Abstract
Survivin, a member of inhibitor of apoptosis (IAP) protein family, is a multifunctional protein expressed in most cancers. In addition to inhibition of apoptosis, it regulates proliferation and promotes migration. Its presence and function in cells is strongly regulated via transcription factors, intracellular localization, and degradation. We analyzed the presence of survivin at protein level in various culture environments and under activation of Src tyrosine kinase in epithelial canine kidney MDCK cells in order to elucidate factors controlling survivin ‘lifespan’. We used untransformed and temperature sensitive ts-Src MDCK cells as a model and forced them to grow in suspension (1D), in 2D on hard and soft surfaces and in soft 3D Matrigel environment with or without EGTA. In addition, we tested the effect of stressful conditions by cultivating the cells in the presence of an anti-cancer drug and a generator of reactive oxygen species (ROS), piperlongumine (PL) with or without an antioxidant, N-acetylcysteine (NAC). We could confirm that inhibition of apoptosis and simultaneous downregulation of survivin in MDCK cells required both intact cell–cell junctions, trans-interactions of E-cadherin and soft 3D matrix environment. In ts-Src-transformed MDCK cells, survivin was upregulated as soon as the cell–cell junctions were disintegrated. ROS generation with PL-induced cell death of ts-Src MDCK cells concomitantly with survivin downregulation. NAC rescued the ts-Src MDCK cells from ROS-induced apoptosis without upregulation of survivin resulting in a situation resembling untransformed MDCK cells in 3D environment and E-cadherin delineating the lateral cell walls.
Similar content being viewed by others
Main
Most tumors are carcinomas, having epithelial origin. They develop in stages, from benign to malignant and the transformation process consists of a sequence of events where the epithelial cells lose their differentiated phenotype and start uncontrolled proliferation, often caused by activation of oncogenes, such as Src or Ras. These trigger signaling cascades leading to activation of transcription factors and subsequent expression of mesenchymal proteins and downregulation of epithelial markers, increased proliferation, altered energy metabolism, and increased resistance to apoptosis. As transformation process continues, the cells turn migratory and acquire metastatic capacity. Expression of E-cadherin is often downregulated in tumor tissues and, conversely, strong E-cadherin adhesion can be considered as a mechanism for tumor suppression.1 In recent years, alternative roles for E-cadherin in tumor progression have become apparent and in many tumors classical epithelial-mesenchymal transition (EMT) does not occur and E-cadherin expression is not downregulated, but instead cell clusters capable of anchorage-independent survival and increased chemo-resistance have been observed.2
An important protein expressed in cancer tissues is survivin, a small member of the inhibitor of apoptosis (IAP) protein family, involved in cell cycle progression and apoptosis inhibition. Expression of survivin is associated with poor prognosis of almost all human tumors, and it may favor tumor development through several pathways. It is a component of the chromosome passenger complex and a key regulator of chromosome segregation and cytokinesis. Another role of survivin is inhibition of apoptosis after DNA damage-induced activation of checkpoint kinase 2. This results in rapid release of survivin from mitochondria, which binds to pro-apoptotic mediator Smac, and sequesters Smac away from the caspase inhibitor XIAP.3, 4 Very recently, new functions for survivin have been found: in androgen-independent prostate cancer PC3 cells it acts as a promoter of tumor cell invasion through enhanced mitochondrial bioenergetics and oxidative phosphorylation,5 whereas the opposite effect has been observed in neuroblastoma NB cell lines where survivin induces mitochondrial fragmentation, reduces mitochondrial respiration and enhances aerobic glycolysis.6 Moreover, the proline rich NH2 terminus of survivin has been shown to regulate activity of c-Src, and promote tumorigenesis in concert with c-Src.7
As survivin promotes proliferation and migration, it is important to keep survivin content low in adult differentiated tissues for the maintenance of the tissue integrity and functionality. Hence, external or internal stimuli are needed for upregulation of its expression when necessary. Several transcription factors have been observed to induce the expression of the survivin gene, eg, NF-κB, the inducible transcription factor signal transducer and activator of transcription-3 (STAT3), and Wnt-induced signaling involving nuclear β-catenin, and T-cell factor-4 (TCF4). Also GATA-1 transcription factor, KLF5 and specificity proteins Sp1 and Sp3 are able to activate survivin expression.3, 8, 9 In addition to gene expression, several post-translational mechanisms exist which regulate survivin levels and localization.3 Survivin is a short-lived protein and its content varies along the cell cycle, being high in the G2/M phase, and this has been shown to be caused by reduced rate of protein degradation instead of increased rate of gene transcription in cells.10 Localization of survivin in cytoplasm, mitochondria or nucleus also has significance in tumor progression.7, 11
Since malignant transformation is a very complex process, it has been an attractive target for bioinformatics. Guebel et al12 have carried out a systems biology analysis of events related to initiation of tumorigenesis in colon tissues either via cancer stem cells or malignant transformation. In their analysis, survivin expression was the lowest in stressed untransformed cells, modest in differentiated cells and high in transformed colon cancer cells. Parameters addressed in the model to the transformation state were eg, TCF/LEF/β-catenin pathway, and to the stressed cells the presence of reactive oxygen species (ROS).12 The authors admit, however, that more experimental data are needed for a reliable analysis of survivin expression.
In epithelial cells, the cadherin interactions and complexes generate ‘outside-in’ and ‘inside-out’ signals which regulate embryonic development and tissue homeostasis, at least via Wnt, receptor tyrosine kinase, Hippo, NF-κB and JAK/STAT signaling pathways.13 Chen and his co-workers14 have constructed a network model to evaluate the dynamic interplay between cadherin-mediated cell adhesion and Wnt signaling pathway, and elucidate the ways β-catenin can act as a functional switch between cadherin-mediated adhesion and gene activation. They could show that cadherins can inhibit Wnt signaling via binding of β-catenin molecules and in this way sequesters them away from participating to the signaling, but may also promote β-catenin signaling to nucleus through intracellular clustering of cadherins at adherens junctions (AJ), which affects the phosphorylation rate of β-catenin.14 Cadherin–catenin complexes can also interact with receptor tyrosine kinases, eg, EGFR resulting in either inhibition of EGFR signaling, thus promoting differentiation, or ligand-independent EGFR activation and subsequent activation of survival and proliferation mechanisms.2, 13
In order to test the validity of these models and analyse the correlation between survivin expression and suppression of apoptosis under various external conditions, a simple and flexible cell culture experimental model is needed. In our previous studies of transformation process we have used a ts-Src MDCK cell line, ie, canine kidney epithelial MDCK cells stably transfected with temperature sensitive viral Src. When cultivated at 40.5 °C, they behave as normal epithelial cells, whereas after a shift to 35 °C, Src tyrosine kinase is activated and the transformation process begins.15 With this model we have monitored changes in gene expression, protein phosphorylation, cadherin internalization, mitochondrial activity, and apoptosis.16, 17
In the present work, we have carried out an extensive analysis of the critical factors affecting survivin expression at protein level in untransformed and ts-Src-transformed MDCK cells in various conditions. We manipulated cell–matrix and cell–cell interactions as environmental parameters. ROS was shown to be a stress factor and influenced survivin expression in the model developed by Guebel et al.12 There are numerous natural and synthetic anti-cancer agents which inhibit tumor growth and induce ROS. Such molecules include eg, curcumin, dehydrozingerone, and PL.18, 19, 20 In the present work we used the ROS-producing agent, PL with or without an antioxidant, N-acetylcysteine (NAC) to test the correlation between survivin expression and oxidative stress.
We monitored the cell morphology, localization of E-cadherin and actin, expression of survivin and occurrence of apoptosis in all these conditions. We could confirm that inhibition of apoptosis and simultaneous downregulation of survivin in MDCK cells required both intact cell–cell junctions, trans-interactions of E-cadherin, and soft extracellular matrix (ECM) environment. In ts-Src-transformed MDCK cells survivin was upregulated as soon as the cell–cell junctions were disintegrated. ROS generation with PL-induced cell death of ts-Src MDCK cells concomitantly with survivin downregulation. NAC rescued the ts-Src MDCK cells from ROS- induced apoptosis without upregulation of survivin resulting in a situation resembling untransformed MDCK cells in 3D environment.
Materials and methods
Cell Lines
Madin-Darby canine kidney cells (MDCK, strain II) were provided by Professor Kai Simons (Max Planck Institute for Molecular Biology, Dresden, Germany), and the ts-Src MDCK cells were provided by Professor Walter Birchmeier and Dr Jürgen Behrens (Max Delbrück Center for Molecular Medicine, Berlin, Germany).15
Both cell lines cells were maintained as described by Töyli et al17 and Capra and Eskelinen.21
Reagents and Antibodies
Hanks’ Balanced Saline Solution (HBSS), ethylene glycol-bis(2-aminoethylether)-N,N,N′,N′-tetraacetic acid (EGTA), NAC and PL were purchased from Sigma-Aldrich (St Louis, MO, USA). CellMask Orange membrane dyes, Alexa 568 phalloidin actin dye as well as DAPI, and Hoechst 33342 nuclear dyes were purchased from Life Technologies (Carlsbad, CA, USA). A FITC-annexin V/Dead Cell Apoptosis Kit with FITC-annexin V and propidium iodide was purchased from Life Technologies.
Survivin anti-rabbit polyclonal antibody (ab469) was purchased from Abcam (Cambridge, UK), 1:500 dilution of antibody was used for both Western blotting and fluorescence imaging of fixed cells. Anti-α/β-tubulin rabbit antibody (#2148, purchased from Cell Signaling Technology (Danvers, MA, USA)) was used as a loading control in western blotting.
β1 integrin blocking antibody (AIIB2-c) and monoclonal rr1 anti-mouse antibody to extracellular domain of E-cadherin were purchased from Developmental Studies Hybridoma Bank (Iowa City, IA, USA).
Anti-β-tubulin mouse antibody (#86298), used in immunofluorescence, was purchased from Cell Signaling Technology.
Alexa 488 (A-1008) and Texas Red anti-rabbit (T2767) and Alexa 488 (A-21200) and Texas Red anti-mouse antibodies (T2767) were all purchased from Life Technologies. HRP-conjugated anti-rabbit secondary antibody was purchased from Jackson ImmunoResearch Laboratories (West Grove, PA, USA) and was used for Western blotting in 1:5000 dilutions. Luminol and p-coumaric acid were purchased from Sigma-Aldrich.
EGTA was dissolved in PBS buffer (without Ca2+) containing 18 mM glucose to the concentration of 1 mM.
Hoechst was diluted into DMSO to a stock concentration of 5 mg/ml. DAPI was diluted into distilled water to a stock concentration of 1 mg/ml. FITC-annexin V, propidium iodide, CellMask Orange and phalloidin were prepared as per manufacturers’ instructions.
The antibodies were diluted into PBS with 0.2 M glycine for confocal microscopy samples (1:50 dilution for primary antibodies, 1:100 for secondary) and into TBS with 5% fat-free milk powder for Western blotting.
Matrigel and Cell Recovery Solution were purchased from Corning (New York, USA). Rat tail high-concentration collagen 1 was purchased from BD Biosciences (Franklin Lakes, NJ, USA). Collagenase A was purchased from Sigma-Aldrich.
Culture Conditions
We cultivated MDCK cells and ts-Src MDCK cells under four different circumstances: in suspension (1D), on cell culture plates (2D), on cell culture plates coated with thick layer of Matrigel (2½D), and in three-dimensional Matrigel or Matrigel-collagen mixtures (3D) and analyzed the morphology and apoptosis using time-lapse imaging of living cells using spinning disc confocal microscopy and fluorescence light microscopy. Expression of survivin was analyzed by immunoblotting. The distribution of junctional complexes and cytoskeletal proteins were analyzed by confocal microscopy of fixed specimens with the aid of specific antibodies and fluorescent markers.
MDCK cells and ts-Src MDCK cells were forced to grow in suspension (1D) by adding 10% β1 integrin antibody (AIIB2) into the culture medium and cultivating the cells in 37 °C and 35 °C, respectively, in 10 cm bacterial culture dishes lacking cell adhesion promoting treatment.
For experiments in 2D environment, the cells were cultivated on coverslips in cell culture dishes and used for confocal microscopy and survivin expression analysis as described by Töyli et al.17
To encourage the cells to polarize, but not yet form cysts, so called 2½D environment was used. For this purpose, the cells were seeded on top of solidified Matrigel for confocal microscopy and multi-well dishes for immunoblotting, but instead of medium containing Matrigel, normal E-MEM medium was used as a supplement.
For 3D experiments, Matrigel was used in dilution of 1:1 with E-MEM. 150 000 cells were seeded on top of a solidified layer of Matrigel on 35 mm glass-bottom dishes, as described earlier17, 22 and cells were allowed to grow for 4 to 7 days, and used for live cell microscopy with a spinning disc confocal microscopy. For confocal microscopy of fixed cell cysts, a 20 mm flexiPERM conB silicon adapter (Greiner Bio-One, Monroe, NC, USA) was attached to an objective slide and Matrigel-E-MEM solution was applied and allowed to solidify. Subsequently, 60 000 cells were seeded on top of the Matrigel, as described by Debnath and co-workers22 and Töyli and co-workers,17 and were allowed to grow for 3–5 days. Cells were fixed and stained as explained by Töyli et al17 and Capra and Eskelinen.21
For analysis of survivin expression induced by the activation of Src by western blotting, ts-Src MDCK cells were seeded as described above, but grown in 40.5 °C for 7 days and then transferred to 35 °C for the indicated time. To confirm the reversibility of the survivin expression, ts-Src MDCK cells were first cultivated at 35 °C and then transferred to 40.5 °C for the indicated time periods.
In some experiments, a mixture of Collagen I and Matrigel (in a ratio of 3:1) was used. Collagen was prepared as described by Torkko et al.23 Ice cold Matrigel and Collagen I were mixed, the cell suspension was added and the mixture pipetted on to the glass-bottom dish and were allowed to solidify in 37 °C in the cell culture incubator.
Spinning Disc Microscopy of Living Cells for Morphological Analysis
We have employed confocal spinning disc time-lapse microscopy to monitor the sequence of events caused by the activation of Src in ts-Src MDCK cells growing in 3D environment with the aid of fluorescent markers Hoechst and CellMask Orange.
The cells were cultivated in a cell culture incubator in Matrigel at 40.5 °C on a 35 mm glass-bottom dish. When the experiment was to be initiated, both Hoechst 33342 and CellMask Orange membrane dye were diluted to HBSS to the concentration of 5 μg/ml, and cells were incubated in the presence of the dyes at 40.5 °C for 30 min. Subsequently, the cells were transferred to the illumination chamber of the Zeiss Cell Observer spinning disc confocal microscope set at 35 °C. From each sample, 5–15 cysts were selected for time-lapse imaging and visualized at 30 min intervals up to 6 h. A Z-stack of each cyst at each time point was collected.
Excitation wavelengths of 405 and 561 nm, EC Plan NeoFluar 40 × /0.75 DIC air objective, and BP 450/50 nm (blue) and BP 629/62 nm (red) emission filters were used. The experiments were repeated at least two times.
EGTA Treatment
The role of intact cell–cell junctions in protecting the untransformed MDCK cells from apoptosis was investigated with the aid of a calcium-chelator EGTA which brings about the opening of calcium-dependent trans-interactions of E-cadherins in adjacent cells. The MDCK cells were cultivated in 2D, 2½D, and 3D environment on the glass-bottom 35 mm dish as described above, and cultivated at 37 °C in a cell culture incubator for given time periods. Before the experiments, the Hoechst 33342 and CellMask Orange dyes were added to the cells. After 30 min incubation with the dyes, the medium was aspirated and the cells were washed twice with calcium-free PBS and subsequently 1 mM EGTA was added. The cells were visualized with a spinning disc microscope as described above.
PL and NAC Treatment
PL was diluted to the concentration of 15 mM in DMSO. To evaluate its effect on MDCK and ts-Src MDCK cells, the cells were grown in Matrigel for 4 days at 37 °C and 35 °C, respectively, and 15 or 50 μM of PL was added to the cells 24 h before visualization. The effect of NAC was tested by cultivating the cells in the presence of 1 μM NAC, added to the cells daily, and the cell morphology and nuclear staining was visualized with or without 15 μM of PL. The cells were illuminated using CellM fluorescence microscope equipped with LUMPPlanFI 40 × /0.80 water immersion objective. Oblique illumination was used for transmitted light and xenon lamp for fluorescence imaging. Excitation filter used for Hoechst was 360/40 nm and 555/28 nm for propidium iodide dye and the images were collected with a Hamamatsu Orca ER CCD camera.
Visualization of Cells Grown in Suspension
MDCK and ts-Src MDCK cells were forced to grow in suspension for 48 h by using bacterial (non-cell-binding) petri dishes and β1 integrin blocking antibody, at temperatures of 37 °C and 35 °C, respectively. Hoechst, propidium iodide and FITC-annexin V were added 15 min before visualization according to the manufacturer’s instructions. The cells were illuminated using CellM fluorescence microscope as described above. Excitation filters used were 360/40 nm for Hoechst, 490/20 nm for FITC, and 555/28 for propidium iodide.
Quantification of Apoptosis
To determine the amount of apoptotic cells in suspension cultures and under treatment with PL, the CellM fluorescence microscope pictures were analyzed using ImageJ software with the Cell Calculator plug-in. Hoechst staining was used to calculate the total number of cells in each image, FITC-annexin V and propidium iodide to determine the number of early and late apoptotic cells, respectively. The percentage of early and late apoptotic cells in each experiment was calculated, mean values, and standard errors were plotted onto a graph using Excel (Microsoft, Redmond, WA, USA).
Statistical Tests
Two-tailed Student’s paired t-test with equal variance was used to assess the significance of the occurrences of apoptosis in different circumstances. P-values were categorized as not significant (P>0.05), significant (**P<0.05) and very significant (***P<0.01).
Preparation of Fixed Samples for Confocal Microscopy
The cells were grown in various environments as described above and fixed and stained as follows: Cells grown in 2D or 2½D were washed twice with room temperature PBS. Fixing was done using 4% paraformaldehyde (PFA) for 15 min and unspecific protein binding sites were blocked with 50 mM NH4Cl solution for 30 min. The cells were then washed four times with PBS at room temperature and permeabilized with 0.1% saponin in PBS for 30 min. After washing the cells with PBS/glycine, a short post-fixation was performed with ethanol at −20 °C as described by Palovuori et al.16 Subsequently, 30 μl of primary antibody solution was added to the cells and incubated at room temperature for 60 min. Cells were washed four times with PBS/glycine for 5 min. For secondary antibody staining, the washing solution was removed and 30 μl of secondary antibody solution (including DAPI and phalloidin) was added and the cells were incubated at room temperature for 30 min. This and all subsequent steps were performed while samples were protected from light. Finally, cells were washed four times with PBS/glycine for five minutes, and twice with distilled water, and mounted onto a microscopy slide using Shandon Immu-Mount (Thermo Scientific, Waltham, MA, USA). The samples were allowed to dry for at least 24 h before microscopy.
For cells grown in 3D, the following fixation method was used: the cysts within the Matrigel or Matrigel-collagen mixture were washed three times with PBS, fixed with 500 μl of 1% PFA for 15 min at room temperature, and carefully washed three times for 10 min with PBS. 500 μl of 0.2% Triton-X in PBS was added and the cysts were incubated at room temperature for 15 min. The Triton-X solution was then replaced with 150 μl of primary antibody solution, and the cysts were incubated for 4 h at room temperature, and washed with PBS three times for 10 min. This procedure was repeated with the secondary antibodies (including DAPI and phalloidin), except the cysts were incubated in the dark for 1 h at room temperature. The cysts were then washed three times for 10 min with PBS and twice with distilled water. After careful aspiration of the water, the silicon ring was removed and excess water was aspirated. Mounting was done as with 2D samples.
The Z-stack images of cells and cysts were captured with an Olympus FluoView 1000 laser scanning confocal microscope using UPLSAPO 60 × /1.35 oil-immersion objective. Excitation wavelengths of 405, 488, and 543 nm and appropriate emission filters were used for DAPI and green and red dyes, respectively.
For cadherin localization studies upon initiation of transformation, ts-Src MDCK cells were grown in Matrigel at 40.5 °C for 7 days. The cells were then transferred to 35 °C for up to 4 h, and fixed with 4% PFA after indicated time periods. Fixed cells were stained with DAPI and E-cadherin specific antibody rr1 as described in the protocol for the cells grown in 3D. The Z-stacks of cysts were visualized with Zeiss LSM780 laser scanning confocal microscope using iPlan-Apochromat 63 × /1.4 DIC oil objective. Spectral detection emission wavelengths of 410–495 and 490–578 were used for DAPI and rr1, respectively.
For the analysis of the reversibility of the transformation, ts-Src MDCK cells were first cultivated in Matrigel at 35 °C for 4 days, and were subsequently transferred to 40 °C for the indicated time periods. Cells were fixed and treated with antibodies as described above.
The experiments describing effects of PL and NAC on cadherin localization in MDCK cells were done as described above.
Immunoblotting analysis of Survivin Expression
To prepare the cell lysate from 2D specimens, the cells were first washed with ice cold PBS and then scraped from dishes, dissolved on ice in cell lysis buffer (50 mM Tris-HCl, pH 7.4, 1% NP-40, 0.25% Na-deoxycholate, 150 mM NaCl, 1 mM EDTA, 1 mM PMSF, 1 mM Na3VO4, 1 mM NaF, 1 μg/ml aprotin, 1 μg/ml leupeptin, and 1 μg/ml pepstatin), sonicated and incubated on ice for 30 min, after which the suspension was centrifuged for 2 min at 10 000 g and 4 °C. The supernatant was then collected for protein determination and gel electrophoresis.
To prepare the cell lysate from Matrigel-collagen cultures, the specimens were first treated with 0.01% collagenase A for 30 min at 37 °C and subsequently with Cell Recovery Solution for 1 h on ice, gently rocking the solution every 10 min. After the Matrigel-collagen matrix had dissolved, the cells were collected by centrifuging at 300 g for 5 min at 4 °C. The supernatant was removed, and the cells were resuspended into ice cold HBSS and centrifuged as above. This wash step was repeated, and the cell pellet was dissolved in the cell lysis buffer as described above.
To obtain the total cell lysates from 3D cultures grown only in Matrigel, the collagenase treatment was omitted, but otherwise the procedure for dissolving Matrigel was identical.
Suspension cultures were harvested by centrifugation (3 min, 200 g), washed with PBS and then resuspended into the lysis buffer as described above.
The protein concentrations of the 1D, 2D, 2½D, and 3D samples were determined using the Bradford Protein Assay kit from Bio-Rad (Hercules, CA, USA) and Tecan Infinite M1000 microplate reader (Tecan, Männedorf, Switzerland) as per the manufacturers’ instructions. For each blot, equal amounts of protein lysate (in μg) in all wells were used. The proteins were resolved on 15% SDS-PAGE and transferred onto nitrocellulose membrane. The blots were incubated with the primary antibodies for 2 h followed by a one hour incubation with horseradish peroxidase-conjugated secondary antibody. The blots were developed for 2 min in light with a detection liquid (10 ml 0.1 M Tris-HCl pH 8.5, 35 μl 250 mM luminol in DMSO, and 15 μl 90 mM p-coumaric acid in DMSO and 3 μl 30% H2O2) and the emitted light collected using FujiFilm LASS 3000 gel imaging device (Fujifilm, Minato, Tokyo, Japan) and blot pictures were acquired using LASS 3000 Image Reader software.
For the visualization of tubulin, blots were stripped with 0.5 M NaOH for 15 min, after which the blocking, antibody treatments and the visualization of the blot were performed as described above. For a more accurate comparison of survivin expression, the band mean intensities were measured with ImageJ software. The ratio of survivin band intensity to tubulin band intensity was calculated, and the highest ratio was set as the maximum intensity (100% max). Other intensity ratios were then compared to this maximum intensity to get the corresponding percentages of maximum intensity.
Post-Modification of Images
For better readability of pictures and the blot from EGTA experiments, some image post-modifications were performed.
Resolution of spinning disc confocal images of EGTA-treated cells suffered from the debris released by apoptotic cells into the Matrigel. Therefore, to better determine the cell and cyst borders and reduce out-of-focus noise, Huygens deconvolution software by Scientific Volume Imaging (Hilversum, The Netherlands) was used with number of maximum iterations set to 40, signal to noise ratio to 20 and quality threshold to 0.1 together with optimized iteration mode and automated brick layout.
In the EGTA-treated cells grown in Matrigel, no increase in survivin expression was observed with immunoblotting. To confirm the result, the blot image was enhanced using Histogram Equalizer, an image contrast adjustment method, where the most frequent intensity values are spread out through the whole histogram, allowing areas of lower local contrast to achieve a higher contrast.
Results
We aimed to analyse the correlation between the phenotype and survivin expression in MDCK cells in various culture conditions and upon activation of Src tyrosine kinase.
Downregulation of Survivin in Well-Differentiated MDCK Cell Cysts in 3D Environment
We analyzed the integrity of epithelial cell layers and cysts with the aid of confocal microscopy, and visualized actin filaments and E-cadherin in MDCK cells grown on two-dimensional hard surface (2D), two-dimensional soft surface (2½D) and within three-dimensional Matrigel and a Matrigel-collagen mixture. Survivin expression was analyzed in the same circumstances using western blotting with a survivin-specific antibody. In 2D environment, the cell monolayer was dense and AJ component E-cadherin and actin localized at the cell–cell contact sites, whereas in 2½D environment E-cadherin was delineating the lateral membranes of the cell layer, but actin was surrounding apical structures which resembled initial sites of lumina (Figure 1a). Survivin expression was high in cells grown on 2D and modest on 2½D surfaces (Figure 1c). In 3D environment, in both Matrigel and Matrigel-collagen mixture, the cells were organized in a spherical cyst with a lumen inside, E-cadherin at lateral and actin at apical membranes (Figure 1a). Still, there were hardly any signs of apoptosis, even if survivin expression varied from modest in Matrigel to nil in the Matrigel-collagen mixture (Figure 1c). Out of curiosity, we stained the MDCK cells grown in 2½D environment with survivin antibody and made an attempt to observe its localization (Figure 1d). In untransformed MDCK cells survivin was visible in chromosomal structures and midbodies of dividing cells, but it was not possible to demonstrate its mitochondrial, nuclear or cytoplasmic localization, presumably due to its low amount in non-proliferating cells.10
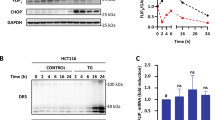
MDCK and ts-Src MDCK cell phenotypes and the expression of survivin in various culture environments. The confocal microscopy images show the localization of cell junction protein E-cadherin and cytoskeleton component actin in MDCK cells, grown on coverslips (2D), coverslips coated with Matrigel (2½D), within Matrigel or mixture of Matrigel and collagen (a). E-cadherin colocalized with actin on the cell membranes in 2D, whilst in both 3D environments, actin lined the apical cell membrane and E-cadherin localized to lateral membranes. In 2½D environment, E-cadherin was delineating the lateral walls, whereas actin was surrounding the initial sites of lumina. Ts-Src MDCK cells were cultivated either at permissive (35 °C) or non-permissive (40.5 °C) temperature (b). In 2D environment, at permissive temperature the cells had mesenchymal phenotype with E-cadherin localizing to the cytoplasm, whereas at non-permissive temperature E-cadherin localized to the cell membranes. Ts-Src-MDCK cells grown in Matrigel at permissive temperature formed cysts without clear apical or basal surfaces, or lumen. E-cadherin was localized both to the cell membranes and cytoplasm. After transferring the cysts, grown in 35 °C, to 40.5 °C, the Src-induced transformation was reversed, and after one day of cultivation in 40.5 °C, the cells had started to form both clear apical surfaces and lumen. After two days in the non-permissive temperature the lumen had expanded considerably, and E-cadherin had started to localize to the lateral membranes. After six days in non-permissive temperature, a cyst consisting of a single cell layer, with clear apical and basal membranes, was formed (b). Expression of survivin was determined using western blotting (c). Higher levels of survivin were detected in both non-transformed MDCK cells and ts-Src-transformed MDCK cells grown in 2D. The survivin expression of MDCK cells decreased to less than 10% of the maximal value (2D environment) when the cells were grown in 2½D or 3D environment. Ts-Src MDCK cells expressed survivin also in 3D environment at the permissive temperature. Temperature shift from 35 °C to 40.5 °C reduced the survivin expression in ts-Src MDCK cells in a time-dependent manner (c). Localization of survivin in MDCK cells grown in 2½D environment was determined using immunofluorescence with the aid of confocal microscope (d). Survivin was visible in the chromosomal structures of the cells undergoing cell division and occasionally in the nucleus (arrows), and occasionally in the midbodies (arrowhead). The scale bar is 20 μm.
Upregulation of Survivin in ts-Src-Transformed MDCK Cells at 35 °C
Ts-Src MDCK cells grown at 40 °C both in 2D and 3D environments showed a similar phenotype to non-transformed MDCK cells, E-cadherin was at lateral membranes and actin delineating apical surfaces, although the cells in 3D environment were thinner than untransformed cells, and, identically, in 3D environment survivin was downregulated (Figure 1b and c). The phenotype was changed, when the cells were grown at 35 °C: on 2D surfaces the cells had elongated mesenchymal morphology and in 3D environment formed an irregular cell cluster without any polarity, typical to transformed cells (Figure 1b). In all circumstances, however, E-cadherin expression was not downregulated, but at permissive temperature it was partly in cytoplasm, partly along cell membranes (Figure 1b). Survivin expression was strong in 2D and moderate in 3D environment at the permissive 35 °C temperature (Figure 1c). The transformation process was reversible and the cells acquired differentiated morphology after returning to 40.5 °C. Figure 1b shows the time course of lumen formation after inactivating Src by temperature shift to 40.5 °C: apico-basal polarity is developed and lumen formed within 24 h at non-permissive temperature which coincides with downregulation of survivin expression (Figure 1b and c).
After shifting the ts-Src MDCK cells from 40.5 to 35 °C, the Src activation has been shown to occur within 1 h.24 In order to monitor the early events in transformation process, we cultivated the cells several days at 40.5 °C in the cell culture incubator and brought them to the spinning disc confocal microscope chamber, set at 35 °C, and visualized their morphology with the aid of fluorescent markers. We could show that the transformation process began by the opening of cell–cell junctions to water within one hour at 35 °C, followed by shrinkage of the lumen, presumably due to leakage of water through opened tight junctions (Figure 2a). Hence, the cells did not migrate towards the lumen, but the lumen collapsed as the intraluminal hydrostatic pressure was relieved. E-cadherin localized at the cell membranes even after 4 h at 35 °C, although the staining was no longer restricted to the lateral walls (Figure 2b).
Live cell imaging of ts-Src MCDK cysts with a spinning disc confocal microscope after shift to permissive 35 °C temperature inducing activation of Src (a). The cysts were stained with CellMask Orange membrane dye and Hoechst 33342 nuclear dye. A stack of images was collected every 30 min. The lumina within the cysts began to shrink after one hour (arrow) and the cyst size was decreasing. Localization of E-cadherin in ts-Src MDCK cysts fixed at various time points after Src activation, visualized using E-cadherin antibody and DAPI nuclear dye (b). In cells grown at 40.5 °C (zero time point), E-cadherin localized at lateral and basal membranes, but after Src activation it is distributed also to all membranes as the epithelial polarity is lost (b). The expression levels of survivin were measured with western blotting (c). Already after two hours survivin levels were increased. The scale bar is 20 μm.
Survivin expression was clearly increased within two hours, much before any sign of cell proliferation could be observed, then it temporarily declined and was strongest after 24 h at permissive temperature (Figure 2c). Hence, survivin expression correlated with the disintegration of junctions and loss of polarity, a phenomenon linked to the phosphorylation of junctional proteins, especially catenins by Src.16
Downregulation of Survivin in Suspension of MDCK, but not ts-Src MDCK Cells
In order to elucidate the role of ECM in the expression of survivin, the MDCK cells and ts-Src MDCK cells were forced to grow in suspension (1D) by adding β1 integrin blocking antibody into the culture medium. Apoptotic cells were visualized using FITC-annexin V, and dead cells with propidium iodide as a marker. MDCK cells entered to apoptosis especially when β1 integrin blocking antibody was present (Figure 3a and b). In these circumstances survivin expression was low (Figure 3e). In contrast, the majority of the ts-Src MDCK cells were proliferating and formed large cell clusters at 35 °C, survivin being expressed with and without blocking antibody (Figure 3c–e).
Cell morphology, apoptosis, and expression of survivin of cells grown in suspension. MDCK (a and b) and ts-Src MDCK (c and d) cells were forced to grow in suspension by supplementing β1 integrin blocking antibody to the culture medium. Early phases of apoptosis was visualized with FITC-annexin V and the late stages of apoptosis using propidium iodide. Hoechst 33342 shows nuclear condensation due to apoptosis. Apoptosis was evident in MDCK cells, especially in the presence of the blocking antibody (b) and survivin expression was low (e). Ts-Src MDCK cells grew in clusters and continued proliferation, and only few cells showed apoptotic markers (c and d). Survivin was expressed in ts-Src MDCK cells both with and without β1 integrin blocking antibody (e), but its levels were dramatically higher than in non-transformed MDCK cells.
Quantitative analysis of apoptotic cells in various circumstances is shown in Figure 4a. More than half of the MDCK cells grown in suspension in the presence of β1 integrin antibody for 48 h showed positive FITC-annexin staining which is a sign of activation of the apoptotic cascade, whereas the corresponding proportion of ts-Src-transformed cells was only 30%. The proportion of dead cells indicated by positive propidium iodide staining was 25% in MDCK cells and 15% in ts-Src-transformed cells in the presence of blocking antibody and 13 and 10% without blocking antibody (Figure 4a).
Quantitative analysis of apoptotic cells grown in suspension (a) or in the presence of piperlongumine (b). To determine the amount of apoptotic cells in suspension cultures and under treatment with PL, the CellM fluorescence microscope pictures were analyzed using ImageJ software with the Cell Calculator plug-in. For analysis of suspension cultures, Hoechst staining was used to calculate the total number of cells in each image, FITC-annexin and propidium iodide to determine the number of early and late apoptotic cells, respectively (a). The percentage of early and late apoptotic cells in each experiment was calculated, mean values and standard errors were plotted onto a graph using Excel. For statistical analysis, values of ts-Src MDCK cells were compared to those of MDCK cell. For analysis of the effects of PL, FITC-annexin V was omitted, and only Hoechst and propidium iodide stainings were quantitated (b). Values were compared to corresponding values in control cells.
Hence, in 1D environment, survivin expression was promoting proliferation of transformed cells, and the lack of integrin signaling induced anoikis in non-transformed cells.
Lack of Survivin Expression in EGTA-Treated MDCK Cells
In order to analyze the behavior of untransformed MDCK cells under stressing conditions and the correlation between intact cell–cell junctions, survivin expression and apoptosis, we tested the behavior of untransformed MDCK cells in 2D, 2½D, and 3D environment in the presence of a calcium-chelator EGTA by monitoring the cells under the spinning disc microscope (Figure 5).
Live cell imaging of MDCK cells grown in various environments after treated with calcium-chelator EGTA. The cells were stained with CellMask Orange membrane dye and Hoechst 33342 nuclear dye, and a stack of images was collected every 30 min. MDCK cells grown in 2D environment detached from the surface of the plate, but retained their adherens junctions and thus remained attached to each other (a). Cells grown on top of a layer of Matrigel (2½D), began to lose cell–cell contacts and started to go into apoptosis (b). Cysts grown in Matrigel began to collapse within 3 h and apoptotic bodies appeared within 4 h after addition of EGTA (c). Expression of survivin in cell cysts after introduction of EGTA was visualized with western blotting (d). The expression of survivin in MDCK cells grown in 2D increased, but decreased with time in the the presence of EGTA in cells grown in 2½D. MDCK cells grown in 3D had extremely weak expression of survivin. To confirm the negative results in cells grown in 3D, cell lysates of ts-Src MDCK cells grown in 2D at 35 °C were used as a positive control (diluted 1:1 and undiluted). Histogram Equalizer algorithm, which allows the low intensity signals to become visible, was used to demonstrate the extremely low survivin expression in MDCK cells grown in Matrigel in the presence of EGTA.
In 2D environment the MDCK cell monolayer remained practically unchanged for 2 h in the presence of EGTA whereafter the whole monolayer was released from the substratum, the cells still remaining connected to each other and the whole cell layers began to deteriorate within 4 h without any signs of apoptosis (Figure 5a). The response to EGTA was completely different in 2½D environment: the cell–cell contacts began to open in 2 h and apoptotic bodies appeared immediately after the cells were released from each other and substratum (Figure 5b). Hence, the classical anoikis process was occurring. In 3D Matrigel environment within 3 h after EGTA supplementation, apoptotic bodies began to appear and the majority of the cells entered apoptosis within 5 h (Figure 5c). On the basis of microscopic images we cannot make conclusions on the integrity of the cell–cell or cell–ECM contacts, but obviously the cells seem to be stressed due to EGTA treatment.
We also tested survivin expression in non-transformed MDCK cells treated with EGTA, and observed it to be in line with the occurrence of apoptosis: survivin expression even increased in 2D environment, markedly decreased in 2½D environment, and remained consistently low in 3D environment (Figure 5d).
In summary, cell stress caused by EGTA treatment induced intrinsic apoptotic cascade of untransformed MDCK cells in 2½D and 3D environment where the cells initially showed differentiated phenotype and low survivin expression, but not in 2D where survivin expression is high and the cells still proliferating at the edges of the monolayer. Hence, there is a correlation between downregulation of survivin and cells’ capacity to enter apoptotic cycle, although we cannot make conclusions on the signaling mechanisms initiating apoptosis.
Downregulation of Survivin by PL in ts-Src MDCK Cells
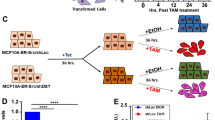
We tested the effect of ROS-producing agent PL on MDCK cells grown at 37 °C and ts-Src MDCK cells grown in Matrigel at 35 °C. In the presence of 15 μM PL, both cell lines showed a distorted phenotype, and ts-Src MDCK cell clusters especially began to disintegrate and apoptotic fragments were seen (Figure 6a and b). Higher concentrations of PL caused both MDCK and ts-Src MDCK cells to bulge and undergo necrosis (Figure 6c). NAC is a reducing agent and antioxidant which is expected to protect the cells from reactive oxidative agents. We tested its effects with or without PL. NAC alone had no visible effect on either of the cell lines, but it rescued ts-Src MDCK cells from PL-induced cell death (Figure 6d and e).
The effects of PL on the morphology of MDCK and ts-Src MDCK cells, grown at 37 °C and 35 °C, respectively. Live cell imaging of MDCK and ts-Src MDCK cells grown in Matrigel in the presence of 15 μM (b) or 50 μM (c) PL, with NAC (d) or with both NAC and 15 μM PL (e). The morphology and Hoechst 33342 nuclear dye of cells in various circumstances (a–e) were visualized with a fluorescence microscopy using water immersion objective. Low concentration of PL had no effect on MDCK cells, but in higher concentrations PL caused the cells to undergo necrosis (a–c). Ts-Src MDCK cells showed nuclear fragmentation already in the presence of low concentrations of PL and high concentrations of PL-induced necrosis (a–c). The addition of NAC rescued the ts-Src MDCK cells from apoptosis and the cell cysts appeared more compact (e).
Quantitative analysis of apoptotic cells in various circumstances is shown in Figure 4b. In 3D Matrigel FITC-annexin fluorescence within the cysts could not be separated from the strong green autofluorescence and, thus, only propidium iodide staining was quantitated. PL-induced slight increase in the number dead MDCK cells, but the effect on ts-Src MDCK cells was much stronger and 25% of cells were dead due to PL treatment (Figure 4b). The number of dead cells in MDCK or ts-Src MDCK cell specimens treated with NAC or NAC and PL was much lower and did not differ significantly from the control cells.
We also monitored the E-cadherin localization in PL- and NAC-treated ts-Src MDCK cells with the aid of confocal microscopy (Figure 7). Normally, ts-Src MDCK cells at 35 °C cultivated in 3D matrix grew in clusters without a clear apico-basal axis or lumen, and E-cadherin located in the cytoplasm and along the cell membranes (Figures 1 and 2). PL changed the cell morphology, and the cells appeared swollen and non-polarized, and E-cadherin staining was delineating the membranes in thick bands (Figure 7b). NAC, however, returned the epithelial phenotype and narrowed E-cadherin staining along lateral membranes, even in the presence of PL (Figure 7a and c).
Confocal microscopy images showing the localization of E-cadherin and nuclei in ts-Src MDCK cells grown in Matrigel at 35 °C after exposure to NAC and PL. In the presence of NAC, the cells had irregular shape, but E-cadherin was localized at the cell membranes (a), PL induced a rounded phenotype, and E-cadherin was in thick clusters along the cell walls (b). When PL was supplemented with NAC, an epithelial appearance was restored and E-cadherin staining was narrower at lateral membranes (c). The expression level of survivin in ts-Src MDCK cells was visualized with Western blotting (d). PL reduced the expression of survivin, and it remained low even after combined treatment with both NAC and PL.
PL diminished survivin expression in ts-Src MDCK cells (Figure 7d). NAC protected the ts-Src MDCK cells from PL-induced cell death even if the expression of survivin remained downregulated in the presence of both PL and NAC (Figure 7d). Hence, the situation resembled that of fully polarized MDCK cysts.
The effects of environmental factors on the cell phenotype, survivin expression, and apoptosis are summarized in Table 1.
Discussion
The balance between apoptosis and proliferation is crucial for epithelial renewal and homeostasis. In adult tissues, apoptosis is induced only when the tissue is growing too crowded or the cells are damaged, whereas proliferation is induced only to replace damaged cells eliminated by apoptosis. It has been shown by several groups that in cell culture models, apoptosis occurs in 3D cultures of MDCK or mammary epithelial cells in order to clear away the cells trapped within the lumen.25, 26 In our previous work, we analyzed gene expression patterns in Src-transformed and untransformed MDCK cell lines in two-dimensional and three-dimensional cell culture environments by means of microarray technology.17 The most significant alterations in gene expression were observed between the untransformed MDCK cells grown either in a 2D or 3D environment, the latter downregulating, eg, expression of survivin. In the present work we explored the presence of survivin and appearance of apoptotic cells more carefully in various external conditions and under influence of the ROS-producing agent PL and antioxidant NAC.
Differentiated Phenotype, Low Survivin Content, no Apoptosis
In our experimental set-up, in 2D environment, the cells were continuously proliferating at the edges of monolayers and consequently, survivin was expressed. In 2½D environment, the cells began to differentiate and formed tiny luminal structures, followed by downregulation of survivin. Still, only in 3D Matrigel or Matrigel-collagen environment, the untransformed MDCK cells displayed a fully polarized phenotype, survivin expression was downregulated and there were no apoptotic cells after the lumen had been cleared. Hence, the MDCK cells in 3D ECM environment were capable of surviving without survivin, the situation corresponding to normal adult tissues.
To remain functional, the tissues must respond to external stimuli and be capable of renewal which requires a highly controlled balance between cell proliferation and apoptosis. In cell culture systems this is evident when the culture is grown too dense which leads to contact inhibition of cell proliferation.27, 28 Kim and co-workers analyzed proliferation of MCF-7 or MCF-10 cells in sparse and dense cultures and showed that homophilic E-cadherin interaction was needed for contact inhibition of proliferation. Moreover, they proved that E-cadherin caused redistribution of YAP from nucleus to cytoplasm in dense cell cultures indicating that Hippo pathway can be activated by E-cadherin, resulting in inactivation of transcriptional effector YAP of the Hippo pathway. A different approach was used by Benham-Pyle and co-workers:28 they plated quiescent MDCK cells on elastic surface and showed that mechanical strain induced rapid cell cycle re-entry which was mediated by nuclear accumulation and transcriptional activity of YAP and beta-catenin, inducing expression of proteins supporting proliferation, eg, cyclin E, Myc, and survivin.29, 30 Maintenance of quiescence, YAP nuclear exclusion and inhibition of beta-catenin transcriptional responses to mechanical strain, in turn, required E-cadherin extracellular engagement.28 Hence, Hippo pathway seems to be important for the maintenance of differentiated non-proliferating phenotype of epithelial tissues together with cadherin–catenin complexes.27, 31, 32
Apoptotic Phenotype, Low Survivin Content, Visible Signs of Apoptosis
When MDCK cells are cultured on 2D cell culture dishes, they do not usually enter apoptosis, unless the cells were kept in a culture dish long enough after reaching confluence which induces first contact inhibition of cell proliferation, followed by confluence-induced apoptosis. After reaching confluence in 2D culture, MDCK cells first start forming domes, fluid-filled multi-cellular structures, but ultimately they enter to the apoptotic cycle. Chang and co-workers33 have shown that confluent-induced cell death after dome formation of MDCK cells is mediated by activation of caspase-8 and inhibited by over-expression of Bcl-2, suggesting mitochondria-dependent pathway for confluence-induced apoptosis. In 3D culture, epithelial cells do not show confluence-induced cell death, but stop proliferation and the differentiated cysts can remain in culture for 10 days.34 In these circumstances, intrinsic apoptosis requires stressing stimuli that are sensed intracellularly.
It has been shown in several works that E-cadherin-mediated cell interactions have a significant role in epithelial cells’ resistance to anoikis, and knockdown of E-cadherin reduces anoikis resistance.35, 36, 37, 38 We tested the MDCK response of the cells to opening of cell–cell junctions with the aid of a calcium-chelator EGTA. Cell stress caused by EGTA treatment induced intrinsic apoptotic cascade in 2½D and 3D environment where the cells initially showed differentiated phenotype and low survivin expression, but not in 2D where survivin expression was high and the cells still proliferating at the edges of the monolayer. Hence, there is a correlation between downregulation of survivin and cells’ capacity to enter apoptotic cycle in response to stress. Different behavior of cells in variable environments is observed, eg, in vivo in intestinal epithelium along the crypt-villus axis. The crypt is occupied by proliferative immature cells, whereas the differentiated cells are located at the villus. These two populations have different mechanisms of apoptosis and this feature has led to the concept of a distinct modulation of cell survival and apoptosis according to the state of differentiation.39
The importance of intact cell–cell contacts to epithelial cell survival was demonstrated by our observation of survivin downregulation and apoptosis of untransformed MDCK cells in 3D environment in the presence of EGTA, even if the cells were in contact with proper ECM environment. Guo et al40 have made an extensive analysis of E-cadherin interacting proteins, the s.c. cadherin adhesome at AJs. They observed that calcium depletion brought about dissociation of cadherin trans-dimers between adjacent cells and promoted the formation of cis-interactions on the membranes within each individual cell. They also showed that many intracellular proteins interacted with E-cadherin in EGTA-treated cells independent of trans-ligation of cadherins, including β-catenin, α-catenin, γ-catenin, and IQGAP. The presence of cis-interactions and the existence of an intracellular cadherin complex was not sufficient to protect the cells from apoptosis in our experimental model and, hence, trans-dimers of E-cadherin seem to be a prerequisite for viable epithelium, although cis-interactions seemed to be sufficient to downregulate survivin expression. Whether this downregulation is mediated by the Hippo pathway, TCF/LEF β-catenin pathway or other transcription factors is out of the scope of the present work.
The second condition in which survivin expression was low and the cells entered apoptosis (in this case anoikis), was in suspension culture. Normally epithelial cells are protected from anoikis when they are adherent on permissive ECM proteins.41, 42 The exact basis for the sensitivity to anoikis of MDCK cells is still not fully elucidated and may be due to multiple factors.38 Integrins seem to have a central role in suppressing apoptosis in attached cells by transmitting anti-apoptotic and pro-survival signals from ECM, although their role is not quite clear. Blocking β1 integrin in collagen matrix results in inverted polarity.43, 44 Moreover, Myllymäki et al45 have shown that α2β1 and α6β4 integrins were required for apical lumen formation in collagen gels, whereas α3β1 integrin function was critical in basal membrane-extract gels, and knocking down the expression of these integrins resulted in multilumina or inverted polarity. Hence, blocking integrin function in MDCK cells grown within ECM gel did not trigger anoikis. In their classical experiments, Wang et al46, 47 showed that MDCK cells grown in a dish in a rotating shaker also exhibited inverted polarity without anoikis. It is known that MDCK cells are capable of secreting matrix proteins and it is quite possible that a contact to any surface activates their synthesis and secretion which will rescue the cells from anoikis. This may explain the inverted polarity of rotating cells described by Wang et al:46 the cells secrete ECM components to the contact surface with the adjacent cell which gives a cue to the formation of the basal surface within the cell cluster leaving ‘vacant’ surface facing the medium as the apical surface. This was evident also in our experimental set-up, since the MDCK cells began to form a monolayer if they were allowed to remain on the bacterial dishes for several days without additional supplement of β1 integrin antibody.
In addition to integrins, the cell–cell interactions are important for cell survival in suspension culture as shown by Hofmann et al and Ma et al.48, 49 In these works cellular adhesion of human embryonic kidney cells or isolated colonic epithelial cell crypts was inhibited by EGTA, integrin peptides or blocking antibodies or HAV-containing cadherin peptides and the cells survived in suspension as long as cell–cell contacts were preserved. Another approach was used by Bergin et al50 who cultivated mouse proximal tubular cells on agarose surface in high density allowing the cells to adhere to each other but being without cell–matrix contacts. In these conditions the cells remained viable unless treated with EGTA or HAV-containing peptide, supporting the conclusion that trans-interactions of cadherins are protecting cells from apoptosis.
All in all, the MDCK cells seem to need both intact cell–cell and cell–matrix contacts in order to differentiate and function as a tight epithelial layer.
Poorly Differentiated or Proliferative Phenotype of Untransformed MDCK Cells, Modest or High Survivin Content, no Apoptosis
There are many studies on the effect of the quality of the cultivation surface on the cell differentiation, and the general view is that rigid surfaces support the mesenchymal phenotype.51, 52, 53 We monitored the levels of survivin expression in MDCK cells grown on hard 2D or soft 2½D surface, and noticed that even if the cells showed differentiated phenotype they expressed survivin. Marlar and co-workers53 have studied the dependence of the EMT process on the surface properties more carefully by regulating the density of an ECM ligand, cyclic arginine-glycine-aspartate (cRGD) on gold-coated glass coverslips. They observed that the MDCK cells grown on the concentrated cRGD surface formed epithelial clusters with intact cell–cell junctions, whereas on the diluted cRGB surface the cells exhibited an elongated fibroblast-like morphology and expressed mesenchymal markers.
There are numerous regulators that participate in the formation of homotypic interactions between E-cadherin molecules of opposing cells. One important player is c-Jun N-terminal kinase (JNK), which is involved in formation of AJs, TJs and gap junctions in epithelial cells.51 JNK phosphorylates β-catenin leading to AJ disassembly, whereas inhibiting JNK induces AJ formation and trans-interactions of E-cadherin molecules. In cancer tissues, a strongly negative correlation between JNK activity and E-cadherin expression has been demonstrated. JNK seems to be a major regulator of the substrate rigidity-mediated balance between cell–cell and cell-substrate adhesion. JNK has been shown to be phosphorylated and upregulated on stiff and dephosphorylated on soft substrates, resulting in the formation of strong FAs and dissolution of AJs on stiff substrates, whereas on soft substrates JNK was downregulated resulting in strong AJs.51 The exact mechanisms of the regulation is still not quite elucidated, but integrin density and integrin clustering might be the players in this signaling cascade.
It seems that the MDCK cells growing on cell culture dishes have a ‘semi-differentiated’ phenotype: E-cadherin is on lateral membranes, the AJs and TJs are formed, but the cells express survivin, which explains why the MDCK cells are incapable to enter apoptosis in 2D cell culture conditions. Thus, survivin expression may serve as an indicator of the level of differentiation. For this reason, it would be important to perform eg, studies on therapeutic agents against survivin expression in 3D environment.
Poorly Differentiated or Proliferative Phenotype of ts-Src-Transformed Cells, Modest or High Survivin Content, no Apoptosis
An important event in malignant transformation is disintegration of epithelial cell–cell junctions and removal of proteins from the junctional site. Several junctional proteins are Src-substrates, including E-cadherin, and β- and p120catenins. Activation of Src kinase by the temperature shift brings about their phosphorylation, relocalization to the cytoplasm and initiation of transformation process.15, 16, 17 In our hands, survivin expression was initiated as soon as Src was activated and cell–cell junctions disintegrated. This phenomenon took place both in 2D and in 3D environment. There is a close link between expression and localization of E-cadherin, β-catenin and p120catenin, and Wnt signaling through the TCF/LEF pathway. Cadherins affect β-catenin signaling by binding to it with a high affinity that competes for its interactions with other partners, such as TCF, axin and APC, and thereby prevents its nuclear import.13 On the other hand, modifications that reduce the affinity of β-catenin for the cadherin and α-catenin may lead to enhanced nuclear signaling activity of β-catenin, eg, by tyrosine phosphorylation of β-catenin at residue 654, which leads to reduced E-cadherin binding both in vitro and in vivo and enhanced nuclear signaling activity.13 Another signaling pathway linking Src activation to increased survivin expression in cancer cell lines and human cancer tissues is STAT3. Elevated activity of STAT3 and Src, as well as survivin expression, has been found in malignant progression of breast cancers.54 Moreover, inhibition of STAT3 and downstream survivin expression has been shown to induce apoptosis in human breast cancer cell lines.54
We observed E-cadherin expression in all culture conditions where Src was activated, but its localization varied from lateral membranes to cytoplasm. Hence, upregulation of survivin and escape from apoptosis were not dependent on the downregulation of E-cadherin, but rather were due to disintegration of cell–cell junctions and subsequent activation of transcription factors promoting survivin expression. Downregulation of E-cadherin, in turn, may be more evident in migratory cells and metastatic cancers.
ROS and Antioxidants: Apoptotic Phenotype, Low Survivin Content, Visible Signs of Apoptosis Without NAC, Differentiated Phenotype, Low Survivin Content, no Apoptosis in the Presence of NAC
Src-transformed MDCK cells are normally resistant to apoptosis. Still, they could not resist the deleterious effect of ROS and entered apoptosis upon PL treatment. Concomitantly, survivin expression was downregulated. Yan et al55 designed a more electrophilic PL derivate, a stronger ROS inducer and brought about a more efficient S-phase arrest and mitochondria-mediated apoptosis induction in A549 cells. Several works have proven that PL and other prooxidants inhibit transcription factors such as STAT3, NF-κB and Sp1, Sp3 and Sp4, and also reduce the levels of target genes, including key regulators of cell growth (EGFR, cyclin D1, c-MET), survival (Bcl-2, survivin), inflammation (NF-κB), angiogenesis (VEGF, VEGFR1, VEGFR2), and apoptosis related genes (Bcl-XL, XIAP and CIAP).20, 56, 57, 58
NAC rescued the cells from PL-induced apoptosis, but did not upregulate expression of survivin. Hence, other reasons than upregulation of transcription factors must lie behind the rescue mechanism. With the aid of synthetic analogs of PL it has been shown that, in addition to ROS production, protein glutathionylation may contribute to induction of apoptosis by PL.59 Glutathione supplementation with the aid of NAC may, in turn, rescue the cells from apoptotic process at several checkpoints of the apoptotic signaling cascades.60
The reappearance of E-cadherin in the lateral membranes by NAC treatment might suggest that ts-Src MDCK cells adopt a differentiated phenotype and E-cadherin-induced apoptosis resistance similar to untransformed MDCK cells. The same protective action of NAC from oxidative stress has been observed in the mouse colon: low doses of gamma-irradiation caused rapid disruption of TJs and AJs and redistribution of occludin, ZO-1, claudin-3, E-cadherin and β-catenin, as well as the actin cytoskeleton, and this effect was sustained for at least 24 h. Supplementing the mice with NAC before irradiation prevented the radiation-induced disruption of the junctions, and the components of TJs and AJs remained in situ.61 NAC has also been demonstrated to induce anti-proliferative and differentiating effects in normal human epidermal keratinocytes (NHEK), as well as in the epithelial colon cancer cell line Caco-2, and in both cell lines E-cadherin expression was increased by NAC.62
A limitation of anti-EMT therapies against cancer is that metastatic cancer cells, which had escaped the anti-EMT treatment, underwent MET upon reaching the target tissues. Thus, ideally, anti-EMT therapy should also be anti-proliferative.63 We have shown in the present work that survivin expression in epithelial MDCK cells is strongly inhibited by interactions of E-cadherin molecules resulting in a decrease in proliferation and apoptosis. Moreover, cis-interaction seems to be sufficient enough to downregulate survivin expression, whereas inhibition of apoptosis required trans-interactions of E-cadherin molecules. These phenomena are observed only in soft 3D gel environment mimicking natural tissue. The deleterious effect of a ROS-producing agent PL in Src-transformed MDCK cells could be overcome by NAC via returning E-cadherin to lateral membranes. Survivin, as a multifunctional protein, is an ideal target for cancer drugs, and its downregulation, in combination with upregulating of E-cadherin and promoting its trans-interactions, may be a powerful asset against epithelial-derived carcinogenesis.
References
Lamouille S, Xu J, Derynck R . Molecular mechanisms of epithelial – mesenchymal transition. Nat Rev Mol Cell Biol 2014;15:178–196.
Rodriguez F, Lewis-Tuffin L, Anastasiadis P . E-cadherin's dark side: Possible role in tumor progression. Biochim Biophys Acta 2012;1826:23–31.
Mobahat M, Narendran A, Riabowol K . Survivin as a preferential target for cancer therapy. Int J Mol Sci 2014;15:2494–2516.
Altieri D . Survivin - The inconvenient IAP. Semin Cell Dev Biol 2015;39:91–96.
Rivadeneira D, Caino M, Seo J et al. Survivin promotes oxidative phosphorylation, subcellular mitochondrial repositioning, and tumor cell invasion. Sci Signal 2015;8:1–12.
Hagenbuchner J, Kuznetsov A, Obexer P et al. BIRC5/Survivin enhances aerobic glycolysis and drug resistance by altered regulation of the mitochondrial fusion/fission machinery. Oncogene 2013;32:4748–4757.
Dunajová L, Cash E, Markus R et al. The N-terminus of survivin is a mitochondrial-targeting sequence and Src regulator. J Cell Sci 2016;129:2707–2712.
Boidot R, Végran F, Lizard-Nacol S . Transcriptional regulation of the survivin gene. Mol Biol Rep 2014;41:233–240.
Rauch A, Hennig D, Schäfer C et al. Survivin and YM155: how faithful is the liaison? Biochim Biophys Acta 2014;1845:202–220.
Cheung C, Huang C, Tsai F et al. Survivin - biology and potential as a therapeutic target in oncology. Onco Targets Ther 2013;6:1453–1462.
Ponnelle T, Chapusot C, Martin L et al. Cellular localisation of survivin: impact on the prognosis in colorectal cancer. J Cancer Res Clin Oncol 2005;131:504–510.
Guebel D, Schmitz U, Wolkenhauer O et al. Analysis of cell adhesion during early stages of colon cancer based on an extended multi-valued logic approach. Mol Biosyst 2012;8:1230–1242.
McCrea P, Maher M, Gottardi C . Nuclear signaling from cadherin adhesion complexes. Curr Top Dev Biol 2015;112:129–196.
Chen J, Xie Z, Wu Y . Computational modeling of the interplay between cadherin-mediated cell adhesion and Wnt signaling pathway. PLoS One 2014;9:e100702.
Behrens J, Vakaet L, Friis R et al. Loss of epithelial differentiation and gain of invasiveness correlates with tyrosine phosphorylation of the E-cadherin/beta-catenin complex in cells transformed with a temperature-sensitive v-SRC gene. J Cell Biol 1993;120:757–766.
Palovuori R, Sormunen R, Eskelinen S . SRC-induced disintegration of adherens junctions of madin-darby canine kidney cells is dependent on endocytosis of cadherin and antagonized by Tiam-1. Lab Invest 2003;83:1901–1915.
Töyli M, Rosberg-Kulha L, Capra J et al. Different responses in transformation of MDCK cells in 2D and 3D culture by v-Src as revealed by microarray techniques, RT-PCR and functional assays. Lab Invest 2010;90:915–928.
Raj L, Ide T, Gurkar A et al. Selective killing of cancer cells by a small molecule targeting the stress response to ROS. Nature 2011;475:231–234.
Sreevalsan S, Safe S . Reactive oxygen species and colorectal cancer. Curr Colorectal Cancer Rep 2013;9:350–357.
Han J, Gupta S, Prasad S et al. Piperlongumine chemosensitizes tumor cells through interaction with cysteine 179 of IκBα kinase, leading to suppression of NF-κB-regulated gene products. Mol Cancer Ther 2014;13:2422–2435.
Capra J, Eskelinen S . MDCK cells are capable of water secretion and re-absorption in response to changes in the ionic environment. Can J Physiol Pharmacol 2017;95:72–83.
Debnath J, Muthuswamy S, Brugge J . Morphogenesis and oncogenesis of MCF-10A mammary epithelial acini grown in three-dimensional basement membrane cultures. Methods 2003;30:256–268.
Torkko J, Manninen A, Schuck S et al. Depletion of apical transport proteins perturbs epithelial cyst formation and ciliogenesis. J Cell Sci 2008;121:1193–1203.
de Diesbach P, Medts T, Carpentier S et al. Differential subcellular membrane recruitment of Src may specify its downstream signalling. Exp Cell Res 2008;314:1465–1479.
Zahir N, Weaver V . Death in the third dimension: apoptosis regulation and tissue architecture. Curr Opin Genet Dev 2004;14:71–80.
Datta A, Bryant D, Mostov K . Molecular regulation of lumen morphogenesis. Curr Biol 2011;21:R126–R136.
Kim N, Koh E, Chen X et al. E-cadherin mediates contact inhibition of proliferation through Hippo signaling-pathway components. Proc Natl Acad Sci USA 2011;108:11930–11935.
Benham-Pyle B, Pruitt B, Nelson W . Mechanical strain induces E-cadherin–dependent Yap1 and β-catenin activation to drive cell cycle entry. Science 2015;348:1024–1027.
Yang C, Graves H, Moya I et al. Differential regulation of the Hippo pathway by adherens junctions and apical-basal cell polarity modules. Proc Natl Acad Sci USA 2015;112:1785–1790.
Huang J, Nagatomo I, Suzuki E et al. YAP modifies cancer cell sensitivity to EGFR and survivin inhibitors and is negatively regulated by the non-receptor type protein tyrosine phosphatase 14. Oncogene 2013;32:2220–2229.
Archibald A, Al-Masri M, Liew-Spilger A et al. Atypical protein kinase C induces cell transformation by disrupting Hippo/Yap signaling. Mol Biol Cell 2015;26:3578–3595.
Macara I, Guyer R, Richardson G et al. Epithelial homeostasis. Curr Biol 2014;24:R815–R825.
Chang Y, Lin H, Wang Y et al. Activation of caspase-8 and Erk-1/2 in domes regulates cell death induced by confluence in MDCK cells. J Cell Physiol 2007;211:174–182.
Engelberg J, Datta A, Mostov K et al. MDCK cystogenesis driven by cell stabilization within computational analogues. PLoS Comput Biol 2011;7 e1002030.
Derksen P, Liu X, Saridin F et al. Somatic inactivation of E-cadherin and p53 in mice leads to metastatic lobular mammary carcinoma through induction of anoikis resistance and angiogenesis. Cancer Cell 2006;10:437–449.
Onder T, Gupta P, Mani S et al. Loss of E-cadherin promotes metastasis via multiple downstream transcriptional pathways. Cancer Res 2008;68:3645–3654.
Kumar S, Park S, Cieply B et al. A pathway for the control of anoikis sensitivity by E-cadherin and epithelial-to-mesenchymal transition. Mol Cell Biol 2011;31:4036–4051.
Frisch S, Schaller M, Cieply B . Mechanisms that link the oncogenic epithelial-mesenchymal transition to suppression of anoikis. J Cell Sci 2013;126:21–29.
Vachon P . Integrin signaling, cell survival, and anoikis: distinctions, differences, and differentiation. J Signal Transduct 2011;2011:1–18.
Guo Z, Neilson L, Zhong H et al. E-cadherin interactome complexity and robustness resolved by quantitative proteomics. Sci Signal 2014;7:1–12.
Frisch S, Francis H . Disruption of epithelial cell-matrix interactions induces apoptosis. J Cell Biol 1994;124:619–626.
Paoli P, Giannoni E, Chiarugi P . Anoikis molecular pathways and its role in cancer progression. Biochim Biophys Acta 2013;1833:3481–3498.
O'Brian C, Ward N, Stewart J et al. Prospects for targeting protein kinase C isozymes in the therapy of drug-resistant cancer—an evolving story. Cancer Metastasis Rev 2001;20:95–100.
Yu W, Datta A, Leroy P et al. Beta1-integrin orients epithelial polarity via Rac1 and laminin. Mol Biol Cell 2005;16:433–445.
Myllymäki S, Teräväinen T, Manninen A . Two distinct integrin-mediated mechanisms contribute to apical lumen formation in epithelial cells. PLoS One 2011;6:e19453.
Wang A, Ojakian G, Nelson W . Steps in the morphogenesis of a polarized epithelium. I. Uncoupling the roles of cell-cell and cell-substratum contact in establishing plasma membrane polarity in multicellular epithelial (MDCK) cysts. J Cell Sci 1990;95:137–151.
Wang A, Ojakian G, Nelson W . Steps in the morphogenesis of a polarized epithelium. II. Disassembly and assembly of plasma membrane domains during reversal of epithelial cell polarity in multicellular epithelial (MDCK) cysts. J Cell Sci 1990;95:153–165.
Hofmann C, Obermeier F, Artinger M et al. Cell-cell contacts prevent anoikis in primary human colonic epithelial cells. Gastroenterology 2007;132:587–600.
Ma X, Wang L, Li Y et al. HAb18G/CD147 cell-cell contacts confer resistance of a HEK293 subpopulation to anoikis in an E-cadherin-dependent manner. BMC Cell Biol 2010;11:27.
Bergin E, Levine J, Koh J et al. Mouse proximal tubular cell-cell adhesion inhibits apoptosis by a cadherin-dependent mechanism. Am J Physiol Renal Physiol 2000;278:F758–F768.
You H, Lei P, Andreadis S . JNK is a novel regulator of intercellular adhesion. Tissue Barriers 2013;1:e26845.
Leight J, Wozniak M, Chen S et al. Matrix rigidity regulates a switch between TGF-beta1-induced apoptosis and epithelial-mesenchymal transition. Mol Biol Cell 2012;23:781–791.
Marlar S, Abdellatef S, Nakanishi J . Reduced adhesive ligand density in engineered extracellular matrices induces an epithelial-mesenchymal-like transition. Acta Biomater 2016;39:106–113.
Diaz N, Minton S, Cox C et al. Activation of stat3 in primary tumors from high-risk breast cancer patients is associated with elevated levels of activated SRC and survivin expression. Clin Cancer Res 2006;12:20–28.
Yan W, Wang Q, Yuan C et al. Designing piperlongumine-directed anticancer agents by an electrophilicity-based prooxidant strategy: a mechanistic investigation. Free Radic Biol Med 2016;97:109–123.
Bharadwaj U, Eckols T, Kolosov M et al. Drug-repositioning screening identified piperlongumine as a direct STAT3 inhibitor with potent activity against breast cancer. Oncogene 2015;34:1341–1353.
Pathi S, Lei P, Sreevalsan S et al. Pharmacologic doses of ascorbic acid repress specificity protein (Sp) transcription factors and Sp-regulated genes in colon cancer cells. Nutr Cancer 2011;63:1133–1142.
Park K, Kundu J, Chae I et al. Carnosol induces apoptosis through generation of ROS and inactivation of STAT3 signaling in human colon cancer HCT116 cells. Int J Oncol 2014;44:1309–1315.
Adams D, Dai M, Pellegrino G et al. Synthesis, cellular evaluation, and mechanism of action of piperlongumine analogs. Proc Natl Acad Sci USA 2012;109:15115–15120.
Franco R, Cidlowski J . Apoptosis and glutathione: beyond an antioxidant. Cell Death Differ 2009;16:1303–1314.
Shukla P, Gangwar R, Manda B et al. Rapid disruption of intestinal epithelial tight junction and barrier dysfunction by ionizing radiation in mouse colon in vivo: protection by N-acetyl-l-cysteine. Am J Physiol Gastrointest Liver Physiol 2016;310:G705–G715.
Gustafsson A, Kupershmidt I, Edlundh-Rose E et al. Global gene expression analysis in time series following N-acetyl L-cysteine induced epithelial differentiation of human normal and cancer cells in vitro. BMC Cancer 2005;5:751–19.
Yu Y, Elble R . Homeostatic signaling by cell-cell junctions and its dysregulation during cancer progression. J Clin Med 2016;5:1–20.
Acknowledgements
We thank Dr Veli-Pekka Ronkainen for assistance with microscopes and deconvolution, Civ. Eng. Antti Viklund for help with image analysis, and M.Sc. Lisette Van Tassel for help with language revision.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
In a soft extracellular matrix environment, MDCK cells resemble adult tissue: they become fully differentiated, survivin is down-regulated, E-cadherin trans-interactions are well-developed, and the cells are susceptible to stress-induced apoptosis. In MDCK cells stably transfected with temperature sensitive viral Src, survivin is up-regulated and E-cadherin is relocated to the cytoplasm. Reactive oxygen species induces survivin down-regulation and apoptosis. The antioxidant N-acetylcysteine rescues the cells by returning E-cadherin to membranes.
Rights and permissions
About this article
Cite this article
Capra, J., Eskelinen, S. Correlation between E-cadherin interactions, survivin expression, and apoptosis in MDCK and ts-Src MDCK cell culture models. Lab Invest 97, 1453–1470 (2017). https://doi.org/10.1038/labinvest.2017.89
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/labinvest.2017.89