestine of many other species, including dogs.82 In mice, half of the TCRαβ-expressing T cells in the IEL population are unconventional, a subset of which has been shown to be immunoregulatory.83 The presence of CD8αα is believed to increase the threshold for T-cell activation and hence to prevent excessive inflammatory responses to commensal bacteria.84 A ligand for CD8αα, thymic leukemia antigen, which is constitutively expressed on the gut epithelia, has only been identified in mice,85 and the role of CD8αα in the human gut has yet to be fully elucidated.
LP T cells
T cells in the LP in both mice and humans are mainly CD4+ T cells and all the classical TH subsets (TH1, TH2, TH17) and T-regulatory cells are represented. There are also populations of natural killer T (NKT) cells. Several reports indicate different NKT subsets in the mice and human intestine, but there is a huge variation in the different studies in part due to inconsistencies in NKT definitions and the fact that conventional T cells can upregulate natural killer markers upon activation. Both variant and invariant NKT cells have been identified in the mice and human intestine (reviewed in the study by Middendorp and Nieuwenhuis86). Invariant NKT cells express Vα14Jα18 in mice and Vα24Jα18 in humans, and recognize glycolipids presented by CD1d on enterocytes and dendritic cells. It should be noted that mice express only CD1d, whereas other isoforms are present in humans (CD1a–e).87 Human iNKT cells also express the C-type lectin, CD161, whereas mice do not. More recently, a population of mucosal invariant T cells (MAIT) has been identified that recognizes as yet undefined ligands (possibly α-mannosylceramide; see the study by Shimamura et al.88) presented by MHC class I-like molecule, MR1. These express Vα19Jα33 paired with Vβ6/Vβ8 in mice and Vα7.2Jα33 paired with Vβ2/Vβ13 in humans. Mouse MAIT cells seem naive, in contrast to the memory phenotype of human MAIT. This may be related to the different levels of the transcription factor, promyelocytic leukemia zinc finger, in mice and humans or may imply a different differentiation pathway between the two species.89
The manner by which T cells get to the gut is still unclear especially for the large intestine with the only known requirement being α4β7. However, it does seem that gut homing to the small intestine is similar in both mice and humans. CD103+ dendritic cells have been found in murine and human mesenteric lymph nodes that confer CCR9 and α4β7 expression and hence the gut-homing ability on responding T cells, suggesting a conserved mechanism between the species.90
T-regs in the Gut
The intestinal immune system is in a constant state of controlled activation to maintain homeostasis and tolerance to dietary antigens and commensal bacteria. Regulatory T cells (T-regs) have a fundamental role in maintaining homeostasis in the gut, and transfer of T-regs can inhibit and cure colitis in the T-cell transfer colitis model of the disease.91 TGFβ can induce FOXP3 expression and suppressive functions in mice, whereas forced expression of FOXP3 or its induction with TGFβ is not sufficient to induce a regulatory function in humans.92, 93 Thus, in contrast to mice, the expression of FOXP3 is not always linked to suppressor function,94, 95 which may go some way to explain a lack of evidence for defects or reductions in human T-regs in IBD pathogenesis. A further difference between murine and human T-regs, particularly important in the mucosa, relates to stimulation through TLR2. Activation of TLR2 reduces murine T-reg-suppressive capacity,96 whereas stimulation of human T-regs through TLR2 increases suppression,97 although the use of different TLR2 ligands in these studies may have activated different TLR2 complexes.
There are additional subsets of CD4+ T-regulatory cells that lack FOXP3 but express interleukin (IL)-10 known as type I T-regs (Tr1), which could also prevent colitis in mice.98 Many different regimens have been identified in mice that induce these Tr1 cells, although the pathways that induce these cells in humans are less well defined. However, the complement regulator, CD46 has been identified as a key factor in humans in the switch from Th1 to Tr1-like cells.99 The ability of CD46 stimulation (with IL-2) to convert human CD4 Th1 cells into IL-10-producing regulatory cells may be of paramount importance in the gut where chronic stimulation produces a milieu conducive to such conversion. However, murine somatic cells lack any expression of CD46, and no molecule that recapitulates TCR-IL-2-CD46-dependent IL-10 production has been identified.100 Recent data have suggested that activation of the aryl hydrocarbon receptor (AHR) in mice promotes differentiation of Tr-1 cells, whereas in humans, both Tr-1 and FOXP3+ T-regs are induced by AHR activation, depending on the cytokine context.101, 102 As it is already known that AHR activation by specific ligands promotes expansion of Th17 cells,103 it is conceivable that AHR ligands, provided by the diet or intestinal flora, influence the differentiation of many different T-cell subsets (such as Tr-1, FOXP3+ T-regs, γδ T cells, and Th17) in vivo and that this differs between mice and humans. Similarly, the fact that human AHR has at least a 10-fold lower affinity for dioxins than AHR in mice may also suggest different response thresholds.104
IL-17-Producing T Cells in the Gut
Closely linked to the differentiation of T-regs in the gut is the differentiation of Th17 cells, not least as T-regs can convert to Th17 in the presence of IL-6 in mice and IL-6 and IL-1β in human cells.105, 106, 107 The Th17 population has received an enormous amount of attention especially relating to the gut mucosa. It has been shown that IL-17 production by Th17 cells can be regulated by specific fractions of the intestinal commensal population68, 69, 70, 108 and that during gut inflammation, many of the functions traditionally attributed to IL-12 and Th1 cells could instead be due to IL-23 and Th17.109 However, there is evidence that Th17 differentiation is differentially regulated in humans and mice. IL-6 and TGFβ can induce murine Th17 differentiation,110, 111 wherein IL-6 signaling activates STAT (signal transducer and activator of transcription) 3 and the lineage transcription factor, RORγt112 and TGFβ indirectly favors the expansion of Th17 cells by inhibiting the expression of transcription factors required for Th1 and Th2 development.113, 114 In contrast, IL-6 plus TGFβ is not sufficient for Th17 differentiation in humans. However, Th17 can be induced in humans with IL-1β together with IL-23 or IL-6.115, 116 Both IL-1β and IL-6 are present in the gut mucosa and are elevated in IBD patients,117 suggesting a milieu that may promote IL-17 production and contribute to disease pathogenesis. Furthermore, recent murine data suggest that this combination of cytokines (IL-1β, IL-6, IL-23) can also induce Th17 differentiation in the mouse, in the absence of TGFβ signaling, suggesting that species-specific differences may not be as pronounced as initially believed. Interestingly, induction of Th17 in the absence of TGFβ induces cells co-expressing RORγt and T-bet and thus capable of producing both IL-17 and interferon-γ, a “double-producing” cell believed to have greater pathogenic potential.118
Unconventional cells are ideally suited to provide an innate source of IL-17 in the earliest stages of the inflammatory response before the cytokine can be produced by antigen-specific TCRαβ T cells.119 TLRs can activate mouse γδ cells directly to induce IL-17120, 121 and it seems that the presence of certain commensals may also be important for specific IL-17-expressing murine γδ T cells as they are for conventional Th17 T cells.122 However, whereas IL-17-producing γδ T cells have been readily identified in mice and appear to be programmed for IL-17 production during thymic differentiation,123 IL-17-producing γδ cells have been hard to identify in humans. In the periphery, very few Vγ9-expressing T cells (1%) produce IL-17, and similarly, a very low percentage of IL-17-expressing γδ T cells is achieved even after polarization in vitro,124 although some IL-17-producing γδ cells have been observed in patients with active pulmonary tuberculosis.125 The Vδ1 γδ population, more usually associated with the gut in humans, is increased in the periphery of patients with HIV (human immunodeficiency virus) and these cells have been shown to produce IL-17 and express CD27,126 in direct contrast to murine γδ IL-17-expressing cells, which are CD27 negative. Whether these Vδ1-expressing cells are also capable of IL-17 expression in their normal niche of the gut is yet to be explored.
It may be the case that other unconventional cells, distinct from γδ T cells, are important for rapid mucosal IL-17 production in the gut. The recently identified innate cell population that mediates IL-23-dependent production of IL-17 and interferon-γ are the obvious candidates.127 Although these cells were described in mice, a population with similar characteristics is also believed to exist in humans.
IL-17R ligation in humans induces IL-8128 and subsequent neutrophil recruitment.129 However, as there is no structural homolog of IL-8 in mice130, 131 (although other chemokines such as KC may have similar properties) and as mice also have reduced neutrophil numbers, the consequences of IL-17 signaling may also be different between the two species.
Th1 Cells in the Gut
The differentiation of Th1 cells was considered to differ between mice and humans due mainly to a microsatellite insertion in murine STAT2 that prevented its interaction with STAT4. In humans, this interaction is intact and allows type 1 interferon induction of Th1 cells.132 However, more recent data suggest that type 1 interferon may not be sufficient, even in human cells, to induce Th1 due to an inability to sustain STAT4 tyrosine phosphorylation133 but may still have a unique role in regulating the development of human IL-2-producing central memory CD4 T cells.134
Same Ballpark, Different Players; Different Rules, Same Score
The salient features of the intestinal immune system are highly conserved between mice and humans. The basic microanatomical features are broadly similar and the outcome of the intestinal immune response is the same: the acquisition of a state of controlled responsiveness to luminal antigen, tolerance of food and the commensal flora, and maintenance of the integrity of the epithelial barrier. The mere fact that cells and pathways that achieve this are different in many ways in mice and humans testifies to the ultimate importance of achieving the common outcome. This also highlights the potential plasticity in the system. The lack of a phenotype in a knockout model may not necessarily indicate a lack of relevance of the target in health; rather the ability of the mucosal immune system to make compensatory changes.
In conclusion, although the value of animal models should not be underestimated, close attention should be given to the detail; the functional outcome may seem the same but the mechanism might be very different.
References
Mestas, J. & Hughes, C.C.W. Of mice and not men: differences between mouse and human immunology. J. Immunol. 172, 2731–2738 (2004).
Ganz, T. Defensins: antimicrobial peptides of innate immunity. Nat. Rev. Immunol. 3, 710–720 (2003).
Ouellette, A.J. & Selsted, M.E. Paneth cell defensins: endogenous peptide components of intestinal host defense. FASEB J. 10, 1280–1289 (1996).
Risso, A. Leukocyte antimicrobial peptides: multifunctional effector molecules of innate immunity. J. Leukocyte Biol. 68, 785–792 (2000).
Doeing, D.C., Borowicz, J.L. & Crockett, E.T. Gender dimorphism in differential peripheral blood leukocyte counts in mice using cardiac, tail, foot, and saphenous vein puncture methods. BMC Clin. Pathol. 3, 3 (2003).
Brandtzaeg, P. & Pabst, R. Let's go mucosal: communication on slippery ground. Trends Immunol. 25, 570–577 (2004).
Brandtzaeg, P. Mucosal immunity: induction, dissemination, and effector functions. Scand. J. Immunol. 70, 505–515 (2009).
Pabst, R. & Gehrke, I. Is the bronchus-associated lymphoid tissue (BALT) an integral structure of the lung in normal mammals, including humans? Am. J. Respir. Cell Mol. Biol. 3, 131–135 (1990).
Spencer, J., Finn, T. & Isaacson, P.G. Gut associated lymphoid tissue: a morphological and immunocytochemical study of the human appendix. Gut 26, 672–379 (1985).
Owen, R.L. Sequential uptake of horseradish peroxidase by lymphoid follicle epithelium of Peyer's patches in the normal unobstructed mouse intestine: an ultrastructural study. Gastroenterology 72, 440–451 (1977).
Kadaoui, K.A. & Corthésy, B. Secretory IgA mediates bacterial translocation to dendritic cells in mouse Peyer's patches with restriction to mucosal compartment. J. Immunol. 179, 7751–7757 (2007).
Bockman, D.E. & Cooper, M.D. Early lymphoepithelial relationships in human appendix. A combined light- and electron-microscopic study. Gastroenterology 68, 1160–1168 (1975).
Cuvelier, C.A. et al. M-cells are damaged and increased in number in inflamed human ileal mucosa. Histopathology 24, 417–426 (1994).
Spencer, J., Finn, T. & Isaacson, P.G. Human Peyer's patches: an immunohistochemical study. Gut 27, 405–410 (1986).
Bjerke, K., Brandtzaeg, P. & Fausa, O. T cell distribution is different in follicle-associated epithelium of human Peyer's patches and villous epithelium. Clin. Exp. Immunol. 74, 270–275 (1988).
Spencer, J., MacDonald, T.T., Finn, T. & Isaacson, P.G. The development of gut associated lymphoid tissue in the terminal ileum of fetal human intestine. Clin. Exp. Immunol. 64, 536–543 (1986).
Yamanaka, T. et al. Microbial colonization drives lymphocyte accumulation and differentiation in the follicle-associated epithelium of Peyer's patches. J. Immunol. 170, 816–822 (2003).
Chabot, S., Wagner, J.S., Farrant, S. & Neutra, M.R. TLRs regulate the gatekeeping functions of the intestinal follicle-associated epithelium. J. Immunol. 176, 4275–4283 (2006).
Litinskiy, M. et al. DCs induce CD40-independent immunoglobulin class switching through BLyS and APRIL. Nat. Immunol. 3, 822–829 (2002).
Tezuka, H. et al. Regulation of IgA production by naturally occurring TNF/iNOS-producing dendritic cells. Nature 448, 929–933 (2007).
Ley, R.E. et al. Evolution of mammals and their gut microbes. Science 320, 1647–5125 (2008).
Syed, S.A., Abrams, G.D. & Freter, R. Efficiency of various intestinal bacteria in assuming normal functions of enteric flora after association with germ-free mice. Infect. Immunol. 2, 376–386 (1970).
Hamada, H. et al. Identification of multiple isolated lymphoid follicles on the antimesenteric wall of the mouse small intestine. J. Immunol. 168, 57–64 (2002).
Bergqvist, P., Gärdby, E., Stensson, A., Bemark, M. & Lycke, N.Y. Gut IgA class switch recombination in the absence of CD40 does not occur in the lamina propria and is independent of germinal centers. J. Immunol. 177, 7772–7783 (2006).
Cornes, J.S. Number, size, and distribution of Peyer's patches in the human small intestine. Part I: The development of Peyer's patches. Gut 6, 225–229 (1965).
Mebius, R.E. Organogenesis of lymphoid tissues. Nat. Rev. Immunol. 3, 292–303 (2003).
Pabst, O. et al. Cryptopatches and isolated lymphoid follicles: dynamic lymphoid tissues dispensable for the generation of intraepithelial lymphocytes. Eur. J. Immunol. 35, 98–107 (2005).
Tsuji, M. et al. Requirement for lymphoid tissue-inducer cells in isolated follicle formation and T cell-independent immunoglobulin A generation in the gut. Immunity 29, 261–271 (2008).
Kanamori, Y. et al. Identification of novel lymphoid tissues in murine intestinal mucosa where clusters of c-kit+ IL-7R+ Thy1+ lympho-hemopoietic progenitors develop. J. Exp. Med. 184, 1449–1459 (1996).
Bouskra, D. et al. Lymphoid tissue genesis induced by commensals through NOD1 regulates intestinal homeostasis. Nature 456, 507–510 (2008).
O'Leary, A.D. & Sweeney, E.C. Lymphoglandular complexes of the colon: structure and distribution. Histopathology 10, 267–283 (1986).
Spencer, J., MacDonald, T.T. & Isaacson, P.G. Heterogeneity of non-lymphoid cells expressing HLA-D region antigens in human fetal gut. Clin. Exp. Immunol. 67, 415–424 (1987).
Moghaddami, M., Cummins, A. & Mayrhofer, G. Lymphocyte-filled villi: comparison with other lymphoid aggregations in the mucosa of the human small intestine. Gastroenterology 115, 1414–1425 (1998).
Wotherspoon, A.C., Ortiz-Hidalgo, C., Falzon, M.R. & Isaacson, P.G. Helicobacter pylori-associated gastritis and primary B-cell gastric lymphoma. Lancet 338, 1175–1176 (1991).
Peng, S.L. Signaling in B cells via Toll-like receptors. Curr. Opin. Immunol. 17, 230–236 (2005).
Conley, M.E., Rohrer, J., Rapalus, L., Boylin, E.C. & Minegishi, Y. Defects in early B-cell development: comparing the consequences of abnormalities in pre-BCR signaling in the human and the mouse. Immunol. Rev. 17, 75–90 (2000).
Spencer, J. & MacDonald, T.T. Ontogeny of human mucosal immunity. Ontogeny of the Immune System of the Gut, CRC Press, Boca Raton, Ann Arbor, Boston p. 23–50 (1990).
Moreau, M.C., Ducluzeau, R., Guy-Grand, D. & Muller, M.C. Increase in the population of duodenal immunoglobulin A plasmocytes in axenic mice associated with different living or dead bacterial strains of intestinal origin. Infect. Immunol. 21, 532–539 (1978).
Crabbe, P.A., Nash, D.R., Bazin, H., Eyssen, H. & Heremans, J.F. Immunohistochemical observations on lymphoid tissues from conventional and germ-free mice. Lab. Invest. 22, 448–457 (1970).
Shroff, K.E., Meslin, K. & Cebra, J.J. Development and maintenance of the gut-associated lymphoid tissue (GALT): the roles of enteric bacteria and viruses. Dev. Immunol. 6, 13–18 (1998).
Talham, G.L., Jiang, H.Q., Bos, N.A. & Cebra, J.J. Segmented filamentous bacteria are potent stimuli of a physiologically normal state of the murine gut mucosal immune system. Infect. Immunol. 67, 1992–2000 (1999).
Hapfelmeier, S. et al. Reversible microbial colonization of germ-free mice reveals the dynamics of IgA immune responses. Science 328, 1705–1709 (2010).
Kroese, F.G. et al. A dual origin for IgA plasma cells in the murine small intestine. Adv. Exp. Med. Biol. 371A, 435–440 (1995).
Stall, A.M., Wells, S.M. & Lam, K.P. B-1 cells: unique origins and functions. Semin. Immunol. 8, 45–59 (1996).
Kroese, F.G., Butcher, E.C., Stall, A.M. & Herzenberg, L.A. A major peritoneal reservoir of precursors for intestinal IgA plasma cells. Immunol. Invest. 18, 47–58 (1989).
Boursier, L., Farstad, I.N., Mellembakken, J.R., Brandtzaeg, P. & Spencer, J. IgVH gene analysis suggests that peritoneal B cells do not contribute to the gut immune system in man. Eur. J. Immunol. 32, 2427–2436 (2002).
Rangel-Moreno, J. et al. Omental milky spots develop in the absence of lymphoid tissue-inducer cells and support B and T cell responses to peritoneal antigens. Immunity 30, 731–743 (2009).
Boursier, L., Montalto, S.A., Raju, S., Culora, G. & Spencer, J. Characterization of cells of the B lineage in the human adult greater omentum. Immunology 119, 90–97 (2006).
Rosado, M.M. et al. From the fetal liver to spleen and gut: the highway to natural antibody. Mucosal Immunol. 2, 351–361 (2009).
Mebius, R.E. & Kraal, G. Structure and function of the spleen. Nat. Rev. Immunol. 5, 606–616 (2005).
Fagarasan, S., Kinoshita, K., Muramatsu, M., Ikuta, K. & Honjo, T. In situ class switching and differentiation to IgA-producing cells in the gut lamina propria. Nature 413, 639–643 (2001).
Rescigno, M. et al. Dendritic cells express tight junction proteins and penetrate gut epithelial monolayers to sample bacteria. Nat. Immunol. 2, 361–367 (2001).
Cerutti, A. The regulation of IgA class switching. Nat. Rev. Immunol. 8, 421–434 (2008).
Bergqvist, P., Stensson, A., Lycke, N.Y. & Bemark, M. T cell-independent IgA class switch recombination is restricted to the GALT and occurs prior to manifest germinal center formation. J. Immunol. 184, 3545–3553 (2010).
Tsuji, M. et al. Requirement for lymphoid tissue-inducer cells in isolated follicle formation and T cell-independent immunoglobulin A generation in the gut. Immunity 29, 261–271 (2008).
Barone, F., Patel, P., Sanderson, J. & Spencer, J. Gut-associated lymphoid tissue contains the molecular machinery to support T-cell-dependent and T-cell-independent class switch recombination. Mucosal Immunol. 2, 495–503 (2009).
He, B., Xu, W. & Cerutti, A. Comment on “Gut-associated lymphoid tissue contains the molecular machinery to support T-cell-dependent and T-cell-independent class switch recombination”. Mucosal Immunol. 3, 92–94 author reply 94–5 (2010).
Zan, H., Cerutti, A., Dramitinos, P., Schaffer, A. & Casali, P. CD40 engagement triggers switching to IgA1 and IgA2 in human B cells through induction of endogenous TGF-beta: evidence for TGF-beta but not IL-10-dependent direct S mu-->S alpha and sequential S mu-->S gamma, S gamma-->S alpha DNA recombination. J. Immunol. 161, 5217–5225 (1998).
McIntyre, T.M., Kehry, M.R. & Snapper, C.M. Novel in vitro model for high-rate IgA class switching. J. Immunol. 154, 3156–3161 (1995).
Litinskiy, M.B. et al. DCs induce CD40-independent immunoglobulin class switching through BLyS and APRIL. Nat. Immunol. 3, 822–829 (2002).
Boursier, L., Gordon, J.N., Thiagamoorthy, S., Edgeworth, J.D. & Spencer, J. Human intestinal IgA response is generated in the organized gut-associated lymphoid tissue but not in the lamina propria. Gastroenterology 128, 1879–1889 (2005).
Stoelm, M. et al. Restricted IgA repertoire in both B-1 and B-2 cell-derived gut plasmablasts. J. Immunol. 174, 1046–1054 (2005).
González-Fernández, A. & Milstein, C. Analysis of somatic hypermutation in mouse Peyer's patches using immunoglobulin kappa light-chain transgenes. Proc. Natl Acad. Sci. USA 90, 9862–9866 (1993).
Roy, B. et al. Somatic hypermutation in peritoneal B1b cells. Mol. Immunol. 46, 1613–1619 (2009).
Spencer, J., Finn, T. & Isaacson, P.G. A comparative study of the gut-associated lymphoid tissue of primates and rodents. Virchows Arch. B. Cell Pathol. Incl. Mol. Pathol. 51, 509–519 (1986).
Beagley, K.W.K. et al. Differences in intraepithelial lymphocyte T cell subsets isolated from murine small versus large intestine. J. Immunol. 154, 5611–5619 (1995).
Maloy, K.J., Mowat, A.M., Zamoyska, R. & Crispe, I.N. Phenotypic heterogeneity of intraepithelial T lymphocytes from mouse small intestine. Immunology 72, 555–562 (1991).
Ivanov, I.I. et al. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell 139, 485–498 (2009).
Ivanov, I.I. et al. Specific microbiota direct the differentiation of IL-17-producing T-helper cells in the mucosa of the small intestine. Cell Host Microbe 4, 337–349 (2008).
Gaboriau-Routhiau, V. et al. The key role of segmented filamentous bacteria in the coordinated maturation of gut helper T cell responses. Immunity 31, 677–689 (2009).
Hayday, A.C. GD cells: a right time and a right place for a conserved third way of protection. Annu. Rev. Immunol. 18, 975–1026 (2000).
Spencer, J., Isaacson, P.G., MacDonald, T.T., Thomas, A.J. & Walker-Smith, J.A. Gamma/delta T cells and the diagnosis of coeliac disease. Clin. Exp. Immunol. 85, 109–113 (1991).
Takagaki, Y., DeCloux, A., Bonneville, M. & Tonegawa, S. Diversity of gamma delta T-cell receptors on murine intestinal intra-epithelial lymphocytes. Nature 339, 712–714 (1989).
Chowers, Y., Holtmeier, W., Harwood, J., Morzycka-Wroblewska, E. & Kagnoff, M.F. The V delta 1 T cell receptor repertoire in human small intestine and colon. J. Exp. Med. 180, 183–190 (1994).
Soderstrom, K. et al. High expression of V gamma 8 is a shared feature of human gamma delta T cells in the epithelium of the gut and in the inflamed synovial tissue. J. Immunol. 15, 6017–6027 (1994).
Groh, V., Steinle, A., Bauer, S. & Spies, T. Recognition of stress-induced MHC molecules by intestinal epithelial gammadelta T cells. Science 279, 1737–1740 (1998).
Hayday, A., Theodoridis, E., Ramsburg, E. & Shires, J. Intraepithelial lymphocytes: exploring the Third Way in immunology. Nat. Immunol. 2, 997–1003 (2001).
Shires, J., Theodoridis, E. & Hayday, A.C. Biological insights into TCRgammadelta+ and TCRalphabeta+ intraepithelial lymphocytes provided by serial analysis of gene expression (SAGE). Immunity 15, 419–434 (2001).
Pennington, D.J. et al. The inter-relatedness and interdependence of mouse T cell receptor gammadelta+ and alphabeta+ cells. Nat. Immunol. 4, 991–998 (2003).
Spencer, J., MacDonald, T.T., Diss, T.C., Walker-Smith, J.A., Ciclitira, P.J. & Isaacson, P.G. Changes in intraepithelial lymphocyte subpopulations in coeliac disease and enteropathy associated T cell lymphoma (malignant histiocytosis of the intestine). Gut 30, 339–346 (1989).
Latthe, M., Terry, L. & MacDonald, T.T. High frequency of CD8 alpha alpha homodimer-bearing T cells in human fetal intestine. Eur. J. Immunol. 24, 1703–1705 (1994).
Luckschander, N. et al. Phenotyping, functional characterization, and developmental changes in canine intestinal intraepithelial lymphocytes. Vet. Res. 40, 58 (2009).
Poussier, P., Ning, T., Banerjee, D. & Julius, M. A unique subset of self-specific intraintestinal T cells maintains gut integrity. J. Exp. Med. 195, 1491–1497 (2002).
Cheroutre, H. & Lambolez, F. Doubting the TCR coreceptor function of CD8alphaalpha. Immunity 28, 149–159 (2008).
Leishman, A.J. et al. T cell responses modulated through interaction between CD8alphaalpha and the nonclassical MHC class I molecule, TL. Science 294, 1936–1939 (2001).
Middendorp, S. & Nieuwenhuis, E.E. NKT cells in mucosal immunity. Mucosal Immunol. 2, 393–402 (2009).
Dutronc, Y. & Porcelli, S.A. The CD1 family and T cell recognition of lipid antigens. Tissue Antigens 60, 337–353 (2002).
Shimamura, M. et al. Modulation of Valpha19 NKT cell immune responses by alpha-mannosyl ceramide derivatives consisting of a series of modified sphingosines. Eur. J. Immunol. 37, 1836–1844 (2007).
Martin, E. et al. Stepwise development of MAIT cells in mouse and human. PLoS Biol. 7, e54 (2009).
Jaensson, E. et al. Small intestinal CD103+ dendritic cells display unique functional properties that are conserved between mice and humans. J. Exp. Med. 205, 2139–2149 (2008).
Uhlig, H.H. et al. Characterization of Foxp3+CD4+CD25+ and IL-10-secreting CD4+CD25+ T cells during cure of colitis. J. Immunol. 177, 5852–5860 (2006).
Tran, D.Q., Ramsey, H. & Shevach, E.M. Induction of FOXP3 expression in naive human CD4+FOXP3 T cells by T-cell receptor stimulation is transforming growth factor-beta dependent but does not confer a regulatory phenotype. Blood 110, 2983–2990 (2007).
Chen, W. et al. Conversion of peripheral CD4+CD25- naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J. Exp. Med. 198, 1875–1886 (2003).
Gavin, M.A. et al. Single-cell analysis of normal and FOXP3-mutant human T cells: FOXP3 expression without regulatory T cell development. Proc. Natl Acad. Sci. USA 103, 6659–6664 (2006).
Wang, J., Ioan-Facsinay, A., van der Voort, E.I., Huizinga, T.W. & Toes, R.E. Transient expression of FOXP3 in human activated nonregulatory CD4+ T cells. Eur. J. Immunol. 37, 129–138 (2007).
Liu, H., Komai-Koma, M., Xu, D. & Liew, F.Y. Toll-like receptor 2 signaling modulates the functions of CD4+ CD25+ regulatory T cells. Proc. Natl Acad. Sci. USA 103, 7048–7053 (2006).
Zanin-Zhorov, A., Cahalon, L., Tal, G., Margalit, R., Lider, O. & Cohen, I.R. Heat shock protein 60 enhances CD4+ CD25+ regulatory T cell function via innate TLR2 signaling. J. Clin. Invest. 116, 2022–2032 (2006).
Groux, H. et al. A CD4+ T-cell subset inhibits antigen-specific T-cell responses and prevents colitis. Nature 389, 737–742 (1997).
Kemper, C., Chan, A.C., Green, J.M., Brett, K.A., Murphy, K.M. & Atkinson, J.P. Activation of human CD4+ cells with CD3 and CD46 induces a T-regulatory cell 1 phenotype. Nature 421, 388–392 (2003).
Cardone, J. et al. Complement regulator CD46 temporally regulates cytokine production by conventional and unconventional T cells. Nat. Immunol. 11, 862–871 (2010).
Gandhi, R. et al. Activation of the aryl hydrocarbon receptor induces human type 1 regulatory T cell-like and Foxp3(+) regulatory T cells. Nat. Immunol. 11, 846–853 (2010).
Apetoh, L. et al. The aryl hydrocarbon receptor interacts with c-Maf to promote the differentiation of type 1 regulatory T cells induced by IL-27. Nat. Immunol. 11, 854–861 (2010).
Esser, C., Rannug, A. & Stockinger, B. The aryl hydrocarbon receptor in immunity. Trends Immunol. 30, 447–454 (2009).
Connor, K.T. & Aylward, L.L. Human response to dioxin: aryl hydrocarbon receptor (AhR) molecular structure, function, and dose-response data for enzyme induction indicate an impaired human AhR. J. Toxicol. Environ. Health B. Crit. Rev. 9, 147–171 (2006).
Kitani, A. & Xu, L. Regulatory T cells and the induction of IL-17. Mucosal Immunol. 1 (Suppl 1), S43–S46 (2008).
Zheng, S.G. The critical role of TGF-beta1 in the development of induced Foxp3+ regulatory T cells. Int. J. Clin. Exp. Med. 1, 192–202 (2008).
Xu, L., Kitani, A., Fuss, I. & Strober, W. Cutting edge: regulatory T cells induce CD4+CD25-Foxp3- T cells or are self-induced to become Th17 cells in the absence of exogenous TGF-beta. J. Immunol. 178, 6725–6729 (2007).
Niess, J.H., Leithauser, F., Adler, G. & Reimann, J. Commensal gut flora drives the expansion of proinflammatory CD4T cells in the colonic lamina propria under normal and inflammatory conditions. J. Immunol. 180, 559–568 (2008).
Sarra, M., Pallone, F., Macdonald, T.T. & Monteleone, G. IL-23/IL-17 axis in IBD. Inflamm. Bowel Dis. 16, 1808–1813 (2010).
Bettelli, E. et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature 441, 235–238 (2006).
Veldhoen, M., Hocking, R.J., Atkins, C.J., Locksley, R.M. & Stockinger, B. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity 24, 179–189 (2006).
Ivanov, I.I. et al. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell 126, 1121–1133 (2006).
Santarlasci, V. et al. TGF-beta indirectly favors the development of human Th17 cells by inhibiting Th1 cells. Eur. J. Immunol. 39, 207–215 (2009).
Das, J. et al. Transforming growth factor beta is dispensable for the molecular orchestration of Th17 cell differentiation. J. Exp. Med. 206, 2407–2416 (2009).
Acosta-Rodriguez, E.V., Napolitani, G., Lanzavecchia, A. & Sallusto, F. Interleukins 1beta and 6 but not transforming growth factor-beta are essential for the differentiation of interleukin 17-producing human T helper cells. Nat. Immunol. 8, 942–949 (2007).
Wilson, N.J. et al. Development, cytokine profile and function of human interleukin 17-producing helper T cells. Nat. Immunol. 8, 950–957 (2007).
Eastaff-Leung, N., Mabarrack, N., Barbour, A., Cummins, A. & Barry, S. Foxp3+ regulatory T cells, Th17 effector cells, and cytokine environment in inflammatory bowel disease. J. Clin. Immunol. 30, 80–89 (2010).
Ghoreschi, K.A. et al. Generation of pathogenic T(H)17 cells in the absence of TGF-beta signalling. Nature 467, 967–971 (2010).
Roark, C.L., Simonian, P.L., Fontenot, A.P., Born, W.K. & O'Brien, R.L. Gammadelta T cells: an important source of IL-17. Curr. Opin. Immunol. 20, 353–357 (2008).
Martin, B., Hirota, K., Cua, D.J., Stockinger, B. & Veldhoen, M. Interleukin-17-producing gammadelta T cells selectively expand in response to pathogen products and environmental signals. Immunity 31, 321–330 (2009).
Reynolds, J.M. et al. Toll-like receptor 2 signaling in CD4(+) T lymphocytes promotes T helper 17 responses and regulates the pathogenesis of autoimmune disease. Immunity 32, 692–702 (2010).
Duan, J., Chung, H., Troy, E. & Kasper, D.L. Microbial colonization drives expansion of IL-1 receptor 1-expressing and IL-17-producing gamma/delta T cells. Cell Host Microbe 7, 140–150 (2010).
Ribot, J.C. et al. CD27 is a thymic determinant of the balance between interferon-gamma- and interleukin 17-producing gammadelta T cell subsets. Nat. Immunol. 10, 427–436 (2009).
Ness-Schwickerath, K.J., Jin, C. & Morita, C.T. Cytokine requirements for the differentiation and expansion of IL-17A- and IL-22-producing human Vgamma2Vdelta2 T cells. J. Immunol. 184, 7268–7280 (2010).
Peng, M.Y. et al. Interleukin 17-producing gamma delta T cells increased in patients with active pulmonary tuberculosis. Cell Mol. Immunol. 5, 203–208 (2008).
Fenoglio, D. et al. Vdelta1T lymphocytes producing IFN-gamma and IL-17 are expanded in HIV-1-infected patients and respond to Candida albicans. Blood 113, 6611–6618 (2009).
Buonocore, S. et al. Innate lymphoid cells drive interleukin-23-dependent innate intestinal pathology. Nature 464, 1371–1375 (2010).
Laan, M., Lotvall, J., Chung, K.F. & Linden, A. IL-17-induced cytokine release in human bronchial epithelial cells in vitro: role of mitogen-activated protein (MAP) kinases. Br. J. Pharmacol. 133, 200–206 (2001).
Pelletier, M. et al. Evidence for a cross-talk between human neutrophils and Th17 cells. Blood 115, 335–343 (2010).
Olson, T.S. & Ley, K. Chemokines and chemokine receptors in leukocyte trafficking. Am. J. Physiol. 283, R7–R28 (2002).
Zlotnik, A. & Yoshie, O. Chemokines: a new classification system and their role in immunity. Immunity 12, 121–127 (2000).
Farrar, J.D., Smith, J.D., Murphy, T.L., Leung, S., Stark, G.R. & Murphy, K.M. Selective loss of type I interferon-induced STAT4 activation caused by a minisatellite insertion in mouse Stat2. Nat. Immunol. 1, 65–69 (2000).
Ramos, H.J., Davis, A.M., George, T.C. & Farrar, J.D. IFN-alpha is not sufficient to drive Th1 development due to lack of stable T-bet expression. J. Immunol. 179, 3792–3803 (2007).
Davis, A.M., Ramos, H.J., Davis, L.S. & Farrar, J.D. Cutting edge: a T-bet-independent role for IFN-alpha/beta in regulating IL-2 secretion in human CD4+ central memory T cells. J. Immunol. 181, 8204–8208 (2008).
Strugnell, R.A. & Wijburg, O.L. The role of secretory antibodies in infection immunity. Nat. Rev. Microbiol. 8, 656–667 (2010).
Norderhaug, I.N., Johansen, F.E., Schjerven, H. & Brandtzaeg, P. Regulation of the formation and external transport of secretory immunoglobulins. Crit. Rev. Immunol. 19, 481–508 (1999).
Krajci, P. et al. Molecular cloning of the human transmembrane secretory component (poly-Ig receptor) and its mRNA expression in human tissues. Biochem. Biophys. Res. Commun. 158, 783–789 (1989).
Klein, U., Küppers, R. & Rajewsky, K. Evidence for a large compartment of IgM expressing memory B cells in humans. Blood 89, 1288–1298 (1997).
Monteiro, R.C. & Van De Winkel, J.G. IgA Fc receptors. Annu. Rev. Immunol. 21, 177–204 (2003).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declared no conflict of interest.
PowerPoint slides
Rights and permissions
About this article
Cite this article
Gibbons, D., Spencer, J. Mouse and human intestinal immunity: same ballpark, different players; different rules, same score. Mucosal Immunol 4, 148–157 (2011). https://doi.org/10.1038/mi.2010.85
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/mi.2010.85
This article is cited by
-
In vivo development of immune tissue in human intestinal organoids transplanted into humanized mice
Nature Biotechnology (2023)
-
Mucosal immunity to poliovirus
Mucosal Immunology (2022)
-
Human gut-associated lymphoid tissues (GALT); diversity, structure, and function
Mucosal Immunology (2021)
-
IgA and the intestinal microbiota: the importance of being specific
Mucosal Immunology (2020)
-
Cholinergic Activation of Primary Human Derived Intestinal Epithelium Does Not Ameliorate TNF-α Induced Injury
Cellular and Molecular Bioengineering (2020)