Abstract
Induced pluripotent stem cell (iPSC) technology was originally developed in 2006. Essentially, it converts somatic cells into pluripotent stem cells by transiently expressing a few transcriptional factors. Once generated, these iPSCs can differentiate into all the cell types of our body, theoretically, which has attracted great attention for clinical research including disease pathobiology studies. Could this technology then become an additional research or diagnostic tool widely available to practicing pathologists? Here we summarize progress in iPSC research toward disease pathobiology studies, its future potential, and remaining problems from a pathologist’s perspective. A particular focus will be on introducing the effort to recapitulate disease-related morphological changes through three-dimensional culture of stem cells such as organoid differentiation.
Similar content being viewed by others
Main
Pathologists seek new research tools that can provide a better understanding of underlying disease mechanisms beyond morphological changes. Standard methods such as immunohistochemistry provide powerful tools not only for diagnosis but also for understanding molecular mechanisms. Genetic analyses such as examination of RNA expression and DNA mutations are valuable for precise molecular diagnosis. Induced pluripotent stem cell (iPSC) technology, the ability to generate stem cells from somatic cells in vitro, was developed a decade ago and since then has been repeatedly implicated for disease pathobiology studies. Could this technology become an additional useful research or diagnostic tool widely available to practicing pathologists? The present review summarizes progress in iPSC research toward disease pathobiology studies, its future potential, and remaining problems particularly from a pathologist’s perspective.
Advantages using ipscs for pathology research
iPSC technology was originally developed by Takahashi and Yamanaka in 2006.1 Essentially, it converts somatic cells into embryonic stem cell (ESC)-like pluripotent stem cells by transiently expressing a few transcriptional factors. Once generated, these iPSCs can differentiate into all the cell types of our body, theoretically, just like ESCs, which has attracted great attention for clinical research. Since iPSC generation technology is powerful, yet relatively straightforward and reproducible, it has spread to labs worldwide and has been intensively utilized for research in the last decade. Its applications are essentially two fold, (1) for cell-based transplantation therapies or (2) providing novel human disease models in a dish; the latter being of great interest to pathologists and others studying disease pathobiology. In a typical study, iPSCs are generated from patient peripheral blood cells or skin fibroblasts, differentiate them into cell types relevant to his/her disease (eg, dopaminergic neurons for Parkinson’s disease), and identify abnormalities caused by a patient’s unique genetic background. Under such a scheme, the best advantage of using iPSC technology is that we can obtain disease-relevant cell types, which are not easily collected from live patients unless they undergo invasive biopsies. Blood cells are often preferred as a starting cell population since they are available in a minimally invasive manner. For some cases, we may want to utilize available skin fibroblasts that have been previously collected for other research purposes. With either cell type, recent progress in reprogramming technology has made it possible to generate iPSCs without exogenous gene integration.2 In most cases, the transient expression of so-called Yamanaka factors (Oct4, Sox2, Klf4 and C-Myc) in somatic cells through plasmid transfection or Sendai-viral vector transduction is sufficient to generate iPSCs. Typically, a few weeks exposure to reprogramming factors, results in the formation of iPSC colonies, which can be isolated individually and expanded to generate clones. Once iPSCs are generated, the cells are naturally immortalized, and moreover can be stored long-term in liquid nitrogen for later use. Thus, the second remarkable merit of iPSCs is their ability to provide a renewable resource for research. Similar to human tissues collected in daily pathology practice, patient-derived iPSCs also carry the genomic information of the original patient. In addition, iPSCs are well suited to genetic manipulation including gene-editing technologies due to their continuous proliferation ability. In particular, gene editing is feasible within iPSCs using the system of clustered regularly interspaced short palindromic repeats/CRISPR-associated protein 9 (CRISPR/Cas9), which have been widely applied to biomedical research because of their accuracy and simplicity. In combination with gene editing, iPSCs have become an ideal in vitro tool to validate the roles of genetic variations in human diseases. The enduring nature of iPSCs makes this resource unique.
Obstacles and limitations for using ipscs for disease modeling
On the other hand, there are many remaining obstacles to utilize iPSCs for disease modeling. An obvious and most serious concern is that iPSCs are an in vitro cellular system, which is a considerable drawback for systemic diseases or organ systems. Indeed, most previous studies successfully utilizing the iPSC system have focused on biochemical or molecular biological analyses of differentiated cells. Cellular functional analyses are certainly possible and have been shown in many previous studies; however, when compared to immense data obtained from tissue samples or animal models, the system provides limited information. In order to address this, researchers have been developing co-culture and 3-dimentional (3D) organized tissues termed 'organoids'. Alternatively, xenographic cellular injection to immunocompromised animals enables to study the effects in vivo. Such efforts make the system more relevant from the standpoint of disease pathology as we discuss below. Another obstacle is a limitation of cell types we can generate from iPSCs. Although iPSCs can theoretically differentiate into any cell type of the mesoderm, endoderm, and ectodermal lineages, feasible protocols have only been established for limited cell types to date. In general, most differentiation methods utilize various growth factors or small molecule inhibitors in a stepwise manner, using cues from normal embryonal development. Although the number of available protocols has significantly increased in the last decade (including for pancreatic beta cells, dopaminergic neurons to CD8 T lymphocytes) many cell types desired are yet to be achieved in vitro. Related to this issue, many differentiated cells resemble embryonic or neonatal cell types and lack the full maturity and functionality of adult cells. In reality, most researchers plan iPSC experiments with practical goals based on availability of differentiation protocols and maturation status to ensure the differentiated cells will be relevant to the study in question.
Effort to recapitulate morphological changes IN VITRO
The field of pathology encompasses study of disease pathogenesis as well as morphology-based cell and tissue diagnosis in the clinical setting. While pathology practice has been revolutionized by molecular diagnostic methods, morphology-based diagnosis is still integral to daily surgical and autopsy pathology. Therefore, it would be of great interest for both research and clinical diagnosis whether the iPSC system could recapitulate disease-relevant morphological changes in vitro.
As previously mentioned, one of the major disadvantages of the iPSC system is that it is an in vitro culture of cells in isolation. From a pathological point of view, morphological changes in cells are less informative than those in tissues. Although cytological morphology is critical especially for cancer screening, there is an obvious limitation to assess disease only by the morphological change of individual cells. In order to overcome this issue, many types of 3D cell culture methods have been developed.3, 4 Under 3D cell culture, individual cells are expected to maintain their natural shapes and functions, promoting interactions with adjacent cells that provide an environment to help create native architecture.
3D culture systems can be broadly categorized into two types; scaffold-free systems and scaffold-based systems.3 A scaffold-free system utilizes the natural character of cells to spontaneously aggregate, which is often referred to as spheroids or multi-cellular aggregates. This method has been used for decades in the fields of developmental biology as well as cancer research.5 While there are various methods available to produce spheroids, they rely on low or non-adherent substrates for formation of cell aggregates. In contrast, scaffold-based systems utilize exogenous scaffolds which lead cells to form a 3D structure. Numerous types of scaffolds are artificially fabricated by bioengineering technology and can be classified into hydrogel technology and solid scaffold-based technologies. The hydrogel technology provides a loose scaffold framework in which cells are allowed to self-migrate, and some hydrogels also support cell growth. One well-known hydrogel is Matrigel, the basement membrane matrix extracted from mouse sarcoma. Hydrogel culture technique is commonly used for angiogenesis assays, in which endothelial cells migrate and form a capillary network.6 On the other hand, solid scaffold-based technologies encourage cells to form 3D structure according to the structure of the scaffolds, which are designed to mimic natural tissue structures. For example, artificial skin can be produced by seeding keratinocytes upon a dermal construct composed of a porous scaffold inoculated with dermal fibroblasts, which form a bilayer structure that mimics the epidermis and dermis.7
In the ESC/iPSC field, these 3D culture systems have been widely utilized. The scaffold-free system is an important technique to generate embryoid bodies, which can be used for differentiation into cells corresponding to all three embryonic germ layers, which provides validation of pluripotency. The angiogenesis assay using hydrogel is frequently used to assess the function of iPSC-derived endothelial cells. For example, in a Moyamoya disease model, poor angiogenetic ability is recapitulated by the angiogenesis assay with iPSC-derived vascular endothelial cells generated from patients harboring the RNF213 R4810K polymorphism.8, 9 With scaffold-based culture systems, there is an effort to utilize iPSCs in the bioengineering field. For example, Zhang YS et al reported an endothelialized myocardium model using a 3D bioprinter.10 They bioprinted a microfibrous scaffold encapsulating endothelial cells. Using a crosslinking processes for stable gelation, it formed a 3D endothelial bed that allowed for migration and expansion of endothelial cells. When cardiomyocytes were seeded onto the 3D endothelial bed, aligned myocardia with spontaneous and synchronous contraction were observed. They applied this technique to human iPSC-derived cardiomyocytes, which successfully presented synchronized beating across entire scaffold at a rate of ~60 bpm for up to 7–10 days.
Organoid culture generated from adult stem cells
Generating organoids may be the ultimate goal of 3D culture in the field of stem cell biology. Organoids, as their name suggests, consist of organized structure containing multiple cell type. Although the term has been used since 1960s in the context of organogenesis in classic developmental experiments by cell dissociation and reaggregation, during the present decade this term has been applied to stem cell-derived aggregates.11, 12 Organoids are defined in recent papers as in vitro 3D cellular clusters derived exclusively from primary tissue, ESCs or iPSCs, are capable of self-renewal and self-organization, and exhibit similar organ functionality to the tissue of origin.13
Currently, organoids are generated in 3D culture conditions using medium supplemented with growth factor cocktails that mimic the organ microenvironment. In these conditions, stem cells spontaneously organize into near-physiological tissue patterns. Organoids are generated from ESCs/iPSCs or organ-restricted adult stem cells (ASCs). The major technological advance was development of small intestinal organoid culture generated from murine small intestine in 2009 by Sato et al.14 They generated intestinal organoids from whole crypts or single stem cells which were identified by Lgr5. Isolated cells were suspended in Matrigel and grown in medium supplemented with R-spondin-1 (Wnt signal amplifier and ligand of Lgr5), Noggin (BMP inhibitor) and EGF. They formed cystic and budding structures lined with a simple epithelial layer containing all cell types corresponding to normal intestinal epithelia. Based on this method, organoids from human ASCs or many other organ tissues have been established, including from colon, stomach, liver, pancreas, lung, prostate, mammary gland, salivary gland, fallopian tube, taste buds and esophagus.11 In some cases, robust protocols that allow for long-term expansion of organoids have been developed. In addition, organoids can be generated from primary tumor tissue using similar methods.15
These ASC-based organoids are applied to model various diseases, often utilizing cells derived from patients with monogenic disorders. Huch et al16 generated liver organoids from liver biopsy of alpha 1-antitripsin deficiency patients. These patient-derived organoids grew for more than 4 months and behaved normally, however they revealed molecular abnormalities including alpha 1-antitripsin protein precipitates within the cytoplasm. They also generated liver organoids from an Alagille syndrome patient, which showed scarce biliary cells that underwent apoptosis in the organoid lumen. Importantly, these organoids reflected disease specific phenotypes and provided important cellular models for scientific study. In another study, gene repair was demonstrated using cystic fibrosis patient-derived intestinal organoids.17 These authors corrected the mutated locus of cystic fibrosis transmembrane conductance receptor gene in organoids using CRISPR/Cas9 system. The corrected allele was expressed and fully functional as verified by a forskolin assay, in which normal intestinal organoids swell by forskolin stimulation while cystic fibrosis organoids did not respond.
In addition to monogenic disease modeling, ASC-based organoids have also been applied to model cancer. Two independent studies using human intestinal organoids were reported as adenoma-carcinoma models.18, 19 In these studies, multiple tumor-related gene mutations were serially introduced into normal organoids derived from normal intestine using the CRISPR/Cas9 system. Upon xenotransplantation into mice, the engineered organoids revealed tumorigenic transformation from adenoma into adenocarcinoma according to the number of introduced mutations.
Organoids also have significant potential as a cellular drug screening model due to their advantages of near-physiological cell organization and expandability. In addition to basic drug screening, patient-derived organoids are suitable for screening in the context of personalized therapy. One such example is an organoid biobank of colorectal cancer patients reported by van de Wetering et al.20 They generated ‘paired organoids’ derived from resected tumor and adjacent healthy mucosa of 20 colorectal cancer patients. Tumor organoids recapitulated the original tumor histology and DNA sequencing results, and their DNA expression conformed to a large cohort data set of colorectal cancer. They applied these healthy and tumor organoids to systematic high-throughput drug screening assays that allowed for detection of gene-drug associations.
Organoid culture generated from pluripotent stem cells
Similar to ASC-based organoids, pluripotent stem cell-derived organoids have been applied to recapitulate human diseases in a dish. While ASC-based organoids mimic the dynamic maintenance of somatic stem cells, ESC/iPSC-based organoids recapitulate the development of the corresponding organ.21 Although both retain the genetic information of original cells, ASC-based organoids tend to reproduce the original tissue phenotype directly. In contrast, organoids derived from ESCs/iPSCs seem to have an ability to form more complex structures, likely due to their pluripotency potential, in contrast to ASC characteristic of multipotency. In a practical sense, ASC-based organoid protocols are simpler than ESC/iPSC-based organoids, because ASCs are already committed to organ-specific differentiation. However, ESCs/iPSCs have an advantage as a cell resource for generating tissues that are difficult to obtain from patients. To date, organoids have been generated from ESCs/iPSCs into such linages as (for neuroectoderm) optic cup,22 cerebrum,23, 24 adenohypophysis25 and cerebellum;26 (for endoderm) stomach,27 small intestine,28 liver,29 lung30 and thyroid;31 and (for intermediate mesoderm) kidney.32 Table 1 illustrates advantages and limitations of ESC/iPSC-and ASC-based organoids.
A representative case of disease modeling using brain organoids generated from ESCs/iPSCs was reported by Lancaster et al.24 They started with floating embryoid bodies followed by embedment in Matrigel and culture in a spinning bioreactor. The obtained brain organoids grew up to 4 mm in diameter in 2 months. Histological analysis revealed self-organized regions reminiscent of cerebral cortex, choroid plexus, retina and meninges. They applied this organoid culture technique for a human microcephaly model. Brain organoids were successfully generated from iPSCs of a microcephaly patient with a CDK5RAP2 mutation, which revealed the smaller neural tissues containing very few progenitor regions with signs of premature neural differentiation. This phenotype was rescued by reintroducing the CDK5RAP2 protein. Their model partially recapitulated microcephaly in vitro. Other disease models have been reported using brain organoids since then, such as Zika virus infection33 and autism spectrum disorder.34 As brain organoids culture resembles fetal human neocortex development,35 current disease models using brain organoids are limited more or less to neurodevelopmental disorders. However, the future improvement of culture conditions may enable researchers to mimic the late stage of neurogenesis to extend the use of organoids to model late onset diseases. As the human central nervous system is very distinct from other human organs in terms of both difficulty of sampling and imperfection of animal models, ESC/iPSC-based organoids should serve as an ideal research model.
Another good example of ESC/iPSC-based organoids is from Takasato et al,32 who generated kidney organoids which reproduced the complex kidney structure consisting of nephrons and the collecting duct network. Individual nephrons included distal and proximal tubules, early loops of Henle and glomeruli containing podocytes and ongoing vascularization. The gene expression analysis from whole kidney organoids resembled first trimester human fetal kidney tissue. They tested the utility of these organoids as a model of drug-related nephrotoxicity. When they treated organoids with cisplatin, immature proximal tubules did not respond, however more mature proximal tubules underwent apoptosis in a dose-depend manner. This result showed the possibility of future applications for nephrotoxicity screening and disease modeling.
In addition to the approaches mentioned above for inducing organoid formation through a combination of specialized media, growth factor, chemicals and extracellular matrices, a genetic engineering approach has been recently reported. Guye et al engineered an iPSC cell line with inducible GATA-binding protein 6 (GATA6) expression, which successfully produced liver bud-like structure made up of multiple cell types including hepatobiliary, hematopoietic and stromal cells as well as a neuronal niche.36
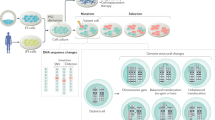
Further, organoid generation can be facilitated by mixing different lineage cell types, such as vessels, nerves or immune cells. Takebe et al29 generated vascularized liver organoids, for example. By co-culture of human iPSC-derived hepatic endoderm cells, human mesenchymal stem cells and human umbilical vein endothelial cells under 3D culture conditions, they were able to generate self-organized hepatic bud structures. Transplanting these onto the brains of immunodeficient mice, vascularization with connection to the host circulation was observed. In addition, transplanted organoids exhibited functionality close to adult liver, suggesting that the formation of vasculature promoted maturation of hepatic endodermal cells. In another example, Nozaki et al37 reported the co-culture of murine intestinal organoids and murine intraepithelial lymphocytes. Time-lapse imaging revealed dynamic movement and migration of intraepithelial lymphocytes into organoids. Organoids containing heterotypic populations reproduced near-physiological conditions in which multiple types of cells interact each other. Such co-culture systems will extend the applications of iPSC-organoids to broader disease modeling of common disorders such as ischemic disease or inflammatory disease in the future. Figure 1 provides an overview of organoid generation and application.
Organoid generation from ESCs/iPSCs and ASCs and their applications. Organoids are generated from ESCs/iPSCs or ASCs. iPSCs can be established from peripheral blood mononuclear cells (PBMCs) or fibroblasts. ASC-based organoids are initiated from organ biopsy samples from normal or tumor tissue, followed by dissociation into epithelia containing stem cells. Both organoids are self-organized via three- dimensional (3D) culture with differentiation and/or expansion medium. They are utilized for disease modeling, drug screening and organoid biobank which can be a unique resource for pathobiology studies.
Perspective of utilizing ipscs for pathological research
Ten years have passed since the first publication of human iPSCs by Takahashi and Yamanaka in 2007,38 and current methods of iPSC generation are well established. Countless numbers of patient-derived iPSCs have been established and reported, originally from hereditary monogenic disorders39 and later from more complexed polygenic diseases.40 However, when it comes to robust cell type specific differentiation, there are still limitations. In reality, this is still the most significant barrier for researchers to utilize these cells as a tool for disease modeling. Further development of efficient differentiation methods is critical for utility of iPSCs.
In this article, we highlighted 3D culture systems as a most promising approach to capture the attention of pathologists who are familiar with the structure of tissues. At this moment, ASC-based intestinal organoids seem exemplary in terms of their application to disease modeling. The established culture protocols allow for long-term expansion and sensitive evaluative assays such as a forskolin assay. Because organoids tend to have complex structures, selecting the appropriate assay to detect significant morphological changes is critical for their evaluation. Obviously, current efforts to generate various organoids from iPSCs are promising as iPSCs are a resource that is easily accessible. Considering that iPSC banks have been established from many normal and disease individuals, creation of iPSC-organoid biobanks would be particularly useful for pathologists. In addition to high-throughput drug screening using adult tissue-derived organoid biobanks by van de Wetering et al20 as mentioned before, recently Fujii et al established 55 colorectal tumor organoids and 41 counterparts from normal mucosa.41 In vitro and in vivo, they reproduced original histological features including rare subtypes like poorly differentiated adenocarcinoma, mucinous adenocarcinoma and neuroendocrine carcinoma. In addition to analyses of DNA sequencing and DNA expression profiling, they investigated niche factor requirements in culture conditions of each tumor organoid and compared them with the clinical stages of the original tumors. Their organoid biobank has several advantages in comparison of other alternative methods. For instance, these organoids replicate the genomic and phenotypic information of individual patients, which is difficult with cancer cell lines. Moreover, they undergo long-term expansion, which is difficult with the culture of tumor sections. Finally, xenotransplantation of tumor sections requires large numbers of mice for propagation. Similarly, iPSC-derived mini-brain or pituitary biobanks, for example, would be extremely useful as sustainable and expandable resources for studying signal transduction or to test efficiency of drug and gene therapy in vitro, which is nearly impossible with formalin-fixed and paraffin-embedded (FFPE) tissues. The combination of FFPE samples, frozen samples and iPSC-derived organoids would enable further diverse and comprehensive pathobiological analyses.
Although there are still numerous obstacles to overcome, we want to emphasize here that considerable and steady progress has been made during the last decade since iPSCs were first created. With further refinement of 3D culture systems using iPSC technology, it may not be long before pathologists use this technique to better understand disease or even in disease diagnosis.
References
Takahashi K, Yamanaka S . Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006;126:663–676.
Santostefano KE, Hamazaki T, Biel NM et al. A practical guide to induced pluripotent stem cell research using patient samples. Lab Invest 2015;95:4–13.
Knight E, Przyborski SJ . Advances in 3D cell culture technologies enabling tissue-like structures to be created in vitro. Anat 2015;227:746–756.
Pampaloni F, Reynaud EG, Stelzer EH . The third dimension bridges the gap between cell culture and live tissue. Nat Rev Mol Cell Biol 2007;8:839–845.
Weiswald LB, Bellet D, Dangles-Marie V . Spherical cancer models in tumor biology. Neoplasia 2015;17:1–15.
Chwalek K, Tsurkan MV, Freudenberg U et al. Glycosaminoglycan-based hydrogels to modulate heterocellular communication in in vitro angiogenesis models. Sci Rep 2014;4:4414.
Auger FA, Berthod F, Moulin V et al. Tissue-engineered skin substitutes: from in vitro constructs to in vivo applications. Biotechnol Appl Biochem 2004;39:263–275.
Hamauchi S, Shichinohe H, Uchino H et al. Cellular functions and gene and protein expression profiles in endothelial cells derived from moyamoya disease-specific iPS Cells. PLoS ONE 2016;11:e0163561.
Hitomi T, Habu T, Kobayashi H et al. Downregulation of Securin by the variant RNF213 R4810K (rs112735431, G>A) reduces angiogenic activity of induced pluripotent stem cell-derived vascular endothelial cells from moyamoya patients. Biochem Biophys Res Commun 2013;438:13–19.
Zhang YS, Arneri A, Bersini S et al. Bioprinting 3D microfibrous scaffolds for engineering endothelialized myocardium and heart-on-a-chip. Biomaterials 2016;110:45–59.
Clevers H Modeling development and disease with organoids. Cell 2016;165:1586–1597.
Kretzschmar K, Clevers H . Organoids: modeling development and the stem cell niche in a dish. Dev Cell 2016;38:590–600.
Fatehullah A, Tan SH, Barker N . Organoids as an in vitro model of human development and disease. Nat Cell Biol 2016;18:246–254.
Sato T, Vries RG, Snippert HJ et al. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature 2009;459:262–265.
Sato T, Stange DE, Ferrante M et al. Long-term expansion of epithelial organoids from human colon, adenoma, adenocarcinoma, and Barrett's epithelium. Gastroenterology 2011;141:1762–1772.
Huch M, Gehart H, van Boxtel R et al. Long-term culture of genome-stable bipotent stem cells from adult human liver. Cell 2015;160:299–312.
Schwank G, Koo BK, Sasselli V et al. Functional repair of CFTR by CRISPR/Cas9 in intestinal stem cell organoids of cystic fibrosis patients. Cell Stem Cell 2013;13:653–658.
Matano M, Date S, Shimokawa M et al. Modeling colorectal cancer using CRISPR-Cas9-mediated engineering of human intestinal organoids. Nat Med 2015;21:256–262.
Drost J, van Jaarsveld RH, Ponsioen B et al. Sequential cancer mutations in cultured human intestinal stem cells. Nature 2015;521:43–47.
van de Wetering M, Francies HE, Francis JM et al. Prospective derivation of a living organoid biobank of colorectal cancer patients. Cell 2015;161:933–945.
Sasai Y, Eiraku M, Suga H . In vitro organogenesis in three dimensions: self-organising stem cells. Development 2012;139:4111–4121.
Eiraku M, Takata N, Ishibashi H et al. Self-organizing optic-cup morphogenesis in three-dimensional culture. Nature 2011;472:51–56.
Eiraku M, Sasai Y . Self-formation of layered neural structures in three-dimensional culture of ES cells. Curr Opin Neurobiol 2012;22:768–777.
Lancaster MA, Renner M, Martin CA et al. Cerebral organoids model human brain development and microcephaly. Nature 2013;501:373–379.
Suga H, Kadoshima T, Minaguchi M et al. Self-formation of functional adenohypophysis in three-dimensional culture. Nature 2011;480:57–62.
Muguruma K, Nishiyama A, Kawakami H et al. Self-organization of polarized cerebellar tissue in 3D culture of human pluripotent stem cells. Cell Rep 2015;10:537–550.
McCracken KW, Cata´ EM, Crawford CM et al. Modelling human development and disease in pluripotent stem-cell-derived gastric organoids. Nature 2014;516:400–404.
Uchida H, Machida M, Miura T et al. A xenogeneic-free system generating functional human gut organoids from pluripotent stem cells. JCI Insight 2017;2:e86492.
Takebe T, Sekine K, Enomura M et al. Vascularized and functional human liver from an iPSC-derived organ bud transplant. Nature 2013;499:481–484.
Dye BR, Hill DR, Ferguson MA et al. In vitro generation of human pluripotent stem cell derived lung organoids. Elife 2015; 4 e05098.
Kurmann AA, Serra M, Hawkins F et al. Regeneration of thyroid function by transplantation of differentiated pluripotent stem cells. Cell Stem Cell 2015;17:527–542.
Takasato M, Er PX, Chiu HS et al. Kidney organoids from human iPS cells contain multiple lineages and model human nephrogenesis. Nature 2015;526:564–568.
Qian X, Nguyen HN, Song MM et al. Brain-region-specific organoids using mini-bioreactors for modeling ZIKV exposure. Cell 2016;165:1238–1254.
Mariani J, Coppola G, Zhang P et al. FOXG1-dependent dysregulation of GABA/glutamate neuron differentiation in autism spectrum disorders. Cell 2015;162:375–390.
Camp JG, Badsha F, Florio M et al. Human cerebral organoids recapitulate gene expression programs of fetal neocortex development. Proc Natl Acad Sci USA 2015;112:15672–15677.
Guye P, Ebrahimkhani MR, Kipniss N et al. Genetically engineering self-organization of human pluripotent stem cells into a liver bud-like tissue using Gata6. Nat Commun 2016;7:10243.
Nozaki K, Mochizuki W, Matsumoto Y et al. Co-culture with intestinal epithelial organoids allows efficient expansion and motility analysis of intraepithelial lymphocytes. J Gastroenterol 2016;51:206–213.
Takahashi K, Tanabe K, Ohnuki M et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 2007;131:861–872.
Spitalieri P, Talarico VR, Murdocca M et al. Human induced pluripotent stem cells for monogenic disease modelling and therapy. World J Stem Cells 2016;8:118–135.
Hamazaki T, El Rouby N, Fredette NC et al. Induced pluripotent stem cell research in the era of precision medicine. Stem Cells 2017;35:545–550.
Fujii M, Shimokawa M, Date S et al. A colorectal tumor organoid library demonstrates progressive loss of niche factor requirements during tumorigenesis. Cell Stem Cell 2016;18:827–838.
Acknowledgements
NT and KES receive partial support by National Institutes of Health (GM119977, DK104194) and American Heart Association (16GRNT30980002). ATY receives partial support from 1Florida ADRC funded by National Institutes of Health (AG047266).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Induced pluripotent stem cell technology has been an integral part of biological research for over a decade. Could this technology become widely available to practicing pathologists for research and diagnostic purposes? The present review emphasizes three-dimensional culture of stem cells as a particularly promising tool for pathobiological studies.
Rights and permissions
About this article
Cite this article
Watanabe, N., Santostefano, K., Yachnis, A. et al. A pathologist’s perspective on induced pluripotent stem cells. Lab Invest 97, 1126–1132 (2017). https://doi.org/10.1038/labinvest.2017.81
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/labinvest.2017.81
This article is cited by
-
Exploring the promising potential of induced pluripotent stem cells in cancer research and therapy
Molecular Cancer (2023)
-
Immortalization of human hepatocytes from biliary atresia with CDK4R24C, cyclin D1, and TERT for cytochrome P450 induction testing
Scientific Reports (2020)
-
Establishment of a Gorlin syndrome model from induced neural progenitor cells exhibiting constitutive GLI1 expression and high sensitivity to inhibition by smoothened (SMO)
Laboratory Investigation (2020)
-
Frequent retrotransposition of endogenous genes in ERCC2-deficient cells derived from a patient with xeroderma pigmentosum
Stem Cell Research & Therapy (2019)