Abstract
We recently characterized Winnie mice carrying a missense mutation in Muc2, leading to severe endoplasmic reticulum stress in intestinal goblet cells and spontaneous colitis. In this study, we characterized the immune responses due to this intestinal epithelial dysfunction. In Winnie, there was a fourfold increase in activated dendritic cells (DCs; CD11c+ major histocompatibility complex (MHC) class IIhi) in the colonic lamina propria accompanied by decreased colonic secretion of an inhibitor of DC activation, thymic stromal lymphopoietin (TSLP). Winnie also displayed a significant increase in mRNA expression of the mucosal TH17 signature genes Il17a, IL17f, Tgfb, and Ccr6, particularly in the distal colon. Winnie mesenteric lymph node leukocytes secreted multiple TH1, TH2, and TH17 cytokines on activation, with a large increase in interleukin-17A (IL-17A) progressively with age. A major source of mucosal IL-17A in Winnie was CD4+ T lymphocytes. Loss of T and B lymphocytes in Rag1-/- × Winnie (RaW) crosses did not prevent spontaneous inflammation but did prevent progression with age in the colon but not the cecum. Adoptive transfer of naive T cells into RaW mice caused more rapid and severe colitis than in Rag1-/-, indicating that the epithelial defect results in an intestinal microenvironment conducive to T-cell activation. Thus, the Winnie primary epithelial defect results in complex multicytokine-mediated colitis involving both innate and adaptive immune components with a prominent IL-23/TH17 response, similar to that of human ulcerative colitis.
Similar content being viewed by others
Introduction
The inflammatory bowel diseases (IBD), Crohn's disease (CD) and ulcerative colitis (UC), are intestinal disorders with complex genetic and environmental etiology. Analyses of animal models generated by genetic manipulation and human disease, together with results from genome-wide association studies, have identified several types of defects that appear to contribute to intestinal inflammation. These include alterations in the mucosal barrier, abnormalities of innate immunity, and inappropriate specific immune responses, particularly activation of effector T cells and increased production of interleukin-17 (IL-17)1, 2 in response to gut microbes culminating in chronic inflammation.
Aberrant mucosal immune responses in IBD could be because of: (i) primary defects in the intestinal epithelium (e.g., conditional knockout mice for IκB kinase-β3 and NFκB essential modulator4 show that loss of some elements of nuclear factor-κB (NF-κB) signaling in intestinal epithelial cells initiate colitis); (ii) a change in the threshold of immune activation to the normal microbiota that could occur at the level of epithelial cell modulation, antigen-presenting cells (APCs), or immune effector cells; and/or (iii) a deviation in the type of immune response generated against luminal microbes. Recent studies suggest that immune deviation from a controlled TH2/Treg-type response5 toward the production of IL-23/TH17 is frequently observed in IBD as well as in mouse models of colitis.6, 7, 8, 9 Genetic evidence indicates that the IL-23/TH17 pathway is important in the pathogenesis of IBD, with polymorphisms in the TH17-related genes IL23R, IL-12B (p40), CCR6, and STAT3 associated with the development of CD and UC.10, 11 Additionally, increased colonic gene expression for IL-23, RORγt, IL-17A, and IL-17F have been reported in UC and CD.1, 8
We recently described a murine model of spontaneous colitis (Winnie) in which chronic intestinal inflammation results from a primary intestinal epithelial defect conferred by a missense mutation in the Muc2 mucin gene.12 Winnie mice show aberrant Muc2 biosynthesis causing endoplasmic reticulum (ER) stress, reduced goblet cell numbers, a depleted mucus layer, increased intestinal permeability, increased crypt proliferation and apoptosis, spontaneous intestinal inflammation, and greatly enhanced susceptibility to luminal inflammation-inducing toxins. The spontaneous intestinal inflammation is most severe in the distal colon and the disease severity increases with age. We originally described increased production of TH1 and TH2 cytokines (tumor necrosis factor-α, interferon-γ (IFN-γ), and IL-13) from mesenteric lymph node (MLN)-derived leukocytes cultured in vitro. Now, we have characterized in detail the immunological events and demonstrate activation of both innate and adaptive immunity and a mixed inflammatory response with progressive development of a mixed TH1, TH2, and prominent TH17 response with age.
Results
Expansion of intestinal lamina propria leukocytes and activation of mucosal CD11c+ dendritic cells (DCs) in Winnie mice
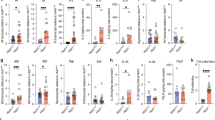
The molecular defect leading to spontaneous colitis in Winnie mice is confined to the intestinal epithelium.12 Nevertheless, an analysis of the spontaneous inflammatory response in Winnie mice revealed a significant increase (threefold, P=0.0001, Figure 1b) in the total number of colonic lamina propria mononuclear cells (LPMCs) in Winnie compared with wild-type mice. An analysis of lymphoid (CD4+, CD8+ T cells, and B cells) and myeloid (CD11b+ and CD11c+) cell populations in LPMCs suggested an increase in the proportion of CD11c+ cells and B cells and a decrease in the proportion of CD8+ T cells. The relative abundance of CD4+, natural killer (NK), NKT, and CD11b+ cells did not differ substantially between the wild-type and Winnie mice (Figure 1a). Mucosal cytokine and chemokine secretions from cultured proximal and distal colon explants were measured using multiplex assays. The most significant increases in cytokines were in the production of IL-1β and IL-12/23p40 in Winnie compared with wild-type mice (Figure 1c; Supplementary Table S1 and Figure S1a online). In the distal colon, where histological inflammation is more severe, these increases were more pronounced (120-fold for IL-1β and 4-fold for IL-12/23p40).
Altered lamina propria (LP) leukocyte composition and cytokine production in Winnie. (a) Lamina propria mononuclear cells (LPMCs; pooled from four mice) from the colon were stained for lymphoid (CD4, CD8 T cells, and B cells) and myeloid markers (CD11b) and analyzed by flow cytometry, and expressed as a proportion of cells from the total live gate. (b) Yield of LPMCs from BL/6 and Winnie (Win) mice. (c) Explants from the proximal and distal colons of C57BL/6 (BL6) and Win mice (N=6, 20–24 weeks) were cultured overnight, and cytokines measured in supernatants using Bio-Plex assay. The most significantly altered cytokines are presented as pg per g of tissue. Supplementary Figure S1 online presents the levels of all the tested cytokines that fell within the detectable range. Statistics: (a) mean; (b, c) median and interquartile range (IQR); Mann–Whitney U-test and P-values are shown.
Mucosal secretions and DC activation point towards a TH17-promoting milieu in Winnie mice
Because the analysis of mucosal immune cell composition and secretions pointed toward DC expansion and activation, we isolated the CD11c+ APC population and investigated their activation status and their cytokine and chemokine profile following stimulation. The frequency of activated DCs (CD11c+ major histocompatibility complex (MHC) class IIhi) in colonic lamina propria was fourfold higher in Winnie mice (Figure 2a). Flow-sorted Winnie colonic LPMC CD11c+ APCs stimulated in vitro with lipopolysaccharide produced more IL-12/23 p40, IL-6, RANTES (regulated upon activation, normal T-cell expressed and secreted), and macrophage inflammatory protein-1α (MIP-1α) (Figure 2b). Production of the chemokines MIP-1α and RANTES confirmed the activated status of the Winnie intestinal APCs, and production of IL-6 and IL-12/23p40 suggested that these APCs may be promoting a mucosal TH17 response. There were slight increases in the secretion of other cytokines and chemokines including IL-12p70, MIP-1β, and keratinocyte chemoattractant in Winnie compared with wild-type APCs (data for all analytes are presented in Supplementary Figure S2 online).
Lamina propria antigen-presenting cell (APC) activation and cytokine production points to a TH17-promoting milieu. (a) Lamina propria mononuclear cells (LPMCs) from wild-type (BL/6) and Winnie (Win) mice (pooled from four 11–12-week-old mice) were stained for APC marker CD11c and dendritic cell (DC) activation marker MHC class II and analyzed by flow cytometry. (b) CD11c+ APCs from LPMCs (pooled from four 11–12-week-old wild-type and Winnie mice) were sorted by flow cytometry and 2 × 105 cells ml–1 cultured with 100 ng ml–1 lipopolysaccharide (LPS). Culture supernatants were collected after 48 h and cytokines measured using a Bio-Plex assay. (c) Proximal and distal colon explant cultures from 7-week-old sex-matched wild-type and Winnie mice were collected after 48 h and thymic stromal lymphopoietin (TSLP) measured using enzyme-linked immunosorbent assay (ELISA); Mann–Whitney U-test and P-values are shown.
Winnie colonic explant cultures from 7-week-old mice showed decreased production of the cytokine, thymic stromal lymphopoietin (TSLP) (Figure 2c). Lowered TSLP, which is secreted by intestinal epithelial cells and represses activation of intestinal APCs,5 suggests that decreased epithelial repression of APC activation may be an early event in the development of colitis.
Winnie intestinal mucosa has increased IL-17- and IFN-γ-secreting cells and displays a TH17 gene expression signature
We postulated that the pattern of DC activation would be conducive for TH17 T-cell differentiation. Therefore, we measured mRNA expression of the TH17 signature genes Il17a, Il17f, Tgfβ, and Ccr6 in the colonic mucosa by quantitative reverse transcriptase-PCR, demonstrating a significant increase in the relative expression of all four genes in Winnie distal colon (Figure 3a). The mean increases in the expression of Il17a, Ill7f, Tgfβ, and Ccr6 in Winnie compared with wild-type distal colon were 28-, 7-, 4-, and 6-fold, respectively, whereas smaller (<3-fold) changes in expression were seen in the Winnie proximal colon. These results are consistent with a TH17 inflammatory response in Winnie colonic mucosa, particularly in the distal colon where histological inflammation is most pronounced. We further characterized the cells that secrete IL-17A and IFN-γ in the colonic lamina propria by intracellular cytokine staining. CD4+ lamina propria lymphocytes in Winnie contained significantly increased frequencies of both IL-17A (mean 2.7 vs. 0.7%, P<0.005) and IFN-γ (mean 7.5 vs. 2.3%, P<0.005) secreting cells compared with age- and sex-matched wild-type mice (Figure 3b). In contrast, there was no alteration in the frequency of CD4– IL-17A- and IFN-γ-producing leukocytes in the Winnie lamina propria, or differences in the frequencies of CD4+ or CD4– leukocytes producing both IL-17A and IFN-γ.
Activation of a TH17 T-cell response in Winnie. (a) Quantitative reverse transcriptase-PCR (RT-PCR) was performed from proximal and distal colon tissue from wild-type (BL/6) and Winnie (Win) mice (N=6, 10–12 weeks). Il17a, Il17f, Tgfb, and Ccr6 mRNA levels were corrected for Gapdh and are presented as fold changes in expression relative to the mean expression in wild-type mice. (b) Lamina propria mononuclear cells (LPMCs) were obtained from wild-type and Win mice (N=8, 10–12 weeks), stimulated with phorbol myristate acetate (PMA; 50 ng ml–1) and ionomycin (500 ng ml–1), treated with GolgiStop (BD Biosciences) for 4 h, then stained immediately for CD4 and intracellular interleukin-17 (IL-17) and interferon-γ (IFN-γ), and analyzed by flow cytometry. Representative fluorescence-activated cell sorting (FACS) plots and the proportion of CD4– and CD4+ cells producing IL-17 and IFN-γ are shown. Statistics: box plots show median, interquartile range (IQR), and range; Mann–Whitney U-test and P-values are shown.
MLN leukocytes secrete elevated TH1, TH2, and TH17 cytokines in an age-dependent manner in Winnie mice
There was a 50% increase in the number of leukocytes recovered from MLNs in 10- to 14-week-old Winnie mice (Supplementary Figure S3a online). The relative frequencies of major lymphoid and myeloid populations (T cells (CD4+, CD8+, and NKT), B cells, and CD11b+ myeloid cells) from MLNs, spleen, bone marrow, and thymus did not differ between wild-type and Winnie mice (Supplementary Figure S3b and c online). There were also similar numbers of memory (CD62L+) T cells in MLNs from Winnie and wild-type mice (Supplementary Figure S4 online). In order to gauge the phenotype of T cells within the MLNs, isolated T cells were restimulated with anti-CD3/anti-CD28 from two age group cohorts of mice (11 and 20–24 weeks) to monitor the evolution of the inflammatory process. Cytokine concentrations from wild-type mice were similar for both age groups, and therefore these data were pooled for analysis. Stimulated lymphocytes from Winnie MLNs (at both 11 weeks and 20–24 weeks) produced significantly elevated levels of multiple TH1, TH2, and TH17 cytokines and chemokines (Figure 4, Supplementary Table S1 online). In cultures from 20- to 24-week-old Winnie mice, there was a highly significant increase (P<0.01) in the production of TH1 (IL-12p70 and tumor necrosis factor-α), TH2 (IL-3, IL-5, and IL-9), and TH17 (IL-17A and IL-12/23p40) cytokines, and the chemokine monocyte chemotactic protein-1, and a less significant increase (P<0.05) in other TH1 (IL-1β and IFN-γ) and TH2 (IL-10 and IL-13) cytokines, and the chemokines, keratinocyte chemoattractant and granulocyte colony-stimulating factor. Particularly striking was a 280-fold increase in median IL-17A concentrations. Generally, MLN lymphocytes from 11-week-old Winnie mice produced concentrations of cytokines intermediate between 20- and 24-week-old Winnie and wild-type mice. Exceptions to this included trends toward higher IL-1β, IFN-γ, MIP-1α, and MIP-1β in cultures from younger Winnie mice. There were trends toward higher concentrations of several cytokines, including IL-23p40 and IL-17A, in the serum of Winnie mice (Supplementary Figure S5 online), which is consistent with production of multiple cytokines at the sites of intestinal and mesenteric lymph node inflammation.
Comprehensive cytokine analysis from mesenteric lymph node (MLN) leukocyte culture supernatants contained significant elevation of multiple TH1, TH2, and TH17 cytokines in Winnie. A total of 0.5 × 106 lymphocytes per ml were activated with anti-CD3/CD28 and cultured for 48 h from wild-type (four individual 11-week-old BL/6 mice, and six 20–24-week-old BL/6 in two pools of three mice) and Winnie (four individual 11-week-old Winnie, and seven 20–24-week-old individual mice). Cytokine concentrations in wild-type mice were similar for both age groups, and therefore they are presented as one group with different symbols representing the different age groups. Cell-free supernatants were analyzed for 23 cytokines and chemokines using a Bio-Plex assay, 21 of which fell within the detectable range of the assay in some samples and are shown here. Statistics: Kruskal–Wallis nonparametric analysis of variance (ANOVA; P-values in Supplementary Table S1 online), Dunn's multiple comparison test; *P<0.05, **P<0.01, ***P<0.001.
Increased frequency of T regulatory cells in Winnie lymphoid tissue
T regulatory cells (Treg), which are known to be key players in basal intestinal immune tolerance, continue to provide tolerogenic control in many mouse models of colitis, and accumulation of Foxp3+ Treg have been described in many chronic inflammatory diseases. We enumerated natural and adaptive Treg (CD4+CD25+Foxp3+) in thymus, spleen, and MLNs. Compared with wild-type mice, there was a small but significant increase in the proportion of Foxp3+ Treg in Winnie spleen (P<0.01) and MLNs (P<0.03) (Figure 5 and Supplementary Figure online), whereas in the thymus Treg frequency did not differ (data not shown).
Increased frequency of T regulatory cells (Treg) in Winnie lymphoid tissues. Enumeration of Treg (CD4+CD25+Foxp3+) from mesenteric lymph nodes (MLNs) and spleen from wild-type (BL/6) and Winnie (Win) mice expressed as a total lymphocytes percentage of Treg in Win and BL/6 MLNs and spleen. Statistics: median and interquartile range (IQR); Mann–Whitney U-test and P-values are shown.
The specialized CD11c+ CD103+ tolerizing DC population also did not differ in relative abundance in MLNs between wild-type and Winnie mice (Supplementary Figure S4 online).
The severity of colitis does not progress in Winnie in the absence of lymphocytes
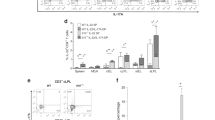
The preceding set of experiments provided evidence of significant T-cell activation in the MLNs, abundant IL-17A, accompanied by an expansion of Treg, although the molecular defect in Winnie is confined to the intestinal epithelium. In order to determine the effect of both effector and regulatory lymphocytes in the Winnie model of colitis, we rendered them deficient in T and B cells by crossing to the Rag-1-/- background (henceforth referred to as RaW mice). There were no differences in clinical symptoms of colitis (loss of body weight, altered behavior, diarrhea, and rectal bleeding) and colon weight between Winnie and RaW mice at 8 weeks of age (controls showed no colitis). However, older Winnie mice (17 weeks) had significantly increased colon weight and more severe histological colitis, whereas older RaW mice (17 weeks) did not show progressive increases in clinical symptoms of colitis, colon weight, or histological colitis scores (Figure 6a–c). The only exception to the lack of progression in older RaW mice was a significant increase in cecal inflammation (Figure 6a). Detailed histological analysis of colons from young Winnie mice showed previously described classical features associated with the spontaneous colitis such as crypt elongation, goblet cell loss, and increased inflammatory infiltrates in the lamina propria, particularly in the distal colon. In older Winnie mice, there was a significant increase in histological colitis scores exhibited through all the classical features described above, particularly an increased number of crypt abscesses, and highest scores in the mid and distal colon. In contrast, the younger RaW mice developed spontaneous colitis scores that trended higher in the proximal and mid colon than younger Winnie mice, whereas the older RaW mice did not have an increase in histological features of colitis in either the proximal, mid, or distal regions of the colon, with colitis scores trending lower than those in young RaW mice.
Spontaneous colonic inflammation does not progress in the absence of lymphocytes in Winnie. (a) Histological colitis scores and (b) colon weight in Winnie (Win) and Winnie × Rag-1-/- (RaW) mice at 8 and 17 weeks of age (n=6–10). (c) Representative hematoxylin and eosin (H&E)-stained sections from the mid colon (MC) of Win and RaW mice and a representative cecal (Ce) section from 17-week-old RaW mice; scale bar=50 μm. Statistics: median and interquartile range (IQR); Mann–Whitney U-test and P-values are shown. Kruskal–Wallis nonparametric analysis of variance (ANOVA), Dunn's multiple comparison test; *P<0.05, **P<0.01, ***P<0.001. *Comparison between age groups within genotypes; #Comparison between the genotypes within age groups.
Taken together, the data from these experiments show, first, that there was no protection from spontaneous colitis in young Winnie mice when there is a deficiency of T and B cells, pointing to a role played by innate cells in mediating spontaneous colitis in this model where epithelial ER stress and a depleted mucus barrier are the primary defects. Second, as RaW mice grew older, the lack of progression of colonic inflammation shows that lymphocytes are necessary for the progression of colitis in this model. Third, as the Rag-1-/- mice also lack Treg, the increased innate inflammation in the proximal and mid colon in younger RaW mice, and the cecal inflammation in older RaW mice, may reflect absence of Treg-mediated suppression of innate immunity. In other words, inflammatory phenotype is dependent on a site-specific balance of innate and adaptive effector and regulatory cells.
Rapid and more severe T cell-mediated colitis in RaW mice
In order to further assess the activation and pathogenicity of effector T cells, we transferred wild-type splenic naive T cells into Rag-1-/- and RaW mice. Rag-1-/- mice displayed symptoms of intestinal disease including hunched back, ruffled fur, diarrhea, and body weight loss, necessitating euthanasia from 46 days post-transfer of naive T cells. In contrast, RaW mice started exhibiting severe body weight loss and other clinical symptoms of colitis as early as 16 days post-transfer. RaW mice showed significantly reduced survival to the point of ethically mandated euthanasia (Figure 7a) and lower body weight compared with Rag-1-/- mice (Figure 7b). Colon weight at the time of ethically mandated euthanasia was significantly greater in RaW mice despite being sampled at earlier time points (Figure 7c). Histological assessment also revealed more severe colonic inflammation in RaW mice compared with Rag-1-/- (Figure 7d). This experiment shows that the Winnie epithelial defect results in enhanced intestinal T-cell activation and that T effector cells can contribute substantially to pathology.
RaW mice develop severe colitis following transfer of naive T cells. (a) Survival to ethically mandated euthanasia (>20% loss of body weight), (b) body weight, (c) examples of histological colitis showing mid colon sections from a Rag1-/- mouse at 54 days and a RaW mouse at 21 days post-transfer of naive T cells mice (scale bar=50 μm), and (d) colon weight at euthanasia in RaW and Rag-1-/- mice receiving naive T cells. Statistics: (a) Log-rank test; (b, d) Mann–Whitney U-test and P-values are shown; ***P<0.001. (d) The different symbols to indicate the day of culling of these mice after naive T-cell transfer are as follows: ▴ 21–30 days; ▾ 31–40 days post-transfer; and ▪ 41–52 days post-transfer.
Discussion
We describe here in detail that mice carrying a single missense mutation in the Muc2 secretory mucin gene, resulting in ER stress in intestinal secretory cells and a depleted mucus barrier, develop a complex inflammatory response involving innate and adaptive immunity not dissimilar to the phenotype of UC. Muc2 is exclusively expressed in epithelial cells but we show in this study that the underlying response to this epithelial defect with a normal immune system involves both innate and adaptive immunity. The Winnie model offers a unique insight into the role of a primary intestinal epithelial defect leading to a progressively escalating immune response where there is less secretion of the mucosal immune system conditioning factor TSLP, an accumulation of activated mucosal DC, elevated IL-17A, and IFN-γ production by the mucosal CD4+ T lymphocytes and increased expression of TH17 genes at the sites of histological inflammation. In addition, leukocytes from the intestinal draining lymph nodes secrete multiple TH1-, TH2-, and TH17-type cytokines in a complex pattern as in IBD.13, 14, 15, 16, 17, 18, 19, 20, 21 Loss of T and B lymphocytes did not completely ameliorate colitis but altered the colonic distribution of inflammation in young mice and prevented progression of colonic inflammation. Taken together with the rapid activation and severe colitis induced following T-cell transfer into Winnie mice lacking lymphocytes, these data show that this primary epithelial defect leads to inflammation driven by both innate and adaptive immunity.
The first description of the missense single-nucleotide polymorphism Winnie model12 demonstrated that intestinal epithelial-restricted defects can initiate inflammation. The list of epithelial defects now includes gene deletions of NFκB essential modulator,22 Muc2,23 secretory cell ER disulfide isomerize Agr2,24 and unfolded protein response transcription factor Xbp1.25 Other epithelial defects that may at least in part induce inflammation include a missense single-nucleotide polymorphism in Mbtps1 that encodes an unfolded protein response transcription factor activating enzyme,26 and deficiency in autophagy genes Atg16l1 and Atg5.27 Interestingly, the majority of these defects affect intestinal secretory cells and are within the ER stress, unfolded protein response, and autophagy pathways. Autophagy is linked to ER stress as it is responsible for clearing accumulated misfolded proteins.27, 28 Defects in autophagy lead to defects in Paneth cells and goblet cells,27 and Paneth cells can also secrete IL-17 under stress. Rare individual gene variations affecting the intestinal epithelium and its interaction with the luminal environment may combine with immune polymorphisms to produce the IBD phenotype. A recent genome-wide association study (GWAS) and meta-analysis of GWAS in UC implicates ORMDL3,29 a gene that is important in responding to ER stress.
The current study provides one of the most detailed descriptions of the nature of inflammation in a murine epithelial defect model. The similarities with the inflammatory response in human IBD provide further support for epithelial defects being a primary driver of at least a subset of IBD. Importantly, it is highly likely that such epithelial defects would combine with common polymorphisms affecting the immune response to initiate and sustain inflammation.
A consistent finding in both UC and CD is markedly elevated intestinal IL-1β as was found in the Winnie epithelial defect model. Similarly, our finding of increased TH17 gene expression and increased IL-17A production by T cells is consistent with findings in IBD.1, 8, 9, 30 Although our detailed cytokine analyses from colonic explants cultures, stimulated CD11c+ APCs, and activated lymph node leukocytes indicated increases in many cytokines, the most highly elevated cytokines were IL-1β, IL-17A, and IL-12/23p40, offering a clue toward a skewing to a IL-23/TH17 response in Winnie as is now emerging in IBD. Our studies in Winnie show the greatest activation of mucosal TH17 responses at the region of maximal histological inflammation, the distal colon, where mRNA expression levels of IL-17A and IL-17F were significantly higher than in the proximal colon, despite the presence of a high density of affected goblet cells in the proximal colon12 and decreased TSLP production in both colonic regions. The major source of IL-17A and IFN-γ production in Winnie colonic lamina propria were CD4+ lamina propria lymphocytes, consistent with findings in other models of murine colitis.31, 32 The inherent differences in immune regulation between the proximal and distal colon, or differences in the distribution of IL-17-producing effector cells, may explain the distal bias in inflammation that is also seen in UC.
Many studies have shown the pathogenic potential of RAR-related orphan receptor C (the master regulator of TH17 cytokines,33 IL-17A, IL-17F,31 and IL-21);34 however, IL-17A and IL-17F have also been shown to be protective in some contexts,35 including suppression of the development of TH1 T cells.35, 36 In Winnie mice, we show an accumulation of CD11c+MHC class IIhi IL-12/23p40-producing APCs in the lamina propria. It is likely that these APCs, which are normally conditioned by their microenvironment including signals from epithelial cells,5 have become activated in Winnie following: (i) direct stimulation by ER-stressed goblet cells,33 (ii) diminished conditioning signals from the stressed epithelium as we show for TSLP, and/or (iii) increased exposure to microbial pattern-recognition receptor ligands because of depletion of the mucus barrier. These factors are likely to act in concert, and the direct link between ER stress and depletion of the mucus barrier make it very difficult to dissect out their relative contribution in this model.
Young RaW mice developed increased histological inflammation in the proximal and mid colon, indicating the importance of innate immune cells (e.g., NK cells, DCs, macrophages, and neutrophils) in the absence of lymphocyte-mediated immunity. However, colitis did not progressively increase in severity in RaW mice, except in the cecum. This demonstrates the importance of T cells in the progression of colitis with age in Winnie mice, and presents an interesting case scenario where innate immune activation seems to have a major role early on, while the effector T cells dictate further exacerbation of chronic intestinal inflammation. The reverse colonic gradient in histological inflammation in the RaW mice vs. Winnie mice suggests that the balance of TH17/Treg at different colonic sites may be a factor in determining the inflammatory phenotype at those sites. The crucial role for T lymphocytes in progressing colonic inflammation is further evidenced by the more rapid and severe colitis seen in RaW mice receiving adoptively transferred naive T cells.
Whether Treg have normal or aberrant function in human colitis is still unclear; however, the protective role of Treg in mouse models of colitis is well established.37 CD4+CD25+Foxp3+ induced Treg accumulate in the colonic mucosa in both affected and unaffected areas in UC.38 The increased innate inflammation in the proximal colon of young RaW mice and in the cecum of the older RaW mice, which lack Treg, is consistent with there being some effective immunosuppression in Winnie. Additionally, we found an elevated frequency of induced Treg in Winnie lymphoid tissues. Specific deletion or interference with effector function or deletion of lymphoid/myeloid or intestinal epithelial cell-derived IL-10, which maintains Foxp3 expression in the context of inflammation,39 would be required to properly assess the efficacy of suppressive activity.
Figure 8 shows our proposed model for the development of inflammation in response to goblet cell ER stress and barrier dysfunction. In the normal intestine, the protective mucosal barrier consists of an outer mucus layer containing microbes and an inner sterile layer.40 The epithelium produces factors such as TSLP41 and IL-10 that condition underlying DCs and macrophages, resulting in non-inflammatory responses (TH2, Tr1, and TH3).42 Draining lymph nodes (MLNs) do not populate the mucosa with activated T cells. In Winnie, the barrier dysfunction due to ER stress in the goblet cells leads to increased proximity between the microbiota and the mucosa, leading to reduced epithelial cell conditioning of DCs and macrophages together with direct epithelial cell stress signals. These combine to activate DCs and macrophages that secrete cytokines including IL-1β, IL-23, and IL-6 that in turn activate TH1- and TH17-type responses, leading to the release of their associated cytokines like IFN-γ and IL-17. Although there is an increase in Treg capable of secreting suppressor cytokines such as IL-10 and transforming growth factor-β (TGF-β), they are overwhelmed by the proinflammatory responses. These events start a cycle of progressive intestinal inflammation.
Schematic representation of the generation of inflammation in Winnie.
In summary, this study of Winnie provides in detail how a primary epithelial defect resulting in goblet cell dysfunction can lead to an inflammatory response involving both innate and adaptive segments of immunity including the IL-23/TH17-type inflammatory response. The model mimics UC in particular, and potentially could provide a better platform for testing IBD therapies.
Methods
Full details are available in the Supplementary Materials and Methods online.
Mice and assessment of colitis. C57BL/6 and Rag-1-/- mice (B6.129S7 Rag1+m1mom) were purchased from the Animal Resource Centre, Australia. Winnie mice were bred in a conventional clean Helicobacter hepaticus-free animal facility used for all the experiments, which were conducted under the directions and approval of the animal ethics committee of the University of Queensland. Winnie and Rag-1-/- mice were crossed to produce littermates homozygous for each and both alleles. Clinical and histological assessment of colitis was conducted as described.12
Leukocyte isolation and culture. Leukocytes were harvested from MLNs, spleen, thymus, and bone marrow using standard techniques, LPMCs) were isolated as described,43 and single cells were used immediately for flow cytometry. Leukocytes were stimulated in culture with anti-CD3 and anti-CD28 and culture supernatants were frozen for analysis. CD11c+ cells were sorted from LPMCs and cultured with 100 ng ml–1 lipopolysaccharide.
Flow cytometry. All antibodies were purchased from BD Biosciences (Franklin Lakes, NJ) unless indicated. Specific clones are given in parentheses. Cells were preincubated with anti-mouse CD16/32 Fc block (93 from eBiosciences, San Diego, CA) before staining with flurochrome-conjugated antibodies against CD4-APC-Cy5 (GK1.5), CD4-FITC (GK1.5), CD8-PE and CD8-PacBlue (53-6.7), B220-FITC (RA3-6B2), CD11b-PE-Cy7 or CD11b-PacBlue (M1/70 from eBiosciences), CD11c-PacBlue (HL3), MHC class II-FITC (2G9), CD3-PE-Cy7 (145-2C11), CD103-PE (M290), CD62L-FITC (MEL-14), and CD44-APC (G44-26), and analyzed using a LSRII flow cytometer (BD BioSciences). Antibodies for intracellular staining for Foxp3-expressing Treg cells were from eBioscience). 7AAD staining was used to restrict analyses to live (negative) cells. Analysis was conducted using Flowjo v7.5 software (Treestar, Ashland, OR).
RNA isolation and quantitative reverse transcriptase-PCR. RNA was extracted in TRIzol (Invitrogen, Carlsbad, CA) and purified using RNEasy columns (Qiagen, Valencia, CA). Complementary DNA was synthesized using an iScript cDNA synthesis kit (Bio-Rad, Hercules, CA). Quantitative PCR was performed using SYBR green PCR master mix (Applied Biosystem, Foster City, CA) on a Rotor-gene 3000 and analyzed with Rotorgene v6 software (Qiagen). Sequences of exon spanning primers are given in Supplementary Methods online. Expression of each gene was normalized to the expression of the housekeeping gene, Gapdh, and relative quantitation based on the Pfaffl equation.44
Transfer model of colitis. Adoptive transfer of naive T cells from normal C57BL/6 donors to Rag-/- and RaW mice was followed as per the methodology described previously.45 Briefly, MACSbead (Miltenyi, Auburn, CA) isolated CD4+ T cells were sorted on FACS ARIA (BD Biosciences) flow sorter to isolate pure CD4+ CD45RBhi naive T cells. Naive T cells per mouse (0.5 × 106) were transferred to 15 Rag-/- and 15 RaW mice. Clinical symptoms were monitored closely and mice reaching the stage of 20% body weight loss or combined symptom scores necessitating euthanasia were culled and assessed for histological inflammation.
Measurement of cytokines. Cytokine concentrations in neat culture supernatants and serum were determined using a mouse Bio-Plex mouse cytokine 23-plex panel kit and analyzed using Luminex 200 (Bio-Rad) and Bio-Plex Manager software (Bio-Rad).
Statistics To compare groups, we used the nonparametric Mann–Whitney U-test or Kruskal–Wallis test with Dunn's multiple comparison test, and the results are presented as median±interquartile range. All statistical analyses were performed using Prism v4.03 (Graphpad Software, La Jolla, CA) or Systat v10.2 (Systat Software, Chicago, IL).
References
Fujino, S. et al. Increased expression of interleukin 17 in inflammatory bowel disease. Gut 52, 65–70 (2003).
Xavier, R.J. & Podolsky, D.K. Unravelling the pathogenesis of inflammatory bowel disease. Nature 448, 427–434 (2007).
Zaph, C. et al. Epithelial-cell-intrinsic IKK-beta expression regulates intestinal immune homeostasis. Nature 446, 552–556 (2007).
Nenci, A. et al. Epithelial NEMO links innate immunity to chronic intestinal inflammation. Nature 446, 557–561 (2007).
Rimoldi, M. et al. Intestinal immune homeostasis is regulated by the crosstalk between epithelial cells and dendritic cells. Nat. Immunol. 6, 507–514 (2005).
Elson, C.O. et al. Monoclonal anti-interleukin 23 reverses active colitis in a T cell-mediated model in mice. Gastroenterology 132, 2359–2370 (2007).
Uhlig, H.H. et al. Differential activity of IL-12 and IL-23 in mucosal and systemic innate immune pathology. Immunity 25, 309–318 (2006).
Kobayashi, T. et al. IL-23 differentially regulates the Th1/Th17 balance in ulcerative colitis and Crohn's disease. Gut 57, 1682–1689 (2008).
Rovedatti, L. et al. Differential regulation of interleukin-17 and interferon-\{gamma\} production in inflammatory bowel disease. Gut 58, 1629–1636 (2009).
Duerr, R.H. et al. A genome-wide association study identifies IL23R as an inflammatory bowel disease gene. Science 314, 1461–1463 (2006).
Barrett, J.C. et al. Genome-wide association defines more than 30 distinct susceptibility loci for Crohn's disease. Nat. Genet. 40, 955–962 (2008).
Heazlewood, C.K. et al. Aberrant mucin assembly in mice causes endoplasmic reticulum stress and spontaneous inflammation resembling ulcerative colitis. PLoS Med. 5, e54 (2008).
Mahida, Y.R., Wu, K. & Jewell, D.P. Enhanced production of interleukin 1-beta by mononuclear cells isolated from mucosa with active ulcerative colitis of Crohn's disease. Gut 30, 835–838 (1989).
Gionchetti, P. et al. Interleukin 1 beta (IL-1 beta) release from fresh and cultured colonic mucosa in patients with ulcerative colitis (UC). Agents Actions Spec No, C50–C52 (1992).
Olson, A.D., Ayass, M. & Chensue, S. Tumor necrosis factor and IL-1 beta expression in pediatric patients with inflammatory bowel disease. J. Pediatr. Gastroenterol. Nutr. 16, 241–246 (1993).
Fuss, I.J. et al. Disparate CD4+ lamina propria (LP) lymphokine secretion profiles in inflammatory bowel disease. Crohn's disease LP cells manifest increased secretion of IFN-gamma, whereas ulcerative colitis LP cells manifest increased secretion of IL-5. J. Immunol. 157, 1261–1270 (1996).
Reimund, J.M. et al. Mucosal inflammatory cytokine production by intestinal biopsies in patients with ulcerative colitis and Crohn's disease. J. Clin. Immunol 16, 144–150 (1996).
Vainer, B., Nielsen, O.H., Hendel, J., Horn, T. & Kirman, I. Colonic expression and synthesis of interleukin 13 and interleukin 15 in inflammatory bowel disease. Cytokine 12, 1531–1536 (2000).
Fuss, I.J. et al. Nonclassical CD1d-restricted NK T cells that produce IL-13 characterize an atypical Th2 response in ulcerative colitis. J. Clin. Invest. 113, 1490–1497 (2004).
Kadivar, K. et al. Intestinal interleukin-13 in pediatric inflammatory bowel disease patients. Inflamm. Bowel. Dis. 10, 593–598 (2004).
Heller, F. et al. Interleukin-13 is the key effector Th2 cytokine in ulcerative colitis that affects epithelial tight junctions, apoptosis, and cell restitution. Gastroenterology 129, 550–564 (2005).
Nenci, A. et al. Epithelial NEMO links innate immunity to chronic intestinal inflammation. Nature 446, 557–561 (2007).
Van der Sluis, M. et al. Muc2-deficient mice spontaneously develop colitis, indicating that MUC2 is critical for colonic protection. Gastroenterology 131, 117–129 (2006).
Park, S.W. et al. The protein disulfide isomerase AGR2 is essential for production of intestinal mucus. Proc. Natl. Acad. Sci. USA 106, 6950–6955 (2009).
Kaser, A. et al. XBP1 links ER stress to intestinal inflammation and confers genetic risk for human inflammatory bowel disease. Cell 134, 743–756 (2008).
Brandl, K. et al. Enhanced sensitivity to DSS colitis caused by a hypomorphic Mbtps1 mutation disrupting the ATF6-driven unfolded protein response. Proc. Natl. Acad. Sci. USA 106, 3300–3305 (2009).
Cadwell, K. et al. A key role for autophagy and the autophagy gene Atg16l1 in mouse and human intestinal Paneth cells. Nature 456, 259–263 (2008).
Bernales, S., McDonald, K.L. & Walter, P. Autophagy counterbalances endoplasmic reticulum expansion during the unfolded protein response. PLoS Biol. 4, e423 (2006).
McGovern, D.P. et al. Genome-wide association identifies multiple ulcerative colitis susceptibility loci. Nat. Genet 42, 332–337 (2010).
Yen, D. et al. IL-23 is essential for T cell-mediated colitis and promotes inflammation via IL-17 and IL-6. J. Clin. Invest. 116, 1310–1316 (2006).
Ito, R. et al. Involvement of IL-17A in the pathogenesis of DSS-induced colitis in mice. Biochem. Biophys. Res. Commun. 377, 12–16 (2008).
O'Connor, W. Jr ., Zenewicz, L.A. & Flavell, R.A. The dual nature of T(H)17 cells: shifting the focus to function. Nat. Immunol. 11, 471–476 (2010).
Leppkes, M. et al. RORgamma-expressing Th17 cells induce murine chronic intestinal inflammation via redundant effects of IL-17A and IL-17F. Gastroenterology 136, 257–267 (2009).
Fina, D. et al. Regulation of gut inflammation and th17 cell response by interleukin-21. Gastroenterology 134, 1038–1048 (2008).
O'Connor, W. Jr . et al. A protective function for interleukin 17A in T cell-mediated intestinal inflammation. Nat. Immunol. 10, 603–609 (2009).
Ogawa, A., Andoh, A., Araki, Y., Bamba, T. & Fujiyama, Y. Neutralization of interleukin-17 aggravates dextran sulfate sodium-induced colitis in mice. Clin. Immunol. 110, 55–62 (2004).
Izcue, A. et al. Interleukin-23 restrains regulatory T cell activity to drive T cell-dependent colitis. Immunity 28, 559–570 (2008).
Yu, Q.T. et al. Expression and functional characterization of FOXP3+ CD4+ regulatory T cells in ulcerative colitis. Inflamm. Bowel. Dis. 13, 191–199 (2007).
Murai, M. et al. Interleukin 10 acts on regulatory T cells to maintain expression of the transcription factor Foxp3 and suppressive function in mice with colitis. Nat. Immunol. 10, 1178–1184 (2009).
Johansson, M.E. et al. The inner of the two Muc2 mucin-dependent mucus layers in colon is devoid of bacteria. Proc. Natl. Acad. Sci. USA 105, 15064–15069 (2008).
Taylor, B.C. et al. TSLP regulates intestinal immunity and inflammation in mouse models of helminth infection and colitis. J. Exp. Med. 206, 655–667 (2009).
Izcue, A., Coombes, J.L. & Powrie, F. Regulatory lymphocytes and intestinal inflammation. Annu. Rev. Immunol. 27, 313–338 (2009).
Weigmann, B. et al. Isolation and subsequent analysis of murine lamina propria mononuclear cells from colonic tissue. Nat. Protoc. 2, 2307–2311 (2007).
Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 29, e45 (2001).
Ostanin, D.V. et al. T cell transfer model of chronic colitis: concepts, considerations, and tricks of the trade. Am. J. Physiol. Gastrointest. Liver Physiol. 296, G135–G146 (2009).
Acknowledgements
This research was supported by the Australian NHMRC project grant 488809. M.A.M. was supported by a NHMRC Senior Research Fellowship; T.H.F. was supported by a NHMRC Practitioner Fellowship.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declared no conflict of interest.
Additional information
SUPPLEMENTARY MATERIAL is linked to the online version of the paper
Rights and permissions
This work is licensed under the Creative Commons Attribution-NonCommercial-No Derivative Works 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/3.0/
About this article
Cite this article
Eri, R., Adams, R., Tran, T. et al. An intestinal epithelial defect conferring ER stress results in inflammation involving both innate and adaptive immunity. Mucosal Immunol 4, 354–364 (2011). https://doi.org/10.1038/mi.2010.74
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/mi.2010.74
This article is cited by
-
Anti-inflammatory effects of Lactobacillus johnsonii L531 in a pig model of Salmonella Infantis infection involves modulation of CCR6+ T cell responses and ER stress
Veterinary Research (2020)
-
Goblet cells: multifaceted players in immunity at mucosal surfaces
Mucosal Immunology (2018)
-
MCC950, a specific small molecule inhibitor of NLRP3 inflammasome attenuates colonic inflammation in spontaneous colitis mice
Scientific Reports (2018)
-
TNFα deficiency results in increased IL-1β in an early onset of spontaneous murine colitis
Cell Death & Disease (2017)
-
High Fat Diets Induce Colonic Epithelial Cell Stress and Inflammation that is Reversed by IL-22
Scientific Reports (2016)