Abstract
Increased generation of reactive oxygen species (ROS) is a common denominative pathogenic mechanism underlying vascular and renal complications in diabetes mellitus. Endothelial NAD(P)H oxidase is a major source of vascular ROS, and it has an important role in endothelial dysfunction. We hypothesized that activation of endothelial NAD(P)H oxidase initiates and worsens the progression of diabetic nephropathy, particularly in the development of albuminuria. We used transgenic mice with endothelial-targeted overexpression of the catalytic subunit of NAD(P)H oxidase, Nox2 (NOX2TG). NOX2TG mice were crossed with Akita insulin-dependent diabetic (Akita) mice that develop progressive hyperglycemia. We compared the progression of diabetic nephropathy in Akita versus NOX2TG-Akita mice. NOX2TG-Akita mice and Akita mice developed significant albuminuria above the baseline at 6 and 10 weeks of age, respectively. Compared with Akita mice, NOX2TG-Akita mice exhibited higher levels of NAD(P)H oxidase activity in glomeruli, developed glomerular endothelial perturbations, and attenuated expression of glomerular glycocalyx. Moreover, in contrast to Akita mice, the NOX2TG-Akita mice had numerous endothelial microparticles (blebs), as detected by scanning electron microscopy, and increased glomerular permeability. Furthermore, NOX2TG-Akita mice exhibited distinct phenotypic changes in glomerular mesangial cells expressing α-smooth muscle actin, and in podocytes expressing increased levels of desmin, whereas the glomeruli generated increased levels of ROS. In conclusion, activation of endothelial NAD(P)H oxidase in the presence of hyperglycemia initiated and exacerbated diabetic nephropathy characterized by the development of albuminuria. Moreover, ROS generated in the endothelium compounded glomerular dysfunctions by altering the phenotypes of mesangial cells and compromising the integrity of the podocytes.
Similar content being viewed by others
Main
Diabetic nephropathy is a major microvascular complication of diabetes, and is a leading cause of end-stage renal disease.1 Previous studies indicate that 30–40% of patients with diabetes mellitus develop overt nephropathy2 by mechanisms other than hyperglycemia that involve genetic factors,3 reactive oxygen species (ROS),4 and advanced glycation end products.5 Moreover, factors such as derangements in lipid profile, abnormal levels of nitric oxide (NO), protein kinase C, and kinin, as well as hypertension, contribute to the pathophysiology of diabetic nephropathy.6 However, there are yet other pathogenetic mechanisms of diabetic nephropathy that remain to be explored.
Endothelial dysfunction is a consistent finding in patients with diabetes.7 Endothelial damage and microcirculatory impairment are the early pathogenetic events in diabetic nephropathy in patients with hyperglycemia.8 Endothelial dysfunction can be defined as increased ROS with reduced bioavailability of endothelial NO. Evidence suggests that in patients with diabetes increased ROS are a common denominator that links diverse pathogenic mechanisms that are involved in the progression of diabetic microvasculopathy.9 We reported that nicotinamide adenine dinucleotide phosphate-oxidase (NAD(P)H oxidase) and the uncoupling of endothelial NO synthase (eNOS) contribute to glomerular ROS production by diabetic rats.10 This is associated with albuminuria, apparently caused by endothelial dysfunctions that is seen in diabetic Zucker fatty rats.11 However, to our knowledge, there is little direct evidence indicating whether or not endothelial-derived generation of ROS in diabetic rats induces albuminuria with exacerbation of diabetic nephropathy.
In the present study, we hypothesized that activation of endothelial NAD(P)H oxidase, in the presence of diabetes, initiates and worsens the outcome of diabetic nephropathy, in particular, the development of albuminuria. To test this hypothesis, we cross-bred endothelial-targeted NAD(P)H oxidase (Nox) 2 overexpression (NOX2TG) mice12 with Akita mice.13 The latter acquire insulin-dependent diabetes mellitus caused by a spontaneous point mutation in Ins2 gene. We demonstrate here that activation of endothelial NAD(P)H oxidase caused albuminuria and accelerated glomerular damage at an early stage of diabetes in Akita mice, indicating that endothelial-derived ROS exacerbates the progression of diabetic nephropathy.
MATERIALS AND METHODS
Mice
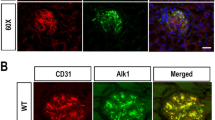
The Animal Research Committee of Kawasaki Medical School approved all the procedures included in the protocols (No. 11-005) that are described in this report. The studies were conducted according to the Guidelines provided by the Care and Use of Laboratory Animals of Kawasaki Medical School, which follows the National Institutes of Health Guide (NIH Publication No. 80-23, revised 1996). Mice were housed in a room with controlled temperature and humidity with a 14–10 h light–dark cycle and provided with unrestricted access to standard laboratory animal chow and tap water. C57BL/6 control (wild-type (WT)) mice and Akita mice (Ins2Akita/Ins2+, C57BL/6 background) were obtained from Japan SLC (Shizuoka, Japan). One of us (KC) provided NOX2TG mice (C57BL/6 background), which harbor a human NOX2 transgene that is primarily expressed in the endothelium under the control of the mouse Tie-2 promoter.12 We verified the TIE-2 expression in renal glomeruli of transgenic mice that harbors the gene encoding green fluorescent protein, which is expressed under the control of the mouse Tie-2 promoter (STOCK Tg(TIE2GFP)287Sato/J; The Jackson Lab, Bar Harbor, ME, USA) (Supplementary Figure S1).
We did not detect significant glomerular structural changes in NOX2TG mice at 30 weeks of age compared with WT mice (Supplementary Figure S2). Male Akita mice were cross-bred with female NOX2TG mice to generate offspring carrying the NOX2 transgene and Akita traits (NOX2TG-Akita). Experiments were carried out using male mice of four groups: WT, NOX2TG, Akita, and NOX2TG-Akita group. Blood pressure, blood glucose, and urinary albumin excretion were assayed at 6, 8, and 10 weeks after birth. Blood pressure was measured using the tail-cuff method with an automatic sphygmomanometer (BP98A; Softron, Tokyo, Japan). Blood samples were obtained using venipuncture of the tail. Blood glucose was measured using a Terumo Finetouch glucose analyzer (Terumo, Tokyo, Japan). Urine samples were collected 24 h after fasting the mice in metabolic cages. Urinary albumin was quantified using Albuwell M (Exocell, Philadelphia, PA, USA), and it was expressed as the ratio of albumin to creatinine.14 For pathological and biochemical analyses, mice 6 weeks of age were anesthetized and then killed using sevoflurane inhalation. Certain NOX2TG-Akita mice were treated with a peptide inhibitor (gp91TAT)15 of NAD(P)H oxidase (10 mg/kg per day, via an osmotic pump) when they were 6–8 weeks old and killed at 8 weeks.
Histopathological Examination
Sections (4 μm thick) were prepared from renal tissue samples embedded in paraffin, and deparaffinized kidney sections were stained with periodic acid-Schiff (PAS) reagent to evaluate glomerular injury. Images of glomeruli were acquired using a BZ-9000 All-in-one Fluorescence Microscope (Keyence, Osaka, Japan). The severity of glomerular injury was evaluated according to a mesangial matrix score from 0 to 4 as described previously.16 Twenty random glomeruli per mouse were blindly evaluated by a renal pathologist who was not informed of the nature of the samples. Ten mice from each group (WT, NOX2TG, Akita, and NOX2TG-Akita group) were evaluated to calculate the mean score.
Immunohistochemical Studies
Kidney cryostat sections (3 μm thick) were prepared and subjected to immunohistochemical analyses for the expression of plasmalemmal vesicle-1 (PV-1), podocin, desmin, and α-smooth muscle actin (α-SMA), using the primary antibodies as follows: PV-1 (no. sc-50169; Santa Cruz Biotechnology, Santa Cruz, CA, USA), podocin (no. ab50339; Abcam, Cambridge, MA, USA), desmin (no. D33; Dako, Carpinteria, CA, USA), and α-SMA (no. 1A4; Sigma-Aldrich, St Louis, MO, USA). Deparaffinized kidney sections (4 μm thick) were stained with Texas Red-conjugated lectin from Lycopersicon esculentum (tomato lectin) (Vector Laboratories, Burlingame, CA, USA). The sections for the expression of PV-1, podocin, desmin, α-SMA, and tomato lectin outlining the podocytes of the glomerular tuft were evaluated to determine the staining score as described previously.16 Briefly, staining was scored as follows (score, % cells stained): 0, 0–5; 1, 5–25; 2, 25–50; 3, 50–75; and 4, >75. At least 20 glomeruli were randomly selected from each mouse (n=10) to calculate the mean score (total >200 glomeruli). The area encompassing the stained glomeruli was measured using Measurement module/Dynamic Cell Count Software (Keyence).
Isolation of Glomeruli
The glomeruli were isolated by taking advantage of their preferential uptake of Dynabeads (M-450 Tosylactivated; Life Technologies Japan, Tokyo, Japan) as described previously17 and processed for RNA extraction, measurements of superoxide levels, and NAD(P)H oxidase activity.
Analysis of Superoxide Production
Superoxide production in glomeruli was detected using 2′,7′-dichlorofluorescein (DCF).18 The isolated glomeruli from each group of mice were incubated for 10 min with RPMI-1640 containing 20 μmol/l DCF diacetate (Molecular Probes, Eugene, OR, USA). Images were obtained using a confocal laser microscope (Leica Microsystems, Tokyo, Japan) at excitation/emission wavelengths of 485/535 nm for DCF. The fluorescence intensity values of 20 different glomeruli isolated from each group were calculated using Leica TCS-NT software (Leica Microsystems), and the mean values are presented.19
Real-Time Quantitative PCR
Total RNA was isolated from the glomeruli using TRIzol (Life Technologies Japan). Reverse transcriptase reactions were performed using a Ready-To-Go T-Primed First-Strand Kit (GE Healthcare Japan, Tokyo, Japan) for first-strand cDNA synthesis. Real-time quantitative PCR was performed using the ABI Prism 7700 sequence detection system (Life Technologies Japan). Data were expressed as copy number relative to that of 18 S rRNA. Primers and probes for TaqMan analysis of human NOX2, mouse Nox2, mouse Nox4, mouse P22, mouse P47, and mouse P67 are included in Supplementary Table S1. TaqMan probes consist of a fluorophore 6-carboxyfluorescein covalently attached to the 5′ end of the oligonucleotide probe and a quencher tetramethylrhodamine at the 3′ end.
Lucigenin Chemiluminescence Assay of NAD(P)H Oxidase Activity
NAD(P)H oxidase activity of glomeruli was measured using lucigenin chemiluminescence (units/min/mg) as described previously.16
Scanning and Transmission Electron Microscopy
For transmission electron microscopy, small fragments of the left kidneys were fixed by immersion fixation in 2.5% glutaraldehyde in 0.1 M cacodylate buffer (pH 7.4) for 3 h. Afterwards, kidneys were treated with 1% OsO4 in 0.1 M cacodylate buffer for 1.5 h, dehydrated with graded ethanol solutions, and embedded in Spurr’s low-viscosity resin (Electron Microscopy Sciences, Hatfield, PA, USA). Semithin sections were stained with toluidine blue stain. Tissues were sectioned using an Ultracut UCT Microtome (Leica Microsystems) equipped with a diamond knife, mounted on copper grids coated with a formvar film, and stained with uranyl acetate and lead citrate. Ultrathin sections were analyzed on a transmission electron microscope (Hitachi H-7100 Electron Microscope; Hitachi, Tokyo, Japan).
For scanning electron microscopy, kidney samples were fixed by immersion in 2.5% glutaraldehyde in 0.1 M cacodylate buffer (pH 7.4) for 3 h. After treatment with 1% OsO4 in 0.1 M cacodylate buffer for 1.5 h, the samples were dehydrated with graded ethanol solutions and immersed in isoamyl acetate solution for 30 min. The kidney samples were dehydrated by critical-point drying with liquefied carbon dioxide. Dried samples were sputter-coated with platinum and examined with a scanning electron microscope (Hitachi S-3400 N electron microscope; Hitachi).
In Vivo Imaging of Macromolecule Filtration
A Laser Scanning Spectral Confocal Microscope (TCS SP2 AOBS MP; Leica Microsystems) was used for in vivo imaging of glomerular microcirculation as described previously.20 For analysis of glomerular permeability, a catheter was used to infuse 70-kDa fluorescein isothiocyanate (FITC)-conjugated dextran (anionic; excitation 494 nm, emission 518 nm; Molecular Probes) through the jugular vein.
Statistical Analysis
Data are expressed as mean±s.e.m. Differences between groups were assessed for statistical significance using a two-tailed unpaired Student's t-test for paired data or one-way analysis of variance for multiple groups. Statistical significance was defined as P<0.05.
RESULTS
Physiological Characterization of Mice
The mean blood glucose concentration of the Akita group was significantly higher compared with that of the WT group and was similar to that of the NOX2TG-Akita group (Table 1). There was no significant difference in mean blood pressure among the groups (Table 2). The NOX2TG-Akita and Akita groups developed albuminuria at 6 and 10 weeks of age, respectively (Figure 1). The NOX2TG groups showed no difference with WT group in blood glucose, blood pressure, and albuminuria.
Analysis of urinary albumin excretion of wild-type mice (WT, open circles), endothelial dominant Nox2 transgenic mice (NOX2TG; open squares), heterozygous Akita mice (Ins2Akita/Ins2+, black solid circles), and heterozygous Akita mice (Ins2Akita/Ins2+) expressing a Nox2 transgene (NOX2TG-Akita, black solid squares), 10 mice per group. *P<0.05 versus WT group; †P<0.05 versus Akita group.
NAD(P)H Oxidase Expression and Activity
The expression of human NOX2 mRNA was detected in the glomeruli of only the NOXTG-Akita group only (Supplementary Figure S3a), whereas the mean expression level of mouse Nox2 mRNA did not differ among the groups (Supplementary Figure S3b). Expression of mRNAs encoding NAD(P)H oxidase components did not differ between the Akita and NOX2TG-Akita groups (Supplementary Figures S3c, e, and f), except for that of p22phox, which was increased in the Akita and NOX2TG-Akita groups compared with that of the WT group (Supplementary Figure S3d). The highest level of NAD(P)H oxidase activity was detected in the glomeruli of the NOX2TG-Akita group followed in decreasing order by the Akita and WT groups (Supplementary Figure S3g).
Glomerular Production of Superoxide
The production of superoxide in glomeruli was determined by staining isolated glomeruli of mice of each group at 6 weeks of age (Figure 2a). The mean fluorescence intensity of the isolated glomeruli of the NOX2TG-Akita group was significantly higher compared with that of the Akita group (Figure 2b).
Glomerular superoxide production and morphological changes in glomeruli of mice from various strains at 6 weeks of age. (a) Reactive oxygen species (ROS) detected by staining with 2′,7′-dichlorofluorescein (DCF) in isolated glomeruli. Magnification × 400. (b) Intensity of DCF staining relative to WT, 10 mice per group. Twenty glomeruli from each mouse were evaluated to calculate the mean intensity. *P<0.05 versus WT group; †P<0.05 versus Akita group. (c) Periodic acid-Schiff (PAS) staining images of glomeruli. Magnification × 400. (d) Glomerular matrix score, 10 mice per group. Twenty glomeruli from each mouse were evaluated to calculate the mean score was calculated. *P<0.05 versus WT group; †P<0.05 versus Akita group. NOX2TG, endothelial dominant Nox2 transgenic mice; NOX2TG-Akita, heterozygous Akita mice (Ins2Akita/Ins2+) expressing a Nox2 transgene; WT, wild-type mice.
Morphology of Glomeruli
PAS staining did not reveal morphological differences between the glomeruli of the WT and NOXTG groups. Akita group showed a slight increase in mesangial matrix deposition (Figure 2c). Mild diffuse expansion of mesangium and a mild increase in mesangial cellularity were observed in NOX2TG-Akita group. The PAS-positive area (mean matrix score) was significantly larger in the Akita and NOX2TG-Akita groups compared with that of the WT group (Figures 2c and d), and the mean matrix score of the NOX2TG-Akita group was significantly higher compared with that of the Akita group.
Characteristics of Glomerular Endothelial Cells
The induction of PV-1 expression coincided with the development of glomerular capillary perturbations and endothelial structural damage.21 PV-1 was detected in the glomeruli of only the NOX2TG-Akita group at 6 weeks of age (Figures 3a and b). The glomerular glycocalyx, which is a main component of endothelial surface layer (ESL), was evaluated by lectin staining. We used tomato lectin to label N-acetyl glucosamine, which residues are present in the surface-bound glycocalyx region.22 Tomato lectin was detected in the glomeruli of the Akita group (Figure 3c), and the area stained by tomato lectin on the capillary wall was significantly smaller in the NOX2TG-Akita group compared with that of the Akita group (Figure 3d). Figures 3e and f shows representative scanning electron micrographs and transmission electron micrographs of glomerular capillary endothelia, respectively. The Akita group showed endothelial cell activation in the form of an increased number of slightly swollen blebs; however, most of the capillary fenestrae and the ridges remained intact (Figure 3f, upper). In contrast, the NOX2TG-Akita group showed more exuberant activation of endothelial cells, as reflected by the appearance of numerous large numbers of swollen blebs, microparticles and the partial effacement of capillary fenestrae and ridges (Figure 3f, lower).
Evaluation of glomerular endothelial injury and morphological changes in mice from various groups at 6 weeks of age. (a) Immunohistochemical analysis of plasmalemmal vesicle-1 (PV-1), a marker of glomerular capillary remodeling. Magnification × 400. (b) PV-1 staining score, 10 mice per group. Ten glomeruli from each mouse were evaluated to calculate the mean score. *P<0.05 versus WT group; †P<0.05 versus Akita group. (c) Assessment of glycocalyx of the endothelial surface layer detected by using tomato lectin staining. Magnification × 400. (d) Lectin-positive areas compared with WT, 10 mice per group. Ten glomeruli from each mouse were evaluated to calculate the mean area. *P<0.05 versus WT group; †P<0.05 versus Akita group. (e) Scanning electron micrographs of activated and swollen endothelial cells (arrows) of NOX2TG-Akita mice. Note the partial occlusion and disappearance of fenestrae (arrowheads). Scale bar=1.0 μm. (f) Transmission electron micrographs of glomerular capillary endothelia. Note the partial disappearance of fenestrae in NOX2TG-Akita mice (arrowheads). Scale bar=1.0 μm. ND, not detected; NOX2TG, endothelial dominant Nox2 transgenic mice; NOX2TG-Akita, heterozygous Akita mice (Ins2Akita/Ins2+) expressing a Nox2 transgene; WT, wild type.
Glomerular Filtration
Glomerular filtration was determined using intravenous injection of 70-kDa FITC-conjugated dextran11, 20 that is poorly filtered by ESL-preserved endothelium. Leakage of the FITC-labeled dextran into the Bowman’s capsule space was undetectable in WT, whereas it was slight and massive in the Akita and NOX2TG-Akita groups, respectively (Figure 4 and Supplementary Movies S1). The leakage was readily appreciated in Supplementary Movies S1, suggesting attenuation of ESL.
Mesangial Cells
Mesangial cells in diverse glomerular diseases become myofibroblast-like, characterized by activation of α-SMA expression.23 Increased α-SMA expression in mesangial cells reflects cellular hypertrophy.24 We analyzed the expression pattern of α-SMA to assess the phenotypic changes and hypertrophy in mesangial cells (Figure 5a). Immunostaining of α-SMA showed that it was distributed over the largest area of the glomeruli of the NOX2TG-Akita group compared with those of the other three groups (Figure 5b). The greatest expansion of the mesangial area occurred in the NOX2T-Akita group (Figure 5c).
Analyses of mesangial lesions in various groups of mice at 6 weeks of age. (a) Immunohistochemical analysis of α-smooth muscle actin (α-SMA) expression in glomeruli. Magnification × 400. (b) α-SMA staining score, 10 mice per group. Ten glomeruli from each mouse were evaluated to calculate the mean score. *P<0.05 versus WT group; †P<0.05 versus Akita group. (c) Electron micrographs of representative glomeruli from Akita and NOX2TG-Akita mice showing notable expansion of the mesangium in the latter group (arrows). Swollen endothelial cells are indicated by the arrowheads. Scale bar=10 μm. NOX2TG, endothelial dominant Nox2 transgenic mice; NOX2TG-Akita, heterozygous Akita mice (Ins2Akita/Ins2+) expressing a Nox2 transgene; WT, wild type.
Podocytes
Immunohistochemical analysis detected desmin, a marker of podocyte damage,25 on the capillaries of the NOX2TG-Akita group (Figure 6a) and occupied an area greater than that of the Akita group (Figures 6a and b). Scanning electron microscopy revealed that podocytes were swollen and showed increased microvilli formation in the NOX2TG-Akita group (Figure 6c). Immunohistochemical analyses revealed that podocin, a slit membrane protein, was present in the capillaries of each group (Supplementary Figure S4a), and there were no significant differences for the area occupied by podocin among the four groups (Supplementary Figure S4b). Interestingly, transmission electron micrographs of the glomerular capillary walls did not reveal podocyte foot-process effacement in the Akita and NOX2T-Akita groups (Supplementary Figure S4c).
Analysis of podocyte injury and morphological changes in mice at 6 weeks of age. (a) Immunohistochemical analysis of desmin expression in glomeruli, which is tremendously increased in NOX2TG-Akita mice group. Magnification × 400. (b) Desmin staining score, 10 mice per group. Ten glomeruli from each mouse were evaluated to calculate the mean score. *P<0.05 versus WT group; †P<0.05 versus Akita group. (c) Electron micrographs depicting ultrastructural changes in podocytes of Akita and NOX2TG-Akita mice. Numerous microvilli-like structures in the podocytes are readily seen (arrows). Scale bar=5.0 μm. NOX2TG, endothelial dominant Nox2 transgenic mice; NOX2TG-Akita, heterozygous Akita mice (Ins2Akita/Ins2+) expressing a Nox2 transgene; WT, wild type.
Inhibiting NOX2 Activity Repairs the Glycocalyx of NOX2TG-Akita Mice
Treatment of NOX2TG-Akita mice at 6–8 weeks of age with the NOX2-specific inhibitor gp91TAT led to recovery of tomato lectin staining of the glomeruli and it was similar to that of WT mice (Figures 7a and b). The staining was not detected in untreated mice. The recovery of lectin staining of glomeruli was associated with a significant reduction in the level of albuminuria in NOX2TG-Akita mice, although blood glucose or blood pressure were not altered (Table 3).
Analysis of the glomerular glycocalyx and urinary albumin excretion in mice NOX2TG-Akita treated with the NOX2 inhibitor gp91TAT. (a) Glycocalyx was detected using tomato lectin. Magnification × 400. (b) Relative lectin-positive area to WT. Treatment with gp91TAT led to the recovery of the lectin-stained area, 10 mice per group. Ten glomeruli from each mouse were evaluated to calculate the mean area. (c) Urinary albumin excretion. Cre, urinary creatinine. *P<0.05 versus WT group; †P<0.05. NOX2TG, endothelial dominant Nox2 transgenic mice; NOX2TG-Akita, heterozygous Akita mice (Ins2Akita/Ins2+) expressing a Nox2 transgene; WT, wild type.
DISCUSSION
The data presented here support the hypothesis that enhanced endothelial oxidative stress promotes the onset and progression of diabetic nephropathy in mice. Albuminuria appeared in mice 6 and 10 weeks of age of the NOX2TG-Akita and Akita groups, respectively. Moreover, the level of ROS generated by NAD(P)H oxidase increased in NOX2TG-Akita mice compared with Akita mice, and caused glomerular microabnormalities. In endothelial cells, decreases in the thickness of the ESL, which apparently has a role in glomerular permeability, and it was greater in NOX2TG-Akita mice. Podocyte activation and mesangial cell matrix expansion were increased in NOX2TG-Akita mice, consistent with increased oxidative stress in endothelial cells. These results indicate that activation of endothelial NAD(P)H oxidase, in particular, accelerated the development of diabetic nephropathy.
Vascular NAD(P)H oxidase, mitochondrial dysfunction, and uncoupled eNOS all the three contribute to ROS generation in diabetes as well as in its microvascular complications, for example, diabetic nephropathy. For example, Gorin et al.26 detected an increase in NAD(P)H-dependent ROS generation in the kidney cortex and isolated glomeruli of diabetic rats. In rats with diabetes induced by streptozotocin (STZ), administration of the NAD(P)H oxidase inhibitor apocynin leads to a reduction in renal ROS and may have a potent therapeutic effect on diabetic nephropathy.27 Deletion of p47 phox28 or NOX4 (ref. 29) attenuates the progression of diabetic nephropathy. Similarly, we reported that NAD(P)H oxidase activity and uncoupling of eNOS contribute to ROS production in the glomeruli of rats with diabetic nephropathy.10, 30, 31 Prolonged NAD(P)H oxidase activation may lead to depletion of intracellular NAD(P)H and impaired ROS scavenging associated with eNOS uncoupling, mitochondrial dysfunction, and diminished defense against antioxidants.32 Therefore, prolonged ROS production by endothelial cells may participate in the onset and progression of diabetic nephropathy.
Enhanced production of endothelial ROS and high glucose concentration induce several structural changes in endothelial cells.33, 34 Endothelial dysfunction is associated with the expression of PV-1, a marker of endothelial structural damage and remodeling.21 PV-1 is not expressed in the mature glomerular endothelial cells but is re-expressed in Thy1.1 nephritis, presumably reflecting a process of restorative remodeling of the glomerular capillary tuft after injury.21 Although the exact functions of PV-1 are not clear, evidence suggests that it is involved in the control of capillary permeability by acting on the capillary wall35 and may have a role in the development of diabetic nephropathy and albuminuria. In the present study, we detected significantly higher levels of PV-1 on the endothelial cells of NOX2TG-Akita mice compared with those of Akita, NOXT2G, and WT mice.
Consistent with these findings are our observations of hypertrophy and activation of endothelial cells in NOX2TG-Akita mice. Blebs were formed on the membranes of activated or injured endothelial cells, which has been also described to be associated with an increased number of microparticles released into the blood stream.36 Further, endothelial microparticles from glucose-treated endothelial cells have been reported to increase vascular inflammation and impair endothelial functions by promoting the activation of endothelium.37 These findings reinforce the idea that the generation of ROS by NAD(P)H oxidase activates the endothelium.
The glomerular endothelium is coated with a polysaccharide-rich layer composed primarily of glycocalyx and a loosely attached endothelial cell coat of secreted proteoglycans, glycosaminoglycans, glycoproteins, and plasma proteins, and it is referred to as the ESL.22, 38, 39 Reduced thickness of the ESL is associated with increased vascular permeability.40 We reported previously that ROS-induced glomerular ESL perturbation coincides with increased glomerular vascular permeability and albuminuria in a rat model of metabolic syndrome.11 Here, we demonstrate that in NOXTG-Akita mice activation of endothelial NAD(P)H oxidase enhanced the reduction of the ESL while increasing the glomerular capillary permeability.
Excessive ROS production activates the transcription of heparanase via nuclear translocation of E-twenty six transcription factor.41 Heparanase has been shown to have a role in the development of proteinuria through the degradation of endothelial glycocalyx enriched with heparan sulfate proteoglycans.37 Therefore, the reduction of endothelial ROS may mitigate the pathophysiological changes observed in diabetic nephropathy through the downregulation of heparanase transcription. In fact, our data show that an NOX2 inhibitor has therapeutic potential, which may have apparently prevented the reduction of glycocalyx on glomerular capillary in rats with experimental diabetes.
In addition to endothelial cell injury, we also show here that overproduction of endothelial ROS-induced mesangial injury in Akita mice with diabetes. Endothelial cells are located adjacent to mesangial cells, and they seem to have direct contact with one another.42, 43 Proliferation of mesangial cells and excessive production of extracellular matrix by mesangial cells are typical findings in the progression of glomerular injury,44 and mesangial cells undergo phenotypic changes in patients with diabetic nephropathy and in rats with STZ-induced diabetes.45 Such changes may be partly associated with the activation of endothelial NAD(P)H oxidase and accumulation of ROS. R ROS generated by NAD(P)H oxidases downregulate subfamily C member of transient receptor potential cation channel in mesangial cells of rats with STZ-induced diabetes.46 This may explain the alterations in mesangial contractile functions observed in diabetic nephropathy. Thus, NAD(P)H oxidase activation associated with endothelial dysfunction may also lead to induction of phenotypic changes in mesangial cells in the early stages of diabetic nephropathy.
Besides glomerular endothelial and mesangial changes, we also demonstrate that enhanced endothelial ROS production induced podocyte injury in Akita mice with diabetes. In diabetic kidney disease, podocytes and glomerular endothelial cells appear to participate in the initiation and progression of nephropathy.47, 48, 49, 50 Integrated functions of glomerular endothelial cells and podocytes are essential to maintain normal homeostasis of glomerular capillaries.51 Podocytes maintain the structure of glomerular endothelial cells via the production of vascular endothelial growth factor (VEGF).52 Moreover, the structure of glomerular endothelial cells is abnormal in podocyte-specific VEGF-deficient mice.53 In humans with diabetic nephropathy, podocyte loss is associated with reduced endothelial cell fenestration.47, 48 In contrast, diabetic mice with a genetic deficiency of eNOS develop podocyte-specific injury and heavy albuminuria,54 and glomerular endothelial mitochondrial oxidative stress determines podocyte depletion in segmental glomerulosclerosis in transgenic mice.55 In view of the above considerations it seems that podocyte–endothelial crosstalk presents new therapeutic opportunities, including the use of endothelial ROS scavengers for the amelioration of diabetic nephropathy.
The observations in this study support the notion that increased endothelial NOX2 sensitizes the kidney to diabetic injury because accelerated renal injury is detected as early as 6 weeks of age in NOX2TG-Akita mice. We also investigated whether endothelial NOX2 transgenic mice showed early progression to advanced diabetic nephropathy. We followed Akita and NOX2TG-Akita mice until 20 weeks. However, the pathological differences between two groups unfortunately disappeared (data not shown). The NOX2 expression was almost same in both Akita and NOX2TG-Akita mice at 20 weeks. High glucose levels in Akita mice might induce the elevation of endothelial Nox2 expression and result in the same degree of diabetic nephropathy lesion. Hence, we cannot make a conclusion on the role of Nox2 in the late phase of diabetic nephropathy, but we believe that our data are important to understand diabetic nephropathy onset because the mechanism of the initiation of diabetic nephropathy has also still been unclear.
In conclusion, it seems that activation of endothelial NAD(P)H oxidase accelerates the initiation as well as the progression of diabetic nephropathy. The present findings highlight the importance of the interactions among endothelial cells, podocytes, and mesangial cells and suggest their importance in the modulation of normal and abnormal endothelial functions. Overall, it is safe to conclude that endothelial dysfunctions associated with increased endothelial ROS production by NAD(P)H oxidase is an important step in the pathogenesis of diabetic nephropathy and this may serve as a potential therapeutic target for the onset of diabetic nephropathy.
References
Alebiosu CO, Ayodele OE . The global burden of chronic kidney disease and the way forward. Ethn Dis 2005;15;418–423.
Krolewski AS, Warram JH, Rand LI et al. Epidemiologic approach to the etiology of type I diabetes mellitus and its complications. N Engl J Med 1987;317;1390–1398.
Maeda S, Kobayashi MA, Araki S et al. A single nucleotide polymorphism within the acetyl-coenzyme A carboxylase beta gene is associated with proteinuria in patients with type 2 diabetes. PLoS Genet 2010;6;e1000842.
Hamada Y, Miyata S, Nii-Kono T et al. Overexpression of thioredoxin1 in transgenic mice suppresses development of diabetic nephropathy. Nephrol Dial Transplant 2007;22;1547–1557.
Raj DS, Choudhury D, Welbourne TC et al. Advanced glycation end products: a Nephrologist's perspective. Am J Kidney Dis 2000;35;365–380.
Qian Y, Feldman E, Pennathur S et al. From fibrosis to sclerosis: mechanisms of glomerulosclerosis in diabetic nephropathy. Diabetes 2008;57;1439–1445.
Avogaro A, Albiero M, Menegazzo L et al. Endothelial dysfunction in diabetes: the role of reparatory mechanisms. Diabetes Care 2011;34;S285–S290.
Hirano T, Ookubo K, Kashiwazaki K et al. Vascular endothelial markers, von Willebrand factor and thrombomodulin index, are specifically elevated in type 2 diabetic patients with nephropathy: comparison of primary renal disease. Clin Chim Acta 2000;299;65–75.
Schaffer SW, Jong CJ, Mozaffari M . Role of oxidative stress in diabetes-mediated vascular dysfunction: unifying hypothesis of diabetes revisited. Vascul Pharmacol 2012;57;139–149.
Satoh M, Fujimoto S, Haruna Y et al. NAD(P)H oxidase and uncoupled nitric oxide synthase are major sources of glomerular superoxide in rats with experimental diabetic nephropathy. Am J Physiol Renal Physiol 2005;288;F1144–F1152.
Kuwabara A, Satoh M, Tomita N et al. Deterioration of glomerular endothelial surface layer induced by oxidative stress is implicated in altered permeability of macromolecules in Zucker fatty rats. Diabetologia 2010;53;2056–2065.
Bendall JK, Rinze R, Adlam D et al. Endothelial Nox2 overexpression potentiates vascular oxidative stress and hemodynamic response to angiotensin II: studies in endothelial-targeted Nox2 transgenic mice. Circ Res 2007;100;1016–1025.
Yoshioka M, Kayo T, Ikeda T et al. A novel locus, Mody4, distal to D7Mit189 on chromosome 7 determines early-onset NIDDM in nonobese C57BL/6 (Akita) mutant mice. Diabetes 1997;46;887–894.
Nagasu H, Satoh M, Kuwabara A et al. Overexpression of klotho protein modulates uninephrectomy-induced compensatory renal hypertrophy by suppressing IGF-I signals. Biochem Biophys Res Commun 2011;407;39–43.
Rey FE, Cifuentes ME, Kiarash A et al. Novel competitive inhibitor of NAD(P)H oxidase assembly attenuates vascular O(2)(-) and systolic blood pressure in mice. Circ Res 2001;89;408–414.
Nagasu H, Satoh M, Yorimitsu D et al. Comparison of combination therapy of olmesartan plus azelnidipine or hydrochlorothiazide on renal and vascular damage in SHR/NDmcr-cp rats. Kidney Blood Press Res 2011;34;87–96.
Takemoto M, Asker N, Gerhardt H et al. A new method for large scale isolation of kidney glomeruli from mice. Am J Pathol 2002;161;799–805.
Koya D, Hayashi K, Kitada M et al. Effects of antioxidants in diabetes-induced oxidative stress in the glomeruli of diabetic rats. J Am Soc Nephrol 2003;14;S250–S253.
Nagasu H, Satoh M, Kuwabara A et al. Renal denervation reduces glomerular injury by suppressing NAD(P)H oxidase activity in Dahl salt-sensitive rats. Nephrol Dial Transplant 2010;25;2889–2898.
Satoh M, Kobayashi S, Kuwabara A et al. In vivo visualization of glomerular microcirculation and hyperfiltration in streptozotocin-induced diabetic rats. Microcirculation 2010;17;103–112.
Ichimura K, Stan RV, Kurihara H et al. Glomerular endothelial cells form diaphragms during development and pathologic conditions. J Am Soc Nephrol 2008;19;1463–1471.
Haraldsson B, Nystrom J, Deen WM . Properties of the glomerular barrier and mechanisms of proteinuria. Physiol Rev 2008;88;451–487.
Patel K, Harding P, Haney LB et al. Regulation of the mesangial cell myofibroblast phenotype by actin polymerization. J Cell Physiol 2003;195;435–445.
Stephenson LA, Haney LB, Hussaini IM et al. Regulation of smooth muscle alpha-actin expression and hypertrophy in cultured mesangial cells. Kidney Int 1998;54;1175–1187.
Hoshi S, Shu Y, Yoshida F et al. Podocyte injury promotes progressive nephropathy in zucker diabetic fatty rats. Lab Invest 2002;82;25–35.
Gorin Y, Block K, Hernandez J et al. Nox4 NAD(P)H oxidase mediates hypertrophy and fibronectin expression in the diabetic kidney. J Biol Chem 2005;280;39616–39626.
Asaba K, Tojo A, Onozato ML et al. Effects of NADPH oxidase inhibitor in diabetic nephropathy. Kidney Int 2005;67;1890–1898.
Liu GC, Fang F, Zhou J et al. Deletion of p47phox attenuates the progression of diabetic nephropathy and reduces the severity of diabetes in the Akita mouse. Diabetologia 2012;55;2522–2532.
Jha JC, Gray SP, Barit D et al. Genetic targeting or pharmacologic inhibition of NADPH oxidase nox4 provides renoprotection in long-term diabetic nephropathy. J Am Soc Nephrol 2014;25;1237–1254.
Satoh M, Fujimoto S, Arakawa S et al. Angiotensin II type 1 receptor blocker ameliorates uncoupled endothelial nitric oxide synthase in rats with experimental diabetic nephropathy. Nephrol Dial Transplant 2008;23;3806–3813.
Kobayashi S, Satoh M, Namikoshi T et al. Blockade of serotonin 2 A receptor improves glomerular endothelial function in rats with streptozotocin-induced diabetic nephropathy. Clin Exp Nephrol 2008;12;119–125.
Gao L, Mann GE . Vascular NAD(P)H oxidase activation in diabetes: a double-edged sword in redox signalling. Cardiovasc Res 2009;82;9–20.
Simionescu M . Implications of early structural–functional changes in the endothelium for vascular disease. Arterioscler Thromb Vasc Biol 2007;27;266–274.
Popov D, Simionescu M . Cellular mechanisms and signalling pathways activated by high glucose and AGE-albumin in the aortic endothelium. Arch Physiol Biochem 2006;112;265–273.
Bodor C, Nagy JP, Vegh B et al. Angiotensin II increases the permeability and PV-1 expression of endothelial cells. Am J Physiol Cell Physiol 2012;302;C267–C276.
Mallat Z, Hugel B, Ohan J et al. Shed membrane microparticles with procoagulant potential in human atherosclerotic plaques: a role for apoptosis in plaque thrombogenicity. Circulation 1999;99;348–353.
Jansen F, Yang X, Franklin BS et al. High glucose condition increases NADPH oxidase activity in endothelial microparticles that promote vascular inflammation. Cardiovasc Res 2013;98;94–106.
Salmon AH, Ferguson JK, Burford JL et al. Loss of the endothelial glycocalyx links albuminuria and vascular dysfunction. J Am Soc Nephrol 2012;23;1339–1350.
Fu J, Lee K, Chuang PY et al. Glomerular endothelial cell injury and crosstalk in diabetic kidney disease. Am J Physiol Renal Physiol 2014;308;F287–F297.
Friden V, Oveland E, Tenstad O et al. The glomerular endothelial cell coat is essential for glomerular filtration. Kidney Int 2011;79;1322–1330.
Lu WC, Liu YN, Kang BB et al. Trans-activation of heparanase promoter by ETS transcription factors. Oncogene 2003;22;919–923.
Singh AK, Gudehithlu KP, Pegoraro AA et al. Vascular factors altered in glucose-treated mesangial cells and diabetic glomeruli. Changes in vascular factors impair endothelial cell growth and matrix. Lab Invest 2004;84;597–606.
Kitahara T, Hiromura K, Ikeuchi H et al. Mesangial cells stimulate differentiation of endothelial cells to form capillary-like networks in a three-dimensional culture system. Nephrol Dial Transplant 2005;20;42–49.
Mao Y, Ootaka T, Saito T et al. The involvement of advanced glycation endproducts (AGEs) in renal injury of diabetic glomerulosclerosis: association with phenotypic change in renal cells and infiltration of immune cells. Clin Exp Nephrol 2003;7;201–209.
Makino H, Kashihara N, Sugiyama H et al. Phenotypic modulation of the mesangium reflected by contractile proteins in diabetes. Diabetes 1996;45;488–495.
Graham S, Ding M, Sours-Brothers S et al. Downregulation of TRPC6 protein expression by high glucose, a possible mechanism for the impaired Ca2+ signaling in glomerular mesangial cells in diabetes. Am J Physiol Renal Physiol 2007;293;F1381–F1390.
Weil EJ, Lemley KV, Mason CC et al. Podocyte detachment and reduced glomerular capillary endothelial fenestration promote kidney disease in type 2 diabetic nephropathy. Kidney Int 2012;82;1010–1017.
Toyoda M, Najafian B, Kim Y et al. Podocyte detachment and reduced glomerular capillary endothelial fenestration in human type 1 diabetic nephropathy. Diabetes 2007;56;2155–2160.
Lindenmeyer MT, Kretzler M, Boucherot A et al. Interstitial vascular rarefaction and reduced VEGF-A expression in human diabetic nephropathy. J Am Soc Nephrol 2007;18;1765–1776.
Siddiqi FS, Advani A . Endothelial–podocyte crosstalk: the missing link between endothelial dysfunction and albuminuria in diabetes. Diabetes 2013;62;3647–3655.
Kanwar YS . Continuum of historical controversies regarding structural–functional relationship of the glomerular ultrafiltration unit (GUU). Am J Physiol Renal Physiol 2014;308;F420–F424.
Eremina V, Cui S, Gerber H et al. Vascular endothelial growth factor a signaling in the podocyte–endothelial compartment is required for mesangial cell migration and survival. J Am Soc Nephrol 2006;17;724–735.
Sison K, Eremina V, Baelde H et al. Glomerular structure and function require paracrine, not autocrine, VEGF-VEGFR-2 signaling. J Am Soc Nephrol 2010;21;1691–1701.
Yuen DA, Stead BE, Zhang Y et al. eNOS deficiency predisposes podocytes to injury in diabetes. J Am Soc Nephrol 2012;23;1810–1823.
Daehn I, Casalena G, Zhang T et al. Endothelial mitochondrial oxidative stress determines podocyte depletion in segmental glomerulosclerosis. J Clin Invest 2014;124;1608–1621.
Acknowledgements
We thank Etsuko Yorimasa and Miki Ishihara for providing animal care and Satomi Hanada and Keiko Satoh for assistance with the in vitro assays. This work was supported, in part, by Grants-in Aid for Scientific Research from the Japan Society of the Promotion of Science (No. 24591220 to MS, Nos 21591047 and 24390218 to NK), the Uehara Memorial Foundation (to NK), the National Institutes of Health grant (DK60635 to YSK) and by a Research Project Grant from Kawasaki Medical School (No. 23B-41 to NK). HN thanks the Japanese Society of Nephrology for a travel grant. Parts of this work were presented at ASN Renal Week 2011, Philadelphia, PA, USA.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Laboratory Investigation website
Activation of endothelial NAD(P)H oxidase in a hyperglycemic milieu initiates and worsens diabetic nephropathy. Superoxide generated in the endothelium compounds glomerular dysfunction by altering the phenotypes of mesangial cells and compromising the integrity of the podocytes. This enzyme may therefore serve as a potential therapeutic target for diabetic nephropathy.
Supplementary information
Rights and permissions
About this article
Cite this article
Nagasu, H., Satoh, M., Kiyokage, E. et al. Activation of endothelial NAD(P)H oxidase accelerates early glomerular injury in diabetic mice. Lab Invest 96, 25–36 (2016). https://doi.org/10.1038/labinvest.2015.128
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/labinvest.2015.128
This article is cited by
-
Importance of wnt-catenin signaling in hypertensive kidney diseases
Hypertension Research (2021)
-
Comprehensive renoprotective effects of ipragliflozin on early diabetic nephropathy in mice
Scientific Reports (2018)
-
APX-115, a first-in-class pan-NADPH oxidase (Nox) inhibitor, protects db/db mice from renal injury
Laboratory Investigation (2017)