Abstract
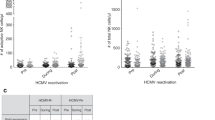
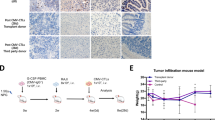
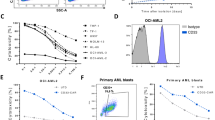
Cytomegalovirus (CMV) reactivation remains a common complication and leads to high mortality in patients who undergo allogeneic hematopoietic stem cell transplantation (allo-HSCT). Early natural killer (NK) cell reconstitution may protect against the development of human CMV (HCMV) infection post-HSCT. Our previous data showed that ex vivo mbIL21/4-1BBL-expanded NK cells exhibited high cytotoxicity against leukemia cells. Nevertheless, whether expanded NK cells have stronger anti-HCMV function is unknown. Herein, we compared the anti-HCMV functions of ex vivo expanded NK cells and primary NK cells. Expanded NK cells showed higher expression of activating receptors, chemokine receptors and adhesion molecules; stronger cytotoxicity against HCMV-infected fibroblasts; and better inhibition of HCMV propagation in vitro than primary NK cells. In HCMV-infected humanized mice, expanded NK cell infusion resulted in higher NK cell persistence and more effective tissue HCMV elimination than primary NK cell infusion. A clinical cohort of 20 post-HSCT patients who underwent adoptive NK cell infusion had a significantly lower cumulative incidence of HCMV infection (HR = 0.54, 95% CI = 0.32–0.93, p = 0.042) and refractory HCMV infection (HR = 0.34, 95% CI = 0.18–0.65, p = 0.009) than controls and better NK cell reconstitution on day 30 post NK cell infusion. In conclusion, expanded NK cells exhibit stronger effects than primary NK cells against HCMV infection both in vivo and in vitro.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Wang Y, Chang YJ, Xu LP, Liu KY, Liu DH, Zhang XH, et al. Who is the best donor for a related HLA haplotype-mismatched transplant? Blood. 2014;124:843–50.
El Chaer F, Shah DP, Chemaly RF. How I treat resistant cytomegalovirus infection in hematopoietic cell transplantation recipients. Blood. 2016;128:2624–36.
Kharfan-Dabaja MA, Boeckh M, Wilck MB, Langston AA, Chu AH, Wloch MK, et al. A novel therapeutic cytomegalovirus DNA vaccine in allogeneic haemopoietic stem-cell transplantation: a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Infect Dis. 2012;12:290–9.
Teira P, Battiwalla M, Ramanathan M, Barrett AJ, Ahn KW, Chen M, et al. Early cytomegalovirus reactivation remains associated with increased transplant-related mortality in the current era: a CIBMTR analysis. Blood. 2016;127:2427–38.
Hill JA, Mayer BT, Xie H, Leisenring WM, Huang ML, Stevens-Ayers T, et al. The cumulative burden of double-stranded DNA virus detection after allogeneic HCT is associated with increased mortality. Blood. 2017;129:2316–25.
Broers AE, van Der Holt R, van Esser JW, Gratama JW, Henzen-Logmans S, Kuenen-Boumeester V, et al. Increased transplant-related morbidity and mortality in CMV-seropositive patients despite highly effective prevention of CMV disease after allogeneic T-cell-depleted stem cell transplantation. Blood. 2000;95:2240–5.
Liu J, Kong J, Chang YJ, Chen H, Chen YH, Han W, et al. Patients with refractory cytomegalovirus (CMV) infection following allogeneic haematopoietic stem cell transplantation are at high risk for CMV disease and non-relapse mortality. Clin Microbiol Infect. 2015;21:1121.e9–15.
Liu J, Chang YJ, Yan CH, Xu LP, Jiang ZF, Zhang XH, et al. Poor CMV-specific CD8+ T central memory subset recovery at early stage post-HSCT associates with refractory and recurrent CMV reactivation. J Infect. 2016;73:261–70.
Zhao XY, Pei XY, Chang YJ, Yu XX, Xu LP, Wang Y, et al. First-line therapy with donor-derived human cytomegalovirus (HCMV)-specific T cells reduces persistent HCMV infection by promoting antiviral immunity after allogenic stem cell transplantation. Clin Infect Dis. 2020;70:1429–37.
Pei XY, Zhao XY, Chang YJ, Liu J, Xu LP, Wang Y, et al. Cytomegalovirus-specific T-cell transfer for refractory cytomegalovirus infection after haploidentical stem cell transplantation: the quantitative and qualitative immune recovery for cytomegalovirus. J Infect Dis. 2017;216:945–56.
Yu XX, Cao XH, Yan H, Luo XY, Zhao XS, Sun YQ, et al. Delay expression of NKp30 on NK cells correlates with long-term mycophenolate mofetil treatment and higher EBV viremia post allogenic hematological stem cells transplantation. Clin Immunol. 2019;205:49–56.
Zhao X, Chang Y, Xu L, Liu D, Liu K, Wang Y, et al. Rapid early recovery of absolute lymphocyte counts at day 30 can predict reduced risk of relapse in acute lymphoblastic leukemia following haploidentcial allogeneic stem cell transplantation. Chin J Organ Transplant. 2014;35:3–7.
Forthal DN, Phan T, Landucci G. Antibody inhibition of cytomegalovirus: the role of natural killer and macrophage effector cells. Transpl Infect Dis. 2001;3:31–4.
Orange JS, Wang B, Terhorst C, Biron CA. Requirement for natural killer cell-produced interferon gamma in defense against murine cytomegalovirus infection and enhancement of this defense pathway by interleukin 12 administration. J Exp Med. 1995;182:1045–56.
Bukowski JF, Woda BA, Habu S, Okumura K, Welsh RM. Natural killer cell depletion enhances virus synthesis and virus-induced hepatitis in vivo. J Immunol. 1983;131:1531–8.
Bukowski JF, Woda BA, Welsh RM. Pathogenesis of murine cytomegalovirus infection in natural killer cell-depleted mice. J Virol. 1984;52:119–28.
Sun JC, Beilke JN, Lanier LL. Adaptive immune features of natural killer cells. Nature. 2009;457:557–61.
Biron CA, Byron KS, Sullivan JL. Severe herpesvirus infections in an adolescent without natural killer cells. N Engl J Med. 1989;320:1731–5.
Orange JS. Natural killer cell deficiency. J Allergy Clin Immunol. 2013;132:515–25.
Kuijpers TW, Baars PA, Dantin C, van den Burg M, van Lier RA, Roosnek E. Human NK cells can control CMV infection in the absence of T cells. Blood. 2008;112:914–5.
Chang YJ, Zhao XY, Huang XJ. Effects of the NK cell recovery on outcomes of unmanipulated haploidentical blood and marrow transplantation for patients with hematologic malignancies. Biol Blood Marrow Transplant. 2008;14:323–34.
Zhao XY, Huang XJ, Liu KY, Xu LP, Liu DH. Reconstitution of natural killer cell receptor repertoires after unmanipulated HLA-mismatched/haploidentical blood and marrow transplantation: analyses of CD94:NKG2A and killer immunoglobulin-like receptor expression and their associations with clinical outcome. Biol Blood Marrow Transplant. 2007;13:734–44.
Barron MA, Gao D, Springer KL, Patterson JA, Brunvand MW, McSweeney PA, et al. Relationship of reconstituted adaptive and innate cytomegalovirus (CMV)-specific immune responses with CMV viremia in hematopoietic stem cell transplant recipients. Clin Infect Dis. 2009;49:1777–83.
Foley B, Cooley S, Verneris MR, Pitt M, Curtsinger J, Luo X, et al. Cytomegalovirus reactivation after allogeneic transplantation promotes a lasting increase in educated NKG2C+ natural killer cells with potent function. Blood. 2012;119:2665–74.
Yu X, Xu L, Chang Y, Huang X, Zhao X. Rapid reconstitution of NK1 cells after allogeneic transplantation is associated with a reduced incidence of graft-versus-host disease. Sci China Life Sci. 2018;61:902–11.
Zhao XY, Luo XY, Yu XX, Zhao XS, Han TT, Chang YJ, et al. Recipient-donor KIR ligand matching prevents CMV reactivation post-haploidentical T cell-replete transplantation. Br J Haematol. 2017;177:766–81.
Fang F, Xiao W, Tian Z. NK cell-based immunotherapy for cancer. Semin Immunol. 2017;31:37–54.
Fang F, Xiao W, Tian Z. Challenges of NK cell-based immunotherapy in the new era. Front Med. 2018;12:440–50.
Zhao XY, Jiang Q, Jiang H, Hu LJ, Zhao T, Yu XX, et al. Expanded clinical-grade membrane-bound IL-21/4-1BBL NK cell products exhibit activity against acute myeloid leukemia in vivo. Eur J Immunol. 2020;50:1374–85.
Yu XX, Shang QN, Liu XF, He M, Pei XY, Mo XD, et al. Donor NKG2C homozygosity contributes to CMV clearance after haploidentical transplantation. JCI Insight. 2022;7:e149120.
Wang Y, Liu QF, Xu LP, Liu KY, Zhang XH, Ma X, et al. Haploidentical vs identical-sibling transplant for AML in remission: a multicenter, prospective study. Blood. 2015;125:3956–62.
Wang Y, Wang HX, Lai YR, Sun ZM, Wu DP, Jiang M, et al. Haploidentical transplant for myelodysplastic syndrome: registry-based comparison with identical sibling transplant. Leukemia. 2016;30:2055–63.
Ojo EO, Sharma AA, Liu R, Moreton S, Checkley-Luttge MA, Gupta K, et al. Membrane bound IL-21 based NK cell feeder cells drive robust expansion and metabolic activation of NK cells. Sci Rep. 2019;9:14916.
Fujisaki H, Kakuda H, Shimasaki N, Imai C, Ma J, Lockey T, et al. Expansion of highly cytotoxic human natural killer cells for cancer cell therapy. Cancer Res. 2009;69:4010–7.
Yang Y, Badeti S, Tseng HC, Ma MT, Liu T, Jiang JG, et al. Superior expansion and cytotoxicity of human primary NK and CAR-NK cells from various sources via enriched metabolic pathways. Mol Ther Methods Clin Dev. 2020;18:428–45.
Wu Z, Sinzger C, Reichel JJ, Just M, Mertens T. Natural killer cells can inhibit the transmission of human cytomegalovirus in cell culture by using mechanisms from innate and adaptive immune responses. J Virol. 2015;89:2906–17.
Bjorklund AT, Carlsten M, Sohlberg E, Liu LL, Clancy T, Karimi M, et al. Complete remission with reduction of high-risk clones following haploidentical NK-Cell therapy against MDS and AML. Clin Cancer Res. 2018;24:1834–44.
Bachanova V, Sarhan D, DeFor TE, Cooley S, Panoskaltsis-Mortari A, Blazar BR, et al. Haploidentical natural killer cells induce remissions in non-Hodgkin lymphoma patients with low levels of immune-suppressor cells. Cancer Immunol Immunother. 2018;67:483–94.
Ciurea SO, Schafer JR, Bassett R, Denman CJ, Cao K, Willis D, et al. Phase 1 clinical trial using mbIL21 ex vivo-expanded donor-derived NK cells after haploidentical transplantation. Blood. 2017;130:1857–68.
Sharma SK, Kumar S, Agrawal N, Singh L, Mukherjee A, Seth T, et al. Cytomegalovirus reactivation following hematopoietic stem cell transplantation. J Infect Dev Ctries. 2013;7:1003–7.
Zhang Y, Wallace DL, de Lara CM, Ghattas H, Asquith B, Worth A, et al. In vivo kinetics of human natural killer cells: the effects of ageing and acute and chronic viral infection. Immunology. 2007;121:258–65.
Merino AM, Kim H, Miller JS, Cichocki F. Unraveling exhaustion in adaptive and conventional NK cells. J Leukoc Biol. 2020;108:1361–8.
Zaghi E, Calvi M, Puccio S, Spata G, Terzoli S, Peano C, et al. Single-cell profiling identifies impaired adaptive NK cells expanded after HCMV reactivation in haploidentical-HSCT. JCI Insight. 2021;6:e146973.
Huenecke S, Zimmermann SY, Kloess S, Esser R, Brinkmann A, Tramsen L. et al. IL-2-driven regulation of NK cell receptors with regard to the distribution of CD16+ and CD16- subpopulations and in vivo influence after haploidentical NK cell infusion. J Immunother. 2010;33:200–10.
Zhao XY, Zhao XS, Wang YT, Chen YH, Xu LP, Zhang XH, et al. Prophylactic use of low-dose interleukin-2 and the clinical outcomes of hematopoietic stem cell transplantation: a randomized study. Oncoimmunology. 2016;5:e1250992.
Hirakawa M, Matos TR, Liu H, Koreth J, Kim HT, Paul NE, et al. Low-dose IL-2 selectively activates subsets of CD4(+) Tregs and NK cells. JCI Insight. 2016;1:e89278.
Borysiewicz LK, Morris S, Page JD, Sissons JG. Human cytomegalovirus-specific cytotoxic T lymphocytes: requirements for in vitro generation and specificity. Eur J Immunol. 1983;13:804–9.
Wang D, Fu B, Shen X, Guo C, Liu Y, Zhang J, et al. Restoration of HBV-specific CD8(+) T-cell responses by sequential low-dose IL-2 treatment in non-responder patients after IFN-alpha therapy. Signal Transduct Target Ther. 2021;6:376.
Acknowledgements
We thank Mr. Chuan-Yu Zhang (iCELL Co., Ltd., Beijing, China) for his help in the organization of the expansion of NK cells. We also thank BeiGene Co., Ltd. (Beijing, China) for providing anti-PD-1, anti-TIGIT and anti-Tim3 antibodies. This work was supported by the National Key Research and Development Program of China (grant 2022YFA1103300), Major of the National Natural Science Foundation of China (No.82293630), Key Program of the National Natural Science Foundation of China (No. 81930004), and National Natural Science Foundation of China (grants 81870140, 82070184, 82270228 and 81370666). It was further supported through the Peking University People’s Hospital Research and Development Funds (grant RDX2019-14; RDL2021-01). We thank the core facilities at the Peking University Institute of Hematology for sample collection.
Author information
Authors and Affiliations
Contributions
Q-NS conducted the in vitro experiments and animal experiments and performed the statistical analyses. X-XY conducted flow cytometry assays and facilitated in vitro experiments. Z-LX, Y-HC, T-TH, Y-YZ, ML, Y-QS, YW, L-PX and X-HZ performed the clinical examinations. X-JH and X-YZ designed the study and interpreted the data. X-JH, X-YZ and Q-NS wrote the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shang, QN., Yu, XX., Xu, ZL. et al. Expanded clinical-grade NK cells exhibit stronger effects than primary NK cells against HCMV infection. Cell Mol Immunol 20, 895–907 (2023). https://doi.org/10.1038/s41423-023-01046-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41423-023-01046-5