Abstract
Interleukin (IL)-18 is a proinflammatory cytokine produced by leukocytes and parenchymal cells (eg, tubular epithelial cells (TECs), mesangial cells, and podocytes). IL-18 receptor (IL-18R) is expressed on these cells in the kidney during ischemia/reperfusion injury (IRI), but its role in this injury is unknown. Fas/Fas ligand (FasL) is also involved in the pathogenesis of renal IRI via tubular apoptosis. In addition, IL-18 enhances the expression of FasL on TECs, but the mechanism underlying this enhancement is not known. Here we used IL-18Rα-deficient mice to explore the pathological role of IL-18Rα in renal IRI. We found that compared to wild-type (WT) mice with renal IRI as an acute kidney injury (AKI), the IL-18Rα-deficient mice demonstrated decreased renal function (as represented by blood urea nitrogen), tubular damage, an increased accumulation of leukocytes (CD4+ T cells, neutrophils, and macrophages), upregulated early AKI biomarkers (ie, urinary kidney injury molecule-1 levels), and increased mRNA expressions of proinflammatory cytokines (IL-1β, IL-12p40, and IL-18) and chemokines (intercellular adhesion molecule-1 and CCL2/monocyte chemoattractant protein-1). The mRNA expression of FasL in the kidney was increased in the IL-18Rα-deficient mice compared to the WT mice. The adoptive transfer of splenocytes by WT mice led to decreased renal IRI compared to the IL-18Rα-deficient mice. In vitro, the mRNA expression of FasL on TECs was promoted in the presence of recombinant IL-18. These data reveal that IL-18Rα has an anti-inflammatory effect in IRI-induced AKI. Above all, IL-18 enhanced the inflammatory mechanisms and the apoptosis of TECs through the Fas/FasL pathway by blocking IL-18Rα.
Similar content being viewed by others
Main
Interleukin (IL)-18 is a proinflammatory mediator that is structurally and functionally related to the IL-1 family, and IL-18 has been implicated in the pathophysiology of a variety of renal inflammatory diseases including ischemia/reperfusion injury (IRI), allograft rejection, autoimmune disease, and, most recently, obstructive nephropathy.1 IL-18 is secreted by antigen-presenting cells (APCs) and signals through the IL-18 receptor (IL-18R), a heterodimer consisting of a ligand-binding IL-18Rα subunit and a signaling IL-18Rβ subunit (also called IL-1RAcPL and IL-1R7). IL-18R is expressed on lymphocytes as well as on accessory cells.2 Although it is firmly established that IL-18 can bind to the IL-18R complex, its affinity for IL-18Rα is weak.3 Natural killer cells and T cells in synergy with IL-12 are necessary to stimulate the production of interferon-gamma (IFN-γ) by IL-18.4 In T cells, IL-18R expression is upregulated by IL-12,5 whereas IL-18 augments the expression of IL-12R.6 Given the protective activity of IL-12, IFN-γ, and tumor necrosis factor (TNF), we therefore sought to identify the functions of IL-18 and IL-18Rα in IRI.
IL-18 is an early and sensitive marker of renal tubular damage,7 and in animal models of renal IRI, IL-18 exacerbated acute tubular necrosis (ATN).8 Several reports indicate that Fas/Fas ligand (FasL) is involved in the pathogenesis of acute ischemic kidney injury via tubular apoptosis and necrosis.9, 10 FasL is a member of the TNF family that induces apoptosis by cross-linking its Fas receptor.11 FasL is believed to have a role in inflammation through the recruitment of inflammatory cells12, 13 and the induction of cytokine production.14 FasL is frequently expressed on lymphocytes and is critical for regulating T-cell homeostasis.15 In the kidney, FasL is frequently expressed on different cell types including mesangial cells, tubular epithelial cells (TECs), fibroblasts, and endothelial cells. In a recent study using HK-2 cells, IL-18 accelerated the expression of FasL on TECs and promoted apoptosis.16
In the present study, we found that IL-18Rα-deficient mice had markedly deteriorated renal function and increased histological injury when they were exposed to an IRI. Our findings revealed that the renal status was deteriorated in the IL-18Rα-deficient mice by the upregulation of proinflammatory cytokines (ie, IL-1β, IL-18, and TNF) and chemokines (ie, intercellular adhesion molecule (ICAM)-1, regulated upon activation, normally T-expressed, and presumably secreted (RANTES), and CCL2/monocyte chemoattractant protein-1 (MCP-1)) compared to wild-type (WT) mice after renal IRI. The expression of FasL was increased in the IL-18Rα-deficient mice and enhanced by recombinant IL-18 in vitro.
However, the effect of proinflammatory cytokine and chemokine expressions on the activation of the IL-18R signaling pathway and Fas/FasL pathway via leukocytes and TECs in AKI remains unknown. In this study, we used both in vivo and in vitro methods to examine the role of IL-18R in IRI model mice.
MATERIALS AND METHODS
Animals
IL-18Rα-deficient (IL-1Rrp−/−) mice (C57BL/6) were kindly provided by Dr S. Akira (Osaka University, Osaka, Japan). The C57BL/6 mice used as the WT controls were purchased from the Shizuoka Laboratory Animal Center (Shizuoka, Japan). All mice were maintained under standard animal care conditions in our specific pathogen-free animal facility. DNA samples were extracted from the tail for polymerase chain reaction (PCR)-based genotyping. Male mice (8–10 weeks old) were used.
Ischemia Protocol
Each mouse in the IRI group was anesthetized with pentobarbital (35 mg/kg, intraperitoneal) and the body temperature was maintained at 37°C for the duration of the ischemia. After performing a midline incision, the mouse was subjected to bilateral renal ischemia for 30 min, during which the renal arteries and veins were occluded by microaneurysm clamps. After the renal clamps were removed, the kidneys were observed for the restoration of blood flow, as demonstrated by a return to their original color. After 1 ml of prewarmed saline was placed in the peritoneal cavity, the abdomen was closed. The mouse was then returned to its cage to recover from the anesthesia. The sham surgery consisted of the same surgical procedure except that the clamps were not applied.
The mice were killed on day 1 (n=6) or day 5 (n=5) after the renal IRI. Kidney and spleen tissues were taken for analysis. Blood was collected from the dorsal aorta in heparinized tubes for the measurement of blood urea nitrogen (BUN), TNF, and IL-18.
Assessment of Renal Injury
One portion of the renal tissue was fixed in 10% buffered formalin, then embedded in paraffin, sectioned, and stained with periodic acid-Schiff (PAS) reagent. Tubular necrosis was evaluated in a semiquantitative manner by determining the percentage of cortical tubules in which epithelial necrosis, loss of the brush border, cast formation, and tubular dilation were evaluated. A five-point scale was used: 0, normal kidney; 1, 1–25%; 2, 26–50%; 3, 51–75%; and 4, 76–100% tubular necrosis.
CD4+ and CD8+ T cells, macrophages, and neutrophils were demonstrated by the immunoperoxidase staining of periodate-lysine-paraformaldehyde (PLP)-fixed frozen 6-μm-thick kidney sections, as described.17 The numbers of CD4+ and CD8+ T cells, macrophages, and neutrophils were assessed in 10 fields per slide at a magnification of × 400, and the results are expressed as cells per high-power field.
A positive tubule cross-section was defined as having two or more stained cells. The primary monoclonal antibodies used were rat monoclonal antibody GK1.5 for CD4+ and 53-6.7 for CD8+ T cells (Pharmingen, San Diego, CA) for macrophages with F4/80 hybridoma culture supernatant (HB198; American Type Culture Collection, Manassas, VA), and RB6-8C5 for neutrophils (anti-Gr-1; Pharmingen).
Immunofluorescence Analysis of Tubular Kidney Injury Molecule-1
Immunohistochemical staining for kidney injury molecule (Kim-1) was performed on 6-μm-thick PLP-fixed kidney sections using a Vectastain Elite ABC kit (Vector, Burlingame, CA). Endogenous peroxidase activity was ablated by incubation in 3% hydrogen peroxidase (20 min). Block sections with avidin/biotin blocking solution (Vector) for 15 min.
After incubation (1 h) with the primary antibody, rat monoclonal antibody Tim-1 (R&D Systems, Minneapolis, MN), biotinylated-conjugated secondary antibody, and biotin-rabbit anti-rat immunoglobulin (DAKO, Glostrup, Denmark) were applied for 40 min, and then the sections were incubated with an ABC kit for 40 min and the color was developed with DAB (Sigma, St Louis, MO). Kim-1 immunostaining was quantified by counting the number of positively stained tubules in the cortex in 10 fields per slide at a magnification of × 400.
Immunofluorescence Analysis of Renal Bax
Tissue samples taken at 24 h after renal IRI were fixed in 10% buffered formalin. Tissues were paraffin-embedded and cut into 4-μm-thick sections. For immunostaining, sections were deparaffinized in xylene and rehydrated in consecutive washes of 100% to 70% ethanol, and washed three times for 5 min each time in wash buffer (0.1% Triton X-100 in phosphate-buffered saline (PBS)). The activation disposal of antigens was carried out with a microwave oven for 15 min (in citrate buffer 0.01 M, pH 6.0). Tissues were permeabilized for 5 min in acetone at 20°C, washed three times, outlined with a Super Pap Pen (Invitrogen, Carlsbad, CA), and blocked with 5% normal goat serum in PBS for 2 h. Sections were incubated in primary antibodies (diluted 1:100, 06–499; Upstate, Lake Placid, NY) overnight at 4°C. After the sections were washed three times for 10 min each time, they were incubated in secondary antibody (diluted 1:100, AP-132c; goat anti-rabbit IgG Cy3) for 1 h at room temperature. The sections were then washed three times with wash buffer and covered with mounting media, and coverslips were applied. Negative controls were incubated without primary antibodies. Images were obtained by using the signal from the Cy3 (excitation 510–560 nm and emission 590 nm) channel with a confocal laser scanning microscope (ECLIPSE E800, Nikon, Tokyo).
Real-Time PCR Analysis
For the measurement of the intrarenal mRNA expressions of TNF-α, IL-1β, CCL2/MCP-1, RANTES, and 18SrRNA, we used a FastStart DNA master, SYBR Green I (Applied Biosystems, Carlsbad, CA) and for the measurement of IFN-γ, IL-10, IL-12p40, IL-18, Kim-1, FasL, and 18SrRNA, we used TaqMan gene (Applied Biosystems), as described.18 The sequences of primers and the gene database numbers are listed in Tables 1 and 2. The relative amount of mRNA was calculated using the comparative Ct (ΔΔCt) method. All specific amplicons were normalized against 18SrRNA, which was amplified in the same reaction as an internal control using commercial reagents (Applied Biosystems) and is expressed as fold differences relative to saline-treated control animals (n=4) and relative to non-treated control TECs in vitro. The expression levels in the sham animals were not different between genotypes.
Urine Kim-1 Level Excretion
Kim-1 was measured in the urine collected from mice after renal IRI on day 1 by enzyme-linked immunosorbent assay as described.19 The antibodies were rat anti-mouse TIM-1 monoclonal antibody (R&D Systems), biotinylated goat anti-mouse TIM-1 (R&D Systems), and streptavidin-HRP (Chemicon International, Billerica, MA). Plates were developed using tetramethylbenzidine substrate, and the OD was read at 450 nm.
Western Blotting
Proteins were extracted by homogenization of the whole kidney on day 1 after IRI and TECs (in vitro) in T-PER tissue protein extraction reagent (Pierce, Rockford, IL) to determine the expression of Bax, Bcl-2, and FasL as described.20 Monoclonal anti-β-actin antibody, Bax antibody, and Bcl-2 antibody were obtained from Cell Signaling Technology (Beverly, MA). Anti-mouse FasL antibody was from Sigma-Aldrich (St Louis, MO). Peroxidase-conjugated goat IgG was from Santa Cruz Biotechnology, (Santa Cruz, CA).
Primary Culture of Mouse Renal TECs
Primary mouse TECs were generated as described.21 In brief, kidneys were flushed with saline in vivo to remove blood cells, then removed. The kidney cortices from WT and IL-18Rα-deficient mice were cut into pieces ∼1 mm3 and then digested in Hanks’ balanced salt solution containing 3 mg/ml of collagenase at 37°C for 25 min and washed in DMEM/F12 medium (Invitrogen). The kidney digest was washed through a series of sieves (mesh diameter 250, 150, 75, and 40 μm). The cortical tubular cells were spun down at 300 g for 5 min and further washed. The cell pellet was resuspended in defined K1 medium.21
The cell suspension was placed on cell culture Petri dishes and incubated at 37°C for 2–3 h to facilitate the adherence of contaminating glomeruli. The nonadherent tubules were then collected and cultured on collagen-coated Petri dishes (BD Biosciences, San Diego, CA) in K1 medium until epithelial colonies were established. The expression of the epithelial cell marker cytokeratin was verified by immunofluorescent staining with an anti-cytokeratin antibody (Sigma-Aldrich). The cells were 96–100% cytokeratin-positive, as reported.21 The experiments were started after the cells had reached 80–90% confluence, which was usually between 5 and 7 days after the isolation procedure.
Induction of Renal TEC Ischemia and IL-18 Stimulation In Vitro
TECs were rendered transiently ischemic by immersing the cellular monolayer in mineral oil according to the protocol of Meldrum et al.22 This immersion induced simulated ischemia by restricting the cellular exposure to oxygen and nutrients as well as by limiting the metabolite washout. We placed mouse renal TECs in serum-free K1 medium for 24 h, washed them twice with PBS, and then immersed them in mineral oil (Sigma-Aldrich) for 60 min at 37°C. After extensive washing with PBS, the cells were incubated in serum-free K1 medium again. TECs were exposed to serum-free K1 medium alone as the nonischemic control. Recombinant mature IL-18 (MBL, Nagoya, Japan) was added to the cells at concentrations of 1, 10, or 100 ng/ml. Cells were then collected at 24 h after medium replacement for the detection of apoptosis.
Fluorescence-Activated Cell Sorter Analysis
In vitro, for the assessment of apoptosis by a fluorescence-activated cell sorting (FACS) system (Becton Dickinson, Lincoln Park, NY), monolayers of TEC were released by a brief incubation with trypsin-EDTA solution (Invitrogen). Apoptosis of the TECs was assessed by an analysis of Annexin V staining (Roche Diagnostics, Indianapolis, IN). Stained cells were analyzed on a FACS Calibur flow cytometer (BD Biosciences, San Jose, CA). Data were analyzed using CellQuest software (BD Biosciences).
Splenocyte Adoptive Transfer
IL-18Rα-deficient mice received an adoptive transfer of splenocytes from WT mice. Splenocytes that were collected from WT mice were minced on a nylon mesh as described.23 Approximately 5 × 106 spleen cells were injected intraperitoneally into each IL-18Rα-deficient mouse 3 weeks before the IRI. IL-18Rα-deficient mice (n=5), WT mice (n=6), and IL-18Rα-deficient mice that received a transfer of splenocytes (n=8) were culled on day 1. To investigate T-cell reconstitution in IL-18Rα-deficient mice, we analyzed splenocytes in a population of IL-18R-positive cells by FACS. FITC-conjugated IL-18R and IgG1 were purchased from R&D Systems.
Statistical Analysis
Data are expressed as means±s.e.m.; the t-test and one-way analysis of variance were used for comparisons of means between experimental groups. Differences were considered to be significant at P<0.05. We analyzed the data using GraphPad Prism software (GraphPad Software, La Jolla, CA).
RESULTS
Functional and Structural Aggravation from IRI
The kidneys of the IL-18Rα-deficient mice developed a severe tubular injury with cast formation, loss of brush border membranes, sloughing of TECs, and dilation of tubules (Figure 1a). In the WT mice, the damage was limited to mild swelling of TECs, and less histological damage was visible. On day 1, the ATN scores in the IL-18Rα-deficient mice were significantly increased compared to those of the WT mice (Figure 1b). On day 5, the ATN scores did not differ between the two groups. As shown in Figure 2a, the IRI caused kidney dysfunction in the IL-18Rα-deficient mice, reflected by a significant elevation of BUN on day 1. Compared with that in IL-18Rα-deficient mice, the BUN values were lower in the WT mice on day 1.
Representative light microscopy of PAS-stained renal sections after ischemia/reperfusion. Kidneys from wild-type (WT) and IL-18 receptor α-deficient (IL-18Rα KO) mice are shown (a). Tubular necrosis (b) was scored in the cortex of the kidney (see the Materials and Methods section for the scoring method). These data are mean scores±s.e. ***P<0.005, the necrosis score in WT and IL-18Rα KO mice on day 1. Original magnifications × 400. Scale bar, 50 μm.
Blood samples from WT and IL-18Rα-deficient (IL-18Rα KO) mice were collected on days 1 and 5 after renal ischemia/reperfusion, and then blood urea nitrogen (BUN) values were measured on day 1 and day 5 (a). The dotted line represents mean values of sham-operated controls. Serum TNF (b), IL-18 (c), and urinary Kim-1 levels (d) were measured as early biomarkers in AKI on day 1. These data are mean values±s.e. *P<0.05, **P<0.01, WT vs IL-18Rα KO mice on day 1.
Serum and Urinary Biomarkers in Renal Injury
We measured the serum TNF, IL-18 (Figure 2b and c), and urinary Kim-1 (Figure 2d) levels as biomarkers of AKI on day 1. The serum levels of TNF and IL-18 and the urinary Kim-1 level in the IL-18Rα-deficient mice were significantly increased compared to those in the WT mice.
The Infiltration of CD4+ and CD8+ T Cells, Macrophages, and Neutrophils in Renal IRI
We investigated the infiltration of inflammatory cells, ie, CD4+ and CD8+ T cells, macrophages, and neutrophils, in the renal interstitium on days 1 and 5 (Figure 3). The numbers of interstitial CD4+ T cells, macrophages, and neutrophils in the IL-18Rα-deficient mice on day 1 were significantly increased compared to those in the WT mice. Although a significant difference was not observed, the numbers of interstitial CD8+ T cells were increased in the IL-18Rα-deficient mice on day 1 compared to the WT mice. On day 5, the numbers of these inflammatory cells were not significantly different between the two groups.
Renal mRNA Expression in Renal IRI
Renal mRNA was measured by real-time quantitative PCR, as described in the Materials and Methods section. Figure 4 shows that the IRI significantly upregulated the expressions of proinflammatory cytokines (IL-1β, IL-12, and IL-18) and chemokines (ICAM-1 and CCL2/MCP-1) in the IL-18Rα-deficient mice on day 1. The IL-10, TNF, and IFN-γ mRNA expressions were increased after IRI compared to the expression in WT mice on day 1. On day 5, the cytokine and chemokine mRNA expressions were not significantly different between the two groups.
Effects of IL-18R on gene expression in the kidney. Cytokine and chemokine gene expression was measured on days 1 and 5 after renal ischemia/reperfusion in WT and IL-18Rα-deficient (IL-18Rα KO) mice by real-time RT–PCR. In each experiment, the expression levels were normalized to the expression of 18SrRNA and are expressed relative to saline-treated control mice, these data are mean fold increase±s.e. *P<0.05, WT vs IL-18Rα KO mice on day 1.
Renal Kim-1 Expression
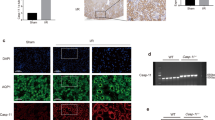
Figure 5 shows the tubular Kim-1 expression after renal ischemia/reperfusion. In the IL-18Rα-deficient mice, the numbers of Kim-1+ cells were dramatically increased on day 1 compared to the WT mice (Figure 5a and b). On day 5, the numbers of Kim-1+ cells in the IL-18Rα-deficient mice were significantly decreased compared to those on day 1. The difference in the number of Kim-1+ cells between the groups was significantly greater on day 1 than on day 5. We also determined the Kim-1 mRNA expressions on days 1 and 5 and found that its expression in the IL-18Rα-deficient mice was increased significantly on day 1 compared to the WT mice (Figure 5c).
Kim-1 expression in the kidney. Representative photographs of Kim-1 expression in the kidney after renal ischemia/reperfusion on days 1 and 5 (a). The number of Kim-1 positive-cells in 10 × 400 fields (b). Expression of Kim-1 mRNA on days 1 and 5 (c). These data are mean numbers and fold increase±s.e. **P<0.01, WT vs IL-18Rα-deficient (IL-18Rα KO) mice on day 1. Scale bar, 50 μm.
Tubular Bax Activation
Figure 6 illustrates the expression of renal Bax as a marker of apoptosis on days 1 and 5 by immunofluorescence and a western blotting analysis. In the IL-18Rα-deficient mice, the levels of Bax-positive tubules were significantly increased on day 1 (Figure 6a). The ratio of Bax/Bcl-2 in the IL-18Rα-deficient mice was significantly higher compared to those in the WT mice on day 1 (Figure 6b and c). By day 5, no significant difference was observed between the two groups.
Expression of Bax in the kidney. Representative photographs of Bax expression in the kidney after renal ischemia/reperfusion on days 1 and 5 (a, white arrow shows the Bax-positive cell in TECs). The ratio of Bax/Bcl-2 (b and c) in WT mice and IL-18Rα-deficient (IL-18Rα KO) mice on day 1 as assayed by western blotting. These data are mean changes±s.e. *P<0.05, WT vs IL-18Rα KO mice. The pictures show a representative band of Bax. Scale bar, 50 μm.
FasL Expression in Renal IRI
Figure 7 shows the protein levels of FasL in the WT and IL-18Rα-deficient mice on day 1 by western blotting analysis. The protein levels of renal FasL in the IL-18Rα-deficient mice were significantly increased compared to those in the WT mice.
Splenocyte Adoptive Transfer Reconstituted the Kidney Susceptibility to IRI
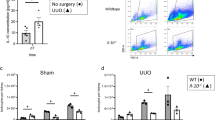
To determine whether IL-18Rα deficiency was indeed an exacerbating factor in IRI-induced AKI, we transferred 5 × 106 splenocytes from WT mice into each of the five IL-18Rα-deficient mice. The successful transfer of splenocytes was confirmed by a FACS analysis with IL-18R staining in a previous study (Figure 8a). The mean population of T cells in the WT mouse spleen was 2.6% of total splenocytes. The IL-18Rα-deficient mice had minimal (1.1%) splenocytes. Three weeks after the transfer, the lymphocytes in the IL-18Rα-deficient mice were reconstituted to 1.8%. The splenocyte transfer thus led to a significant improvement of the renal dysfunction in IL-18Rα-deficient mice. There were also significant improvements in BUN levels (Figure 8b) and ATN scores (Figure 8d) in the IL-18Rα-deficient mice that received a transfer of splenocytes compared to the IL-18Rα-deficient mice without transfer on day 1 after IRI.
Effect of splenocyte transfer on renal function and histological damage in renal ischemia/reperfusion injury on day 1. Splenocytes were isolated from WT mice that are killed, stained with FITC-conjugated anti-mouse IL-18R antibody and analyzed by FACS (a). BUN was measured from blood samples (b) and tubular necrosis was scored in the kidney (c and d) in WT, IL-18Rα KO mice, and IL-18Rα KO mice with splenocyte transfer. These data are mean values±s.e. *P<0.05, IL-18Rα KO mice vs IL-18Rα KO mice with splenocyte transfer and IL-18Rα KO mice with splenocyte transfer vs WT. Representative light microscopy of PAS-stained renal sections after ischemia/reperfusion. Kidneys in WT, IL-18Rα KO, and IL-18Rα KO mice with splenocyte transfer are shown. Original magnifications × 400. Scale bar, 50 μm.
IL-18-Induced Apoptosis in TECs
We next evaluated IL-18’s ability to stimulate TECs by exposing renal TECs to various concentrations of recombinant IL-18 in vitro. A significant increase in the percentage of apoptosis as determined by FACS analysis (Figure 9a) was detected in the TECs stimulated with recombinant IL-18 after oxygen blocking (except the control group), in a dose-dependent manner. The ratio of Bax/Bcl-2 was significantly higher in the IL-18Rα-deficient mice exposed to recombinant IL-18 stimulation (100 ng/ml for 24 h) compared to those in the WT mice (Figure 9b and c). These findings, in conjunction with our in vivo observations, suggest that IL-18 is an important mediator of renal TEC apoptosis.
Flow cytometric analysis (a) of tubular epithelial cells (TECs) following IL-18 stimulation and the expression of Bax in TECs. A graphic representation of the TECs’ percentage apoptosis in response to recombinant mouse IL-18 stimulation (0–100 ng/ml) for 24 h after oxygen blocking. The ratio of Bax/Bcl-2 in TECs (b and c) of WT mice and IL-18Rα-deficient (IL-18Rα KO) mice exposed to recombinant IL-18 stimulation (100 ng/ml for 24 h) after oxygen blocking as assayed by western blotting. These data are mean values±s.e. *P<0.05, **P<0.01, WT vs IL-18Rα-deficient (IL-18Rα KO) mice.
FasL Expression on TECs during IL-18 Stimulation
To evaluate FasL as a mediator of IL-18-induced TEC apoptosis, we measured the FasL mRNA expression in TECs exposed to recombinant IL-18 after oxygen blocking. The FasL mRNA expression was significantly higher in the TECs exposed to recombinant IL-18 stimulation (100 ng/ml for 24 h) compared to the controls (Figure 10a). This result provides evidence that IL-18 induces tubule apoptosis by enhancing FasL expression. In addition, the IL-18 and IL-12 mRNA expressions in TECs after oxygen blocking were significantly increased in the IL-18Rα-deficient mice compared to the WT mice (Figure 10b and c).
The expressions of FasL, IL-18, and IL-12 in TECs. FasL gene expression was measured by real-time RT–PCR after stimulation with recombinant mouse IL-18 (100 ng/ml for 24 h) after oxygen blocking (a). IL-18 and IL-12 gene expression were measured by real-time RT–PCR after an oxygen blocking (b and c). In each experiment, the expression level was normalized to the expression of 18SrRNA, and were expressed relative to non-treated control TECs, these data are mean fold increase±s.e. †P<0.05, recombinant mouse IL-18 concentration 0 ng/ml vs 100 ng/ml. *P<0.05, **P<0.01, WT vs IL-18Rα-deficient (IL-18Rα KO) mice.
DISCUSSION
Recent experimental data suggest that IRI rapidly activates innate immune responses. The pathophysiological mechanisms of acute ischemic renal failure involve multiple mediators, such as proinflammatory cytokines, reactive oxygen species, adhesion molecules/chemokines, and the activation of leukocytes and endothelial cells that lead to tubular injury, endothelial dysfunction, and inflammation.24, 25, 26, 27 In the present study, IL-18 was observed to be upregulated in renal IRI.
In the interstitial cells, the number of CD4+ T cells, neutrophils, and macrophages in IL-18α-deficient mice was increased significantly compared to those in WT mice on day 1 (Figure 3). The mechanisms by which IL-18 promotes kidney damage in this setting seem to involve the generation of inflammatory cytokines and chemokines in the promotion of CD4+ and CD8+ T cells and macrophage and neutrophil infiltration. IL-18 could induce chemokine expression while the chemoattractive properties of IL-18 promote cellular infiltration, local inflammation, and tissue damage.28 This result indicated that CD4+ T cells and neutrophils play a key role in accelerating the infiltration of leukocytes in the early phase after IRI.
As noted above, we demonstrated that IL-18Rα-deficient mice had markedly decreased renal function and increased histological injury on day 1 after the exposure to IRI. However, there were no differences in renal function or histological injury on day 5 between the IRI and WT groups. The mechanism of how the IL-18/IL-18R interaction mediates renal repair injury in IRI mice is still unknown. We found previously that the nephrotoxicity was exacerbated in cisplatin-induced AKI and the survival rate of lupus-prone mice was improved in an IL-18Rα-deficient mouse model.29, 30
We hypothesize that the IL-18/IL-18R interaction regulates a dynamic balance between destructive and repair signals in acute and/or chronic renal injury. As inflammation during a transient insult sets the stage for injury, and since IL-18Rα deficiency is implicated in tissue damage, we selected a model of transient renal damage leading to IRI as an AKI. We found that by knocking out IL-18Rα, the IL-18 and TNF production were significantly increased as destructive signals on day 1. After day 1, the IL-18 and TNF production was decreased rapidly by the knockout of IL-18Rα as a repair signal.
This hypothesis is supported by the report from Ghose et al31 that the IL-18/IL-18R interaction would enhance protective immunity as well as abrogate the immune-mediated pathology that causes multiorgan failure following infections with intracellular pathogens.
IL-18 induces the gene expression of several proapoptotic factors including TNF, IL-1, FasL, and multiple chemokines,32 and IL-18 has been shown to stimulate apoptotic cell death in a variety of cells through both TNF- and Fas-dependent mechanisms.33, 34, 35 Some studies reported that FasL has a greater impact than TNF on apoptosis and inflammation in IRI.36 FasL, whose expression is normally highly restricted, is frequently expressed on different cell types in the kidney including mesangial cells, TECs, fibroblasts, endothelial cells, and leukocytes.
An increased expression of Fas and FasL has been documented in various renal diseases, and the pathway of Fas/FasL is believed to have a role in promoting renal injury through the induction of apoptosis in glomerular cells and TECs. In a recent study using HK-2 cells, IL-18 accelerated the expression of FasL on tubular cells, and the cells exposed to the siRNA knockdown of FasL before IL-18 stimulation exhibited a significant reduction in FasL gene expression, apoptotic cell death, and active caspase-3 expression.35
In the present study, we observed that the expressions of renal IL-18 and FasL in IL-18Rα-deficient mice were significantly increased compared to those in WT mice through the apoptosis pathway. In addition, in our immunofluorescence and western blotting analyses, the expression of Bax was significantly increased in the IL-18Rα-deficient mice compared to the WT mice. Bax is known as an apoptotic promotion marker. The binding of Fas and FasL leads to the activation of caspases and subsequent apoptosis. Bax translocation is dependent on caspase activation, and it increases the release of cytochrome c from mitochondria, thereby promoting apoptosis.37 In vitro, the FasL expression after an oxygen blockade in IL-18Rα-deficient mice was increased compared to the WT mice. A significant increase in the percentage of apoptosis was detected in TECs stimulated with recombinant IL-18, in a dose-dependent manner.
We previously found that MRL-Faslpr mice cross-bred with mice deficient in IL-18Rα exhibited a reduction in autoantibodies, nephritis, and death.30 However, paradoxical IL-18 findings have been reported.38, 39 Proinflammatory cytokines and chemokines are associated with the pathogenesis of the nephrotoxicity, but it was reported that IL-1, TNF, IL-18, and ICAM-1 are similarly involved in renal IRI.25, 32 TNF upregulation leads to an inflammatory cascade that exacerbates tissue damage and includes the production of the chemokines MIP-2 and MCP-1, which recruit neutrophils and macrophages, respectively, into the kidney.
In addition, IL-18 is able to induce chemokine expression.40 This ability together with the chemoattractive properties of IL-18 promote cellular infiltration, local inflammation, and tissue damage. IL-18Rα also affects the suppressors of cytokine signaling (SOCS) 1 and SOCS3 expression. Nold-Petry et al41 reported that SOCS1 and SOCS3 mRNA expression and protein production were greatly reduced in mouse embryonic fibroblasts from IL-18Rα-deficient mice compared to the corresponding cells from WT mice. We also observed the downregulation of splenic/renal SOCS1 and SOCS3 in IL-18Rα-deficient mice compared to WT mice in cisplatin-induced AKI.29
Taken together, the present and previous findings suggest that the antiapoptotic pathway is mediated by Fas/FasL expression results in Bax activation, and that the cytokine signaling pathway is mediated by SOCS activation, which was enhanced through IL-18 expression by blocking IL-18Rα expression in AKI.
IL-18 is a proinflammatory cytokine that stimulates the production of IFN-γ by natural killer cells and T cells in synergy with IL-12.4 IL-18 signaling is known to involve the activation of IL-1 receptor-associated kinase (IRAK).42 Signaling defects in IRAK-deficient Th1 cells resulted in a dramatic decrease in IFN-γ expression, but, a strong synergistic effect in IFN-γ induction and cell proliferation was observed in IRAK-deficient Th1 cells when treated with combination of IL-12 and IL-18.42 In addition, the simultaneous addition of IL-12 and IL-18 resulted in a marked upmodulation of IL-12Rβ1 chain expression.43 Moreover, the expression of FasL was enhanced by IL-12.44
IL-12 is a key cytokine that induces the development of an effective Th1-type immune response in various inflammatory and infectious disorders. Renal TECs are an important source of IL-12.45 We observed here that IL-12 mRNA expression in TECs after oxygen blocking was significantly increased.
The mechanism underlying the upregulation of FasL expression in TECs through IL-18Rα signaling is not still clear, but this upregulation may contribute to the proapoptotic pathway by the augmentation of IL-18 and IL-12 expression through IL-18Rα blockade as shown in the present study.
In conclusion, the onset of the experimental AKI in IRI model mice was more rapid and severe in the IL-18Rα-deficient mice compared to the WT mice. IL-18Rα plays an important role in the development of renal dysfunction and inflammation via its effects on T- and B-cell responses, neutrophils, and macrophages. IL-18Rα also affects the apoptosis pathway via FasL expression on TECs. No definitive cure for AKI will be possible until its etiology is elucidated, but the suppression of proinflammatory cytokines, chemokines, and apoptosis by the manipulation of IL-18Rα gene represents a novel therapeutic strategy for preventing the renal injury associated with ischemia.
References
Striz I, Krasna E, Honsova E et al. Interleukin 18 (IL-18) upregulation in acute rejection of kidney allograft. Immunol Lett 2005;99:30–35.
Sareneva T, Julkunen I, Matikainen S . IFN-α and IL-12 induce IL-18 receptor gene expression in human NK and T cells. J Immunol 2000;165:1933–1938.
Torigoe K, Ushio S, Okura T et al. Purification and characterization of the human interleukin-18 receptor. J Biol Chem 1997;272:25737–25742.
Okamura H, Tsutsi H, Komatsu T et al. Cloning of a new cytokine that induces IFN-gamma production by T cells. Nature 1995;378:88–91.
Timo S, Ilkka J, Sampsa M . IFN-α and IL-12 induce IL-18 receptor gene expression in human NK and T cells. J Immunol 2000;165:1933–1938.
Laura F, Patrizia P, Barbara V et al. IFN-α and IL-18 exert opposite regulatory effects on the IL-12 receptor expression and IL-12-induced IFN-g production in mouse macrophages: novel pathways in the regulation ofthe inflammatory response of macrophages. J Leukoc Biol 2000;68:707–714.
Parikh CR, Abraham E, Ancukiewicz M et al. Urine IL-18 is an early diagnostic marker for acute kidney injury and predicts mortality in the intensive care unit. J Am Soc Nephrol 2005;16:3046–3052.
Bani-Hani AH, Leslie JA, Asanuma H et al. IL-18 neutralization ameliorates obstruction-induced epithelial-mesenchymal transition and renal fibrosis. Kidney Int 2009;76:500–511.
Nogae S, Miyazaki M, Kobayashi N et al. Induction of apoptosis in ischemia-reperfusion model of mouse kidney: possible involvement of Fas. J Am Soc Nephrol 1998;9:620–631.
Ortiz A, Lorz C, Egido J . The Fas ligand/Fas system in renal injury. Nephrol Dial Transplant 1999;14:1831–1834.
Brunner T, Mogil RJ, LaFace D et al. Cell-autonomous Fas (CD95)/Fas-ligand interaction mediates activation-induced apoptosis in T-cell hybridomas. Nature 1995;373:441–444.
Dupont PJ, Warrens AN . Fas ligand exerts its pro-inflammatory effects via neutrophil recruitment but not activation. Immunology 2007;120:133–139.
Cruise MW, Melief HM, Lukens J et al. Increased Fas ligand expression of CD4− T cells by HCV core induces T celldependent hepatic inflammation. J Leukoc Biol 2005;78:412–425.
Park DR, Thomsen AR, Frevert CW et al. Fas (CD95) induces proinflammatory cytokine responses by human monocytes and monocyte-derived macrophages. J Immunol 2003;170:6209–6216.
Hamad AR, Schneck JP . Antigen-induced T cell death is regulated by CD4 expression. Int Rev Immunol 2001;20:535–546.
Zhang H, Hile KL, Asanuma H et al. IL-18 mediates proapoptotic signaling in renal tubular cells through a Fas ligand-dependent mechanism. Am J Physiol Renal Physiol 2011;301:171–178.
Arany I, Safirstein RL . Cisplatin nephrotoxicity. Semin Nephrol 2003;23:460–464.
Phoon RK, Kitching AR, Odobasic D et al. T-bet deficiency attenuates renal injury in experimental crescentic glomerulonephritis. J Am Soc Nephrol 2008;19:477–485.
Nozaki Y, Nikolic-Paterson DJ, Snelgrove SL et al. Endogenous Tim-1 (Kim-1) promotes T-cell responses and cell-mediated injury in experimental crescentic glomerulonephritis. Kidney Int 2011;81:844–855.
Nozaki Y, Tamaki C, Yamagata T et al. All-trans-retinoic acid suppresses interferon-gamma and tumor necrosis factor-alpha; a possible therapeutic agent for rheumatoid arthritis. Rheumatol Int 2006;26:810–817.
Wu H, Chen G, Wyburn KR et al. TLR4 activation mediates kidney ischemia/reperfusion injury. J Clin Invest 2007;117:2847–2859.
Meldrum KK, Meldrum DR, Hile KL et al. A novel model of ischemia in renal tubular cells which closely parallels in vivo injury. J Surg Res 2001;99:288–293.
Liu M, Chien CC, Burne-Taney M et al. A pathophysiologic role for T lymphocytes in murine acute cisplatin nephrotoxicity. J Am Soc Nephrol 2006;17:765–774.
Fiorina P, Ansari MJ, Jurewicz M et al. Role of CXC chemokine receptor 3 pathway in renal ischemic injury. J Am Soc Nephrol 2006;17:716–723.
Devalaraja-Narashimha K, Singaravelu K, Padanilam BJ . Poly (ADPribose) polymerase-mediated cell injury in acute renal failure. Pharmacol Res 2005;52:44–59.
Bonventre JV, Zuk A . Ischemic acute renal failure: an inflammatory disease? Kidney Int 2004;66:480–485.
Rabb H, O’Meara YM, Maderna P et al. Leukocytes, cell adhesion molecules and ischemic acute renal failure. Kidney Int 1997;51:1463–1468.
Wyburn K, Wu H, Yin J et al. Macrophage-derived interleukin-18 in experimental renal allograft rejection. Nephrol Dial Transplant 2005;20:699–706.
Nozaki Y, Kinoshita K, Yano T et al. Signaling through the interleukin-18 receptor α attenuates inflammation in cisplatin-induced acute kidney injury. Kidney Int 2012;82:892–902.
Kinoshita K, Yamagata T, Nozaki Y et al. Blockade of IL-18 receptor signaling delays the onset of autoimmune disease in MRL-Faslpr mice. J Immunol 2004;173:5312–5318.
Ghose P, Ali AQ, Fang R et al. The interaction between IL-18 and IL-18 receptor limits the magnitude of protective immunity and enhances pathogenic responses following infection with intracellular bacteria. J Immunol 2011;187:1333–1346.
Dinarello CA, Novick D, Puren AJ et al. Overview of interleukin-18: more than an interferon-gamma inducing factor. J Leukoc Biol 1998;63:658–664.
Finotto S, Siebler J, Hausding M et al. Severe hepaticinjury in interleukin 18 (IL-18) transgenic mice: a key role for IL-18 in regulating hepatocyte apoptosis in vivo. Gut 2004;53:392–400.
Mariño E, Cardier JE . Differential effect of IL-18 on endothelial cell apoptosis mediated by TNF-alpha and Fas (CD95). Cytokine 2003;22:142–148.
Ohtsuki T, Micallef MJ, Kohno K et al. Interleukin 18 enhances Fas ligand expression and induces apoptosis in Fas-expressing human myelomonocytic KG-1 cells. Anticancer Res 1997;17:3253–3258.
Furuichi K, Kokubo S, Hara A et al. Fas ligand has a greater impact than TNF-α on apoptosis and inflammation in ischemic acute kidney injury. Nephron Extra 2012;2:27–38.
Murphy KM, Streips UN, Lock RB . Bax membrane insertion during Fas (CD95)-induced apoptosis precedes cytochrome c release and is inhibited by Bcl-2. Oncogene 1999;18:5991–5999.
Takagi H, Kanai T, Okazawa A et al. Contrasting action of IL-12 and IL-18 in the development of dextran sodium sulphate colitis in mice. Scand J Gastroenterol 2003;38:837–844.
Lewis EC, Dinarello CA . Responses of IL-18- and IL-18 receptor-deficient pancreatic islets with convergence of positive and negative signals for the IL-18 receptor. Proc Natl Acad Sci USA 2006;103:16852–16857.
Fehniger TA, Shah MH, Turner MJ et al. Differential cytokine and chemokine gene expression by human NK cells following activation with IL-18 or IL-15 in combination with IL-12: implications for the innate immune response. J Immunol 1999;162:4511–4520.
Nold-Petry CA, Nold MF, Nielsen JW et al. Increased cytokine production in interleukin-18 receptor alpha-deficient cells is associated with dysregulation of suppressors of cytokine signaling. J Biol Chem 2009;284:25900–25911.
Kanakaraj P, Nqo K, Wu Y et al. Defective interleukin (IL)-18-mediated natural killer and T helper cell type 1 responses in IL-1 receptor-associated kinase (IRAK)-deficient mice. J Exp Med 1999;189:1129–1138.
Fantuzzi L, Puddu P, Varano B et al. IFN-alpha and IL-18 exert opposite regulatory effects on the IL-12 receptor expression and IL-12-induced IFN-gamma production in mouse macrophages: novel pathways in the regulation of the inflammatory response of macrophages. J Leukoc Biol 2000;68:707–714.
Kitaura H, Nagata N, Fujimura Y et al. Effect of IL-12 on TNF-α-mediated osteoclast formation in bone marrow cells: apoptosis mediated by Fas/Fas ligand interaction. J Immunol 2002;169:4732–4738.
Fan X, Oertli B, Wüthrich RP . Up-regulation of tubular epithelial interleukin-12 in autoimmune MRL-Fas (lpr) mice with renal injury. Kidney Int 1997;51:79–86.
Acknowledgements
We thank K. Furukawa, E. Honda, S. Kurashimo, K. Okumoto, and N. Mizuguchi for their expert technical assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Acute kidney injury in mice with ischemia/reperfusion injury is more rapid and severe in IL-18 receptor (IL-18R)-deficient mice than wild type mice. The authors show that IL-18R affects T and B-cell responses, increases accumulation of leukocytes, and affects apoptosis via Fas/Fas ligand expression. Suppression of pro-inflammatory molecules and apoptosis through IL-18Rα represents a novel therapeutic strategy for ischemia-related renal injury.
Rights and permissions
About this article
Cite this article
Yano, T., Nozaki, Y., Kinoshita, K. et al. The pathological role of IL-18Rα in renal ischemia/reperfusion injury. Lab Invest 95, 78–91 (2015). https://doi.org/10.1038/labinvest.2014.120
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/labinvest.2014.120
This article is cited by
-
The adaptive immune response in cardiac arrest resuscitation induced ischemia reperfusion renal injury
Journal of Biological Research-Thessaloniki (2020)
-
Protective Effects and Mechanisms of Shenhua Tablet (肾华片) on Toll-Like Receptors in Rat Model of Renal Ischemia-Reperfusion Injury
Chinese Journal of Integrative Medicine (2019)
-
Role of IL-18 induced Amphiregulin expression on virus induced ocular lesions
Mucosal Immunology (2018)
-
The effect of whole-body cooling on renal function in post-cardiac arrest patients
BMC Nephrology (2017)
-
Haemoadsorption reduces the inflammatory response and improves blood flow during ex vivo renal perfusion in an experimental model
Journal of Translational Medicine (2017)