Abstract
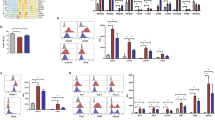
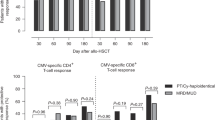
We have recently described a specialized subset of human natural killer (NK) cells with a CD56dimCD57+NKG2C+ phenotype that expand specifically in response to cytomegalovirus (CMV) reactivation in hematopoietic cell transplant (HCT) recipients and exhibit properties characteristic of adaptive immunity. We hypothesize that these cells mediate relapse protection and improve post-HCT outcomes. In 674 allogeneic HCT recipients, we found that those who reactivated CMV had lower leukemia relapse (26% (17–35%), P=0.05) and superior disease-free survival (DFS) (55% (45–65%) P=0.04) 1 year after reduced intensity conditioning (RIC) compared with CMV seronegative recipients who experienced higher relapse rates (35% (27–43%)) and lower DFS (46% (38–54%)). This protective effect was independent of age and graft-vs-host disease and was not observed in recipients who received myeloablative regimens. Analysis of the reconstituting NK cells demonstrated that CMV reactivation is associated with both higher frequencies and greater absolute numbers of CD56dimCD57+NKG2C+ NK cells, particularly after RIC HCT. Furthermore, expansion of these cells at 6 months posttransplant independently trended toward a lower 2-year relapse risk. Together, our data suggest that the protective effect of CMV reactivation on posttransplant relapse is in part driven by adaptive NK cell responses.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Cooley S, Weisdorf DS . Natural killer cells and tumor control. Curr Opin Hematol 2010; 17: 514–521.
Jacobs R, Stoll M, Stratmann G, Leo R, Link H, Schmidt RE . CD16- CD56+ natural killer cells after bone marrow transplantation. Blood 1992; 79: 3239–3244.
Nguyen S, Dhedin N, Vernant JP, Kuentz M, Al Jijakli A, Rouas-Freiss N et al. NK-cell reconstitution after haploidentical hematopoietic stem-cell transplantations: immaturity of NK cells and inhibitory effect of NKG2A override GvL effect. Blood 2005; 105: 4135–4142.
Foley B, Cooley S, Verneris MR, Curtsinger J, Luo X, Waller EK et al. NK cell education after allogeneic transplantation: dissociation between recovery of cytokine-producing and cytotoxic functions. Blood 2011; 118: 2784–2792.
Vago L, Forno B, Sormani MP, Crocchiolo R, Zino E, Di Terlizzi S et al. Temporal, quantitative, and functional characteristics of single-KIR-positive alloreactive natural killer cell recovery account for impaired graft-versus-leukemia activity after haploidentical hematopoietic stem cell transplantation. Blood 2008; 112: 3488–3499.
Min-Oo G, Kamimura Y, Hendricks DW, Nabekura T, Lanier LL . Natural killer cells: walking three paths down memory lane. Trends Immunol 2013; 34: 251–258.
Elmaagacli AH, Steckel NK, Koldehoff M, Hegerfeldt Y, Trenschel R, Ditschkowski M et al. Early human cytomegalovirus replication after transplantation is associated with a decreased relapse risk: evidence for a putative virus-versus-leukemia effect in acute myeloid leukemia patients. Blood 2011; 118: 1402–1412.
Ito S, Pophali P, Co W, Koklanaris EK, Superata J, Fahle GA et al. CMV reactivation is associated with a lower incidence of relapse after allo-SCT for CML. Bone Marrow Transplant 2013; 48: 1313–1316.
Green ML, Leisenring WM, Xie H, Walter RB, Mielcarek M, Sandmaier BM et al. CMV reactivation after allogeneic HCT and relapse risk: evidence for early protection in acute myeloid leukemia. Blood 2013; 122: 1316–1324.
Kaplan EL, Meier P . Nonparametric-estimation from incomplete observations. J Am Stat Assoc 1958; 53: 457–481.
Chang IM, Gelman R, Pagano M . Corrected group prognostic curves and summary statistics. J Chronic Dis 1982; 35: 669–674.
Fine JP, Gray RJ . A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 1999; 94: 496–509.
Foley B, Cooley S, Verneris MR, Pitt M, Curtsinger J, Luo X et al. Cytomegalovirus reactivation after allogeneic transplantation promotes a lasting increase in educated NKG2C+ natural killer cells with potent function. Blood 2012; 119: 2665–2674.
Rolle A, Pollmann J, Ewen EM, Le VT, Halenius A, Hengel H et al. IL-12-producing monocytes and HLA-E control HCMV-driven NKG2C+ NK cell expansion. J Clin Invest 2014; 124: 5305–5316.
Thomson KJ, Mackinnon S, Peggs KS . CMV-specific cellular therapy for acute myeloid leukemia? Blood 2012; 119: 1088–1090, author reply 1090–1091.
Manjappa S, Bhamidipati PK, Stokerl-Goldstein KE, DiPersio JF, Uy GL, Westervelt P et al. Protective effect of cytomegalovirus reactivation on relapse after allogeneic hematopoietic cell transplantation in acute myeloid leukemia patients is influenced by conditioning regimen. Biol Blood Marrow Transplant 2014; 20: 46–52.
DeCook LJ, Thoma M, Huneke T, Johnson ND, Wiegand RA, Patnaik MM et al. Impact of lymphocyte and monocyte recovery on the outcomes of allogeneic hematopoietic SCT with fludarabine and melphalan conditioning. Bone Marrow Transplant 2013; 48: 708–714.
Sun JC, Madera S, Bezman NA, Beilke JN, Kaplan MH, Lanier LL . Proinflammatory cytokine signaling required for the generation of natural killer cell memory. J Exp Med 2012; 209: 947–954.
Gerosa F, Baldani-Guerra B, Nisii C, Marchesini V, Carra G, Trinchieri G . Reciprocal activating interaction between natural killer cells and dendritic cells. J Exp Med 2002; 195: 327–333.
Marin R, Ruiz-Cabello F, Pedrinaci S, Méndez R, Jiménez P, Geraghty DE et al. Analysis of HLA-E expression in human tumors. Immunogenetics 2003; 54: 767–775.
Lo Monaco E, Tremante E, Cerboni C, Melucci E, Sibilio L, Zingoni A et al. Human leukocyte antigen E contributes to protect tumor cells from lysis by natural killer cells. Neoplasia 2011; 13: 822–830.
Schlums H, Cichocki F, Tesi B, Theorell J, Beziat V, Holmes TD et al. Cytomegalovirus infection drives adaptive epigenetic diversification of NK cells with altered signaling and effector function. Immunity 2015; 42: 443–456.
Lee J, Zhang T, Hwang I, Kim A, Nitschke L, Kim M et al. Epigenetic modification and antibody-dependent expansion of memory-like NK cells in human cytomegalovirus-infected individuals. Immunity 2015; 42: 431–442.
Acknowledgements
We thank the following shared resources within the Masonic Cancer Center at the University of Minnesota: The Translational Therapy Laboratory, Flow Cytometry Shared Resource, Clinical Trials Office, Oncology Medical Informatics and Services, and the Biostatistical Core. This work was supported by NIH P30 CA77598, R01 CA72669 (to BRB), 1R01 CA181045 (to DJD), 5R01 CA077544 (to DJD) and P30 CA033572 (City of Hope Cancer Center). FC is an Amy Strelzer Manasevit Research Program Scholar and is supported by a National Marrow Donor Program Award (CON000000052310).
Author contributions
FC and SC contributed to the study design, analyzed and interpreted data and wrote the manuscript. ZD analyzed data. TED performed the biostatistical analyses for the study. HS contributed to the conception of the study. BZ performed NK cell function assays. CGB, BRB, JS and MRV contributed to the study design and sample procurement. DJD reviewed and edited the manuscript. YTB contributed to the conception of the study and interpretation of results. DJW and JSM contributed to the study design and sample procurement, analyzed and interpreted data and wrote the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Cichocki, F., Cooley, S., Davis, Z. et al. CD56dimCD57+NKG2C+ NK cell expansion is associated with reduced leukemia relapse after reduced intensity HCT. Leukemia 30, 456–463 (2016). https://doi.org/10.1038/leu.2015.260
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/leu.2015.260
This article is cited by
-
The effect of varicella-zoster virus reactivation on the long-term outcomes of patients undergoing allogeneic hematopoietic stem cell transplantation
Journal of Health, Population and Nutrition (2023)
-
High-throughput characterization of HLA-E-presented CD94/NKG2x ligands reveals peptides which modulate NK cell activation
Nature Communications (2023)
-
Activating NKG2C Receptor: Functional Characteristics and Current Strategies in Clinical Applications
Archivum Immunologiae et Therapiae Experimentalis (2023)
-
The Role of NK Cells and Their Exosomes in Graft Versus Host Disease and Graft Versus Leukemia
Stem Cell Reviews and Reports (2023)
-
Adaptive NK cells undergo a dynamic modulation in response to human cytomegalovirus and recruit T cells in in vitro migration assays
Bone Marrow Transplantation (2022)