Abstract
The accumulation of extracellular matrix proteins in the interstitial area is the final common feature of chronic kidney diseases. Accumulating evidence suggests that transforming growth factor (TGF)-β1 promotes the development of renal fibrosis. Heat shock protein (Hsp) 90 inhibitors have been shown to repress TGF-β1 signaling, but whether they inhibit renal fibrosis is unknown. The purpose of this study is to determine the therapeutic efficacy of Hsp90 inhibitor on renal fibrosis. In TGF-β1-treated HK2 cells and unilateral ureteral obstruction (UUO) kidneys, we found that 17-allylamino-17-demethoxygeldanamycin (17AAG), an Hsp90 inhibitor, decreased the expression of α-smooth muscle actin, fibronectin, and collagen I and largely restored the expression of E-cadherin. 17AAG inhibited TGF-β1-mediated phosphorylation of Smad2, Akt, glycogen synthase kinase-3β, and extracellular signal-regulated kinase in HK2 cells. Inhibition of Hsp90 also blocked TGF-β1-mediated induction of snail1. This 17AAG-induced reduction was completely restored by simultaneous treatment with proteasome inhibitor MG132. Furthermore, 17AAG blocked the interaction between Hsp90 and TGF-β type II receptor (TβRII) and promoted ubiquitination of TβRII, leading to the decreased availability of TβRII. Smurf2-specific siRNA reversed the ability of 17AAG to inhibit TGF-β1 signaling. The effect of 17AAG on TβRII expression and renal fibrosis was confirmed in UUO kidneys. These findings suggest that Hsp90 inhibitor prevents the development of renal fibrosis via a mechanism dependent on Smurf2-mediated degradation of TβRII.
Similar content being viewed by others
Main
Tubulointerstitial fibrosis is the final common manifestation of various chronic kidney diseases, and progressive accumulation of extracellular matrix (ECM) proteins in the interstitial area is a key feature. Although interstitial fibroblasts are considered the principal source of matrix production,1, 2 the role of epithelial-to-mesenchymal transition (EMT) of renal tubular epithelial cells has been implicated in accelerating fibrogenesis.3 Among several growth factors and cytokines, transforming growth factor (TGF)-β1 has been identified as the most potent mediator and convergent pathway in renal fibrosis.4, 5 On TGF-β ligand binding, the type II receptor (TβRII) dimer and the type I receptor (TβRI) dimer form a stable complex, in which the TβRII phosphorylates the TβRI on serine–threonine residues in the GS domain. Activated TβRI then phosphorylates Smad2/3, which partner with Smad4 and translocate to the nucleus where they ultimately regulate gene transcription.6, 7, 8 In addition to the Smad pathway, the activated TβRI also activates non-Smad signaling pathways, such as Akt, glycogen synthase kinase (GSK)-3β, and mitogen-activated protein kinases.8, 9, 10 Both Smad and non-Smad pathways are known to contribute to TGF-β1-driven renal fibrosis.9, 10
Heat shock protein (Hsp) 90 is one of the most abundant molecular chaperone proteins that are involved in protein folding and stabilization.11 A variety of signaling proteins involved in cell survival, growth, and differentiation are known as Hsp90 client proteins.11 Hsp90 requires several interacting co-chaperone proteins to exert its function on client proteins. On ATP-binding, the Hsp90-client complex associates with co-chaperones such as CDC37 and p23 to facilitate client stabilization.12 In contrast, in its ADP-bound form, Hsp90 associates with different co-chaperones such as Hsp70 and Hop (Hsp70 and Hsp90 organizing protein), resulting in enhanced proteasomal degradation of the Hsp90 client proteins.12 Hsp90 inhibitors suppress the progression of the Hsp90 complex toward the stabilizing form and shift it to the proteasome-targeting form, which result in ubiquitin–proteasome degradation of the client. As Hsp90 inhibitors display remarkable selectivity for client oncoproteins,13 several Hsp90 inhibitors are now in various stages of clinical developments as anticancer therapeutics.12, 14 Interestingly, a recent study has identified TβRI and TβRII as Hsp90 client proteins.15 However, the clinical significance of Hsp90 inhibitors in disease models with aberrant TGF-β responses remains to be determined. Here, we examined whether Hsp90 might regulate TGF-β1 signaling in renal cells and determined the effect of Hsp90 inhibitors in cultured renal cells and in a model of renal fibrosis.
MATERIALS AND METHODS
Cell Culture and Treatments
Cells were purchased from American Type Culture Collection (Rockville, MD, USA). HK2 cells were cultured at 37 °C in a 5% carbon dioxide atmosphere in DMEM (Gibco, Grand Island, NY, USA) mixed 1:1 (vol:vol) with F12 medium (Gibco) supplemented with 10% FBS. NRK49F rat renal fibroblasts were grown in DMEM with 4.5 g/l glucose (Gibco) containing 5% bovine calf serum. Near confluent cells were incubated with serum-free media for 24 h to arrest and synchronize cell cycle. Before stimulation with recombinant human TGF-β1 (R&D Systems, Minneapolis, MN, USA), cells were pre-treated with 17-allylamino-17-demethoxygeldanamycin (17AAG) (LC Laboratories, Woburn, MA, USA), MG132 (Calbiochem, Darmstadt, Germany), Wortmannin (Sigma, St Louis, MO, USA), or PD98059 (Calbiochem) for the specified duration.
Animals
All animal studies were conducted with the approval of the Institutional Care and Use Committee of Soon Chun Hyang University Hospital in Seoul, Korea, and our study complied with the National Institutes of Health guidelines for the care and use of experimental animals. The unilateral ureteral obstruction (UUO) model was established in male CD1 mice that weighed 20–25 g (Charles River Laboratory, Wilmington, MA, USA). Briefly, after a mid-abdominal incision under anesthesia using tiletamine (15 mg/kg, Virbac Laboratories, Carros, France)/zolazepam (15 mg/kg, Virbac Laboratories)/xylazine (9 mg/kg, Bayer, Korea, Seoul, Korea), the left ureter was isolated and ligated. Sham-operated mice were used as controls. 17AAG (2.5 and 25 mg/kg) or vehicle was administered by daily intraperitoneal injection immediately after ureteral ligation. After 2 weeks, the animals were killed and the kidneys were removed for various analyses.
Immunoblot Analysis
Tissue and cell lysates were centrifuged to remove cell debris, and supernatant was mixed with SDS loading buffer. Samples were then heated at 100 °C for 5 to 10 min before loading, separated through SDS-polyacrylamide gels, and subjected to western blot. Antibodies to E-cadherin (BD Transduction Laboratories, San Jose, CA, USA), α-smooth muscle actin (SMA) (Sigma), snail1 (Cell Signaling, Danvers, MA, USA), collagen I (Southern Biotech, Birmingham, AL, USA), phosphorylated Smad2 (Cell Signaling), Smad2/3 (Cell Signaling), phosphorylated Akt (Cell Signaling), Akt (Cell Signaling), phosphorylated GSK-3β (Cell Signaling), GSK-3β (Cell Signaling), phosphorylated extracellular signal-regulated kinase (ERK) (Cell Signaling), ERK (Cell Signaling), TβRI (Cell Signaling), and TβRII (Cell Signaling) were used. For fibronectin, peroxidase-conjugated rabbit anti-human fibronectin (DAKO, Glostrup, Denmark) was used. In some experiments, membrane fractionation was performed using commercially available kit (Pierce, Rockford, IL, USA).
Immunoprecipitation
For some experiments, membrane fraction of HK2 cell lysates was prepared according to the manufacturer’s instruction (Pierce). Approximately, 1 mg of total proteins from membrane fraction or whole cell lysates were incubated overnight at 4 °C with anti-Hsp90 or anti-TβRII, followed by precipitation with 20 μl of protein A/G-Plus-Agarose (Santa Cruz) for 4 h at 4 °C. The precipitated complexes were immunoblotted with anti-Hsp90, anti-TβRII, or anti-ubiquitin.
siRNA Transfection
An effective predesigned siRNA of Smurf2 (Applied Biosystems, Carlsbad, CA, USA) was selected through preliminary study. The sequences were as follows: sense 5′-CACACUUGCUUCAAUCGAATT-3′, antisense 5′-UUCGAUUGAAGCAAGUGUGGG-3′. Cells were transfected with siRNA (50 nM per well) using Lipofectamine (Invitrogen) reagent under serum- and antibiotic-free condition for 24 h and treated with TGF-β1. Several concentrations of siRNA were tested to determine the optimal knock-down conditions. Scramble RNAi was used as control. The transfection efficiency was determined using FAM-labeled Smurf2 siRNA (Applied Biosystems) by flow cytometry.
Histologic Analysis
The kidneys were initially perfused briefly with PBS through the heart to rinse away blood. Then, the kidneys were perfused with periodate-lysine-2% paraformaldehyde for 10 min, cut into slices of 1- to 2-mm thickness, and postfixed overnight in the periodate-lysine-2% paraformaldehyde solution at 4 °C. Paraffin-embedded sections (3 μm thickness) were subjected to immunohistochemical staining and Masson’s trichrome staining. Immunohistochemical staining was performed using anti-TβRII (Cell Signaling) and E-cadherin (BD Transduction Laboratories) antibodies. Masson’s trichrome staining was performed according to the protocol provided by the manufacturer (Sigma). The positive area was quantitatively measured using Image Scope software (Aperio, Vista, CA, USA). For double immunofluorescence, rabbit polyclonal anti-aquaporin 1 antibody (1:500, Chemicon, Temecula, CA, USA) and mouse monoclonal anti-E-cadherin antibody (1:200, BD Transduction Laboratories) were mixed and then allowed to react overnight at 4 °C. After washing with PBS, labeling was visualized using FITC-conjugated donkey anti-rabbit antibody (1:1000, Jackson ImmunoResearch Laboratories, West Grove, PA, USA) and Cy3-conjugated donkey anti-mouse antibody (1:1000, Jackson ImmunoResearch Laboratories). Tissues were mounted in Vectashield mounting media (Vector Laboratories). Images were acquired using a Zeiss LSM 510 confocal microscope (Carl Zeiss).
Statistical Analyses
The mean values obtained from each group were compared by ANOVA with subsequent Fisher’s least significant difference method. Unpaired two-tailed Student’s t-test and non-parametric analyses were also used where appropriate. Data are presented as mean±s.e.m. A P-value<0.05 was used as the criterion for a statistically significant difference.
RESULTS
Hsp90 Inhibitor Blocks TGF-β1-Induced ECM Production and EMT
We first examined the effect of TGF-β1 on ECM production and EMT in HK2 human proximal tubular epithelial cells. The dose of TGF-β1 was determined through a preliminary study. Treatment of HK2 cells with TGF-β1 (2 ng/ml) induced downregulation of their epithelial marker, E-cadherin (Figures 1, 2a and ), and upregulation of mesenchymal markers including α-SMA (Figures 1, 2a and ), fibronectin, and collagen I (compare lanes 1 with 2 in Figures 2c and d) in a time-dependent manner. We also observed a prompt increase in the expression of snail1, a transcription factor that has a critical role in the regulation of EMT by downregulating E-cadherin expression (Figures 1, 2a and ). In the presence of 17AAG, an Hsp90 inhibitor, all of these changes induced by TGF-β1 were abolished in a dose-dependent manner (Figure 2). A qualitatively similar effect of 17AAG on TGF-β1-induced ECM production and snail1 expression was observed in renal fibroblasts NRK49F (Figures 2e and f).
TGF-β1 induces downregulation of epithelial marker and upregulation of mesenchymal markers. HK2 cells were incubated with TGF-β1 at the concentration of 2 ng/ml for various periods of time as indicated. (a) Representative immunoblot analyses of E-cadherin, α-SMA, and snail1 protein. (b) Quantification of immunoblot results expressed as the mean±s.e. of three independent experiments. *P<0.05 vs control.
Hsp90 inhibitor 17AAG ameliorates TGF-β1-induced ECM and EMT marker expression in renal cells. HK2 cells (a–d) or NRK49F cells (e, f) were preincubated with 17AAG at the indicated doses for 6 h and then stimulated with TGF-β1 (2 ng/ml) for 48 h. (a, c, e) Representative immunoblot analyses of E-cadherin, α-SMA, snail1, fibronectin, and collagen I. (b, d, f) Quantification of immunoblot results expressed as the mean±s.e. of three independent experiments. *P<0.05 vs control, †P<0.05 vs TGF-β1 only.
Hsp90 Inhibitor Blocks TGF- β 1-Induced Smad and Non-Smad Phosphorylation
As both Smad and non-Smad pathways have been implicated in TGF-β1-induced ECM accumulation and EMT, we next examined the effect of 17AAG on Smad and non-Smad intracellular signaling molecules. As shown in Figure 3, TGF-β1 induced rapid and significant phosphorylation of Smad2, Akt, GSK-3β, and ERK. Phosphorylation of Smad2, Akt, and GSK-3β started to increase as early as 5 min and sustained until 1 h. Increased phosphorylation of ERK was detected as early as 15 min. As phosphorylation of Smad and non-Smad signaling molecules were continuously increased up to 1 h in our experimental setting, this time point was used in subsequent experiments. Pre-treatment with 17AAG significantly inhibited phosphorylation of Smad2, Akt, GSK-3β, and ERK in a time-dependent manner (Figures 4a and b). This finding was similarly observed in NRK49F cells (Figures 4c and d). To investigate how 17AAG suppresses Akt and ERK phosphorylation, we determined how long 17AAG took to reduce TGF-β1-induced Akt, GSK-3β, and ERK phosphorylation as compared with the phosphatidylinositol 3-kinase (PI3K) inhibitor wortmannin or the MAP kinase/ERK kinase inhibitor PD98059. As expected, treatment with wortmannin immediately blocked TGF-β1-induced phosphorylation of both Akt and GSK-3β (Figure 5a). In contrast, a 1- to 2-h treatment with 17AAG did not significantly alter the phosphorylation of Akt and GSK-3β. 17AAG took 4 to 6 h to decrease Akt and GSK-3β phosphorylation (Figure 5a). Similarly, TGF-β1-induced ERK phosphorylation was reduced immediately after PD98059 treatment, but only after >4 h of 17AAG treatment (Figure 5b). These findings suggest that 17AAG may act via different mechanisms from known kinase inhibitors to block TGF-β1-induced signaling.
TGF-β1 induces Smad and non-Smad phosphorylation. HK2 cells were treated with TGF-β1 at the concentration of 2 ng/ml for various periods of time as indicated. (a) Representative immunoblot analyses of Smad, Akt, GSK-3β, and ERK signaling. (b) Quantification of immunoblot results expressed as the mean±s.e. of three independent experiments. *P<0.05 vs control.
Hsp90 inhibitor 17AAG decreases TGF-β1-induced Smad and non-Smad phosphorylation in renal cells. HK2 cells (a, b) or NRK49F cells (c, d) were preincubated with 17AAG (1 μM) for various periods of time as indicated and then stimulated with TGF-β1 (2 ng/ml) for 1 h. (a, c) Representative immunoblot analyses of Smad, Akt, GSK-3β, and ERK signaling. (b, d) Quantification of immunoblot results expressed as the mean±s.e. of three independent experiments. *P<0.05 vs control, †P<0.05 vs TGF-β1 only.
Hsp90 inhibitor 17AAG blocks Akt, GSK-3β, or ERK phosphorylation at different rates from PI3K inhibitor wortmannin or MAP kinase/ERK kinase (MEK) inhibitor PD98059. (a) HK2 cells were treated with wortmannin or 17AAG for the indicated time and then stimulated with TGF-β1 for 1 h. Cells were harvested for immunoblot analysis. (b) Cells were treated with PD98059 or 17AAG for the indicated time and then stimulated with TGF-β1 for 1 h. Cells were harvested for immunoblot analysis. Data are mean±s.e. of three experiments. *P<0.05 vs control, †P<0.05 vs TGF-β1 only.
We next sought to determine whether 17AAG might decrease Smad and non-Smad phosphorylation via a mechanism dependent on ubiquitin–proteasome degradation. HK2 cells were treated with 17AAG in the presence or absence of MG132, a proteasome inhibitor, before TGF-β1 stimulation. As shown in Figures 6a and b, TGF-β1 significantly induced phosphorylation of Smad2, Akt, GSK-3β, and ERK. 17AAG effectively prevented all of these changes, but this 17AAG-mediated reduction was completely restored by simultaneous treatment with MG132, suggesting that 17AAG might decrease TGF-β1-induced Smad and non-Smad phosphorylation via a mechanism dependent on proteasome-mediated degradation. The inhibitory effect of 17AAG on TGF-β1-induced enhancement of snail1 expression was also restored by the treatment with MG132 (Figures 6a and b). In contrast, a pathway that is not TGF-β1 dependent such as phosphatase and tensin homolog was not affected by MG132 (Figure 6c), showing specificity for TGF-β1-dependent signaling.
Hsp90 inhibitor 17AAG-induced loss of TGF-β1 signaling is restored by MG132. Cells were treated with 17AAG with or without MG132 for 6 h and then stimulated with TGF-β1 (2 ng/ml) for 1 h. (a) Representative immunoblot analyses of Smad, Akt, GSK-3β, ERK, and snail1 expression. (b) Quantification of immunoblot results expressed as the mean±s.e. of three independent experiments. *P<0.05 vs no treatment, †P<0.05 vs corresponding control without 17AAG, #P<0.05 vs corresponding control with 17AAG. (c) Representative immunoblot analysis of phosphatase and tensin homolog (PTEN). Quantification of immunoblot results expressed as the mean±s.e. of three independent experiments.
Hsp90 Inhibitor Induces Degradation of T β RII
As 17AAG inhibited multiple signaling pathways induced by TGF-β1 at the same time, we anticipated that 17AAG might block TGF-β1 signaling at or close to the receptor level. To examine the effect of 17AAG on the expression of TGF-β receptors, immunoblot analysis using membrane fraction was performed. As shown in Figure 7a, TβRI abundance did not change by the treatment with TGF-β1 or 17AAG. However, TβRII abundance was modestly but significantly induced by TGF-β1 and reduced by 17AAG. Furthermore, immunoprecipitation analysis revealed that the interaction between Hsp90 and TβRII was significantly decreased by 17AAG, and which was almost completely restored by simultaneous treatment with MG132 (Figure 7b). In addition, 17AAG resulted in more pronounced formation of polyubiquitinated, higher-moleculer-weight forms of TβRII in the presence or absence of MG132 (Figure 7c). These data indicate that 17AAG probably blocks the interaction between Hsp90 and TβRII and subsequently induces ubiquitin–proteasome degradation of TβRII.
(a) Hsp90 inhibitor 17AAG decreases TβRII expression. Membrane fraction was used for immunoblot analysis. HK2 cells were treated with TGF-β1 at the concentration of 2 ng/ml for 1 h with or without pre-treatment with 17AAG for the indicated time. Data are mean±s.e. of three experiments. *P<0.05 vs control, †P<0.05 vs TGF-β1 only. (b) Immunoprecipitation analysis using membrane fraction revealed that the interaction between Hsp90 and TβRII was significantly decreased by 17AAG, which was almost completely restored by simultaneous treatment with MG132. Cells were treated with 17AAG for 6 h and then stimulated with TGF-β1 (2 ng/ml) for 1 h. Cells were cotreated with MG132 in parallel with 17AAG as indicated. Data are mean±s.e. of three experiments. *P<0.05 vs no treatment, †P<0.05 vs corresponding control without 17AAG, #P<0.05 vs corresponding control with 17AAG. (c) 17AAG significantly increased ubiquitination of TβRII in the presence or absence of MG132. Cells were treated for 6 h with 17AAG, MG132, both agents and vehicle. Whole cell lysates were used for immunoprecipitation with TβRII antibody and were blotted for ubiquitin. Data are mean±s.e. of three experiments. *P<0.05 vs no treatment, †P<0.05 vs MG132 alone.
Hsp90 Inhibitor-Induced Downregulation of TGF-β1-Signaling is Mediated by Smurf2
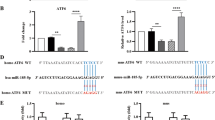
Ubiquitin–proteasome degradation has been implicated in the regulation of the stability of the TGF-β receptor complex. As Smurf2 has been shown to be an E3 ubiquitin ligase for TβRII,16 we next tested whether siRNA targeting Smurf2 can block 17AAG-mediated downregulation of TGF-β1-signaling. With 96.7% of transfection efficiency of siRNA (Figure 8a), Smurf2-specific siRNA could efficiently knockdown Smurf2 protein expression by 44% (Figure 8b). As shown in Figure 8c, Smurf2 siRNA restored TβRII expression in the membrane. In parallel, the ability of 17AAG to inhibit Smad2, Akt, and ERK phosphorylation-induced by TGF-β1 was reversed by Smurf2 siRNA compared with scrambled oligonucleotides (Figures 8c and d). These results suggest that Smurf2 is required for 17AAG-mediated downregulation of TGF-β1 signaling.
Hsp90 inhibitor 17AAG-induced downregulation of TGF-β1 signaling is mediated by Smurf2. (a) Transfection efficiency was determined by flow cytometry using FAM-labeled Smurf2 siRNA. (b–d) Effect of Smurf2 silencing on TGF-β1 signaling. HK2 cells were transfected with scramble control siRNA (C-siRNA) or Smurf2 siRNA using Lipofectamine reagent for 24 h and treated with 17AAG for 6 h. TGF-β1 (2 ng/ml) was added for 15 min (Akt and ERK) or for 1 h (Smad and TβRII). The protein levels for Smurf2, Smad2/3, phosphorylated Smad2, Akt, phosphorylated Akt, ERK, and phosphorylated ERK were analyzed by immunoblot analysis using whole cell lysates. Membrane fraction was used for TβRII immunoblot analysis. Data are mean±s.e. of three experiments. *P<0.05 vs without TGF-β1 in the same condition, †P<0.05 vs corresponding control without 17AAG, #P<0.05 vs corresponding control with 17AAG.
Hsp90 Inhibitor Blocks Renal Fibrosis in Obstructed Kidney
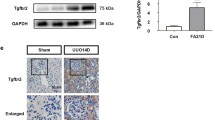
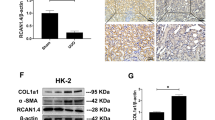
To investigate the ability of Hsp90 inhibitor to suppress renal fibrosis in vivo, we examined the effect of 17AAG on ECM accumulation and the expression of TβRII in UUO kidneys. 17AAG was administered into mice by daily intraperitoneal injection at 2.5 or 25 mg/kg body weight for 14 days. We observed significant increase in expression of α-SMA, fibronectin, and collagen I in the UUO kidneys (Figure 9). We also observed increased expression of snail1 (Figures 9a and b). Treatment with 17AAG attenuated the induction of snail1, as well as α-SMA, fibronectin, and collagen I in UUO kidneys while it had no effect in sham controls (Figure 9). In contrast, E-cadherin expression was significantly decreased in renal tubules of UUO kidneys as compared with control (Figures 9a, b). 17AAG almost completely restored E-cadherin expression in a dose-dependent manner (Figures 9a, b). Phosphorylation of Smad2 induced by UUO was significantly decreased by the treatment with 17AAG, suggesting that all of these changes were TGF-β1 dependent (Figures 9c and d). As we used proximal tubular cells in our in vitro studies, we performed parallel studies of proximal tubules with double staining for proximal tubular marker aquaporin 1 and E-cadherin. In control mice, E-cadherin expression was strongly positive in aquaporin 1-negative cells, however, E-cadherin-positive proximal tubular cells could be identified. We observed that UUO reduced E-cadherin expression in proximal tubular cells and that 17AAG restored it (Figure 10e). Of note, immunohistochemical staining revealed that UUO kidneys have increased expression of TβRII and that 17AAG effectively prevented UUO-induced enhancement of TβRII by 52% and 78% at 2.5 and 25 mg/kg, respectively (Figures 10a and d). Finally, UUO caused a marked increase in collagen accumulation, as shown by Masson’s trichrome staining (Figures 10c and d). Treatment with 17AAG, however, reduced collagen deposition (Figures 10c and d). Together, these in vivo results indicate that administration of 17AAG suppresses UUO-induced TβRII expression and reduces ECM accumulation and renal fibrosis.
Hsp90 inhibitor 17AAG reduces ECM and EMT marker expression in obstructed kidney. Protein expression of E-cadherin, α-SMA, snail1, fibronectin, collagen I, phosphorylation of Smad2, and Smad2 was analyzed by immunoblot analysis. Data are presented as mean±s.e. of four animals per group. *P<0.05 vs control, †P<0.05 vs UUO. 17AAG low: 2.5 mg/kg, 17AAG high: 25 mg/kg.
Hsp90 inhibitor 17AAG suppresses UUO-induced enhancement of TβRII, loss of E-cadherin, and renal fibrosis. (a, b) Immunohistochemical staining was performed using anti-TβRII (a), and E-cadherin antibodies (b). Original magnification, × 200. Staining specificity was tested without primary antibody (negative control, right panel of a, b). (c) Representative kidney tissue sections stained with Masson’s trichrome. Original magnification, × 200. (d) Bar graphs show data obtained by computer-based morphologic analysis. (e) Double staining for proximal tubular marker aquaporin 1 (green) and E-cadherin (red). Original magnification, × 400. Data are presented as mean±s.e. of four animals per group. *P<0.05 vs control, †P<0.05 vs UUO. 17AAG low: 2.5 mg/kg, 17AAG high: 25 mg/kg.
DISCUSSION
In this study, we have demonstrated that an Hsp90 inhibitor suppresses TGF-β1-induced Smad and non-Smad signaling via a mechanism dependent on proteasome-mediated degradation of TβRII. Smurf2 appears to be essential for Hsp90 inhibitor-induced degradation of TβRII because Smurf2 deficiency reversed the ability of the Hsp90 inhibitor to block TGF-β1 signaling. Furthermore, this study, for the first time, clearly showed the therapeutic effects of Hsp90 inhibitor in a model of renal fibrosis.
Hsp90, which accounts for 1–2% of cytosolic proteins, controls the folding and intracellular trafficking of diverse cellular proteins involved in several signal transduction pathways. Hsp90 has been shown to form complexes with many cellular proteins that are important for cell growth, survival, and differentiation.11 As numerous oncoproteins have been shown to be Hsp90 client proteins, more than a dozen Hsp90 inhibitors are currently undergoing clinical evaluation in cancer patients.14 As 17AAG has less toxicity and higher selectivity for client proteins, 17AAG is the first Hsp90 inhibitor to enter clinical trials. Other than the cancer area, Hsp90 inhibitors have also been shown to be effective as neuroprotective agents in animal models of Parkinson disease,17 stroke,18 autoimmune encephalitis,19 and spinal and bulbar muscular atrophy.20 In this study, we have shown the efficacy of 17AAG in tubulointerstitial fibrosis induced by UUO, and considerably extended the therapeutic application of 17AAG beyond cancer and inflammatory and degenerative diseases.
TGF-β1 has been identified as the single most important growth factor that can induce and mediate renal fibrosis.4, 5 Upregulation of TGF-β1 is a common finding in virtually every type of progressive chronic kidney disease, in both animals and humans. In cell culture systems, TGF-β1 stimulates mesangial cells,21 interstitial fibroblasts,22, 23 and tubular epithelial cells9, 10, 24 and induces matrix expression through its interaction with TGF-β receptors. Thus, TGF-β1 has been studied as a target to prevent the progression of renal fibrosis. Previous studies showed that anti-TGF-β strategies with natural inhibitor, monoclonal antibody or antisense oligonucleotides could attenuate kidney injury.25, 26, 27 However, none of these strategies has been implemented clinically so far because of the potential of untoward inhibition of the anti-inflammatory actions of TGF-β1.28 Therefore, from a clinical point of view, it is important to develop alternate novel therapies to more specifically suppress downstream targets of maladaptive TGF-β signaling events with relatively low toxicity.
Our data showing the ability of 17AAG to inhibit TGF-β1-induced Smad and non-Smad targets in renal tubular epithelial cells and fibroblasts suggested that this Hsp90 inhibitor could negatively regulate TGF-β signaling at a convergent point such as the TGF-β receptor complexes. Indeed, we show that TβRII interacts with Hsp90 and that the Hsp90 inhibitor blocks their interaction, and subsequently promotes the ubiquitination and degradation of TβRII. Although regulation at the level of the receptor and its role in the complexity of the TGF-β response has not been extensively studied, the availability and function of TGF-β receptors are crucial determinants of TGF-β signaling. Upregulation of TβRII has been shown in experimental animal models of type 129 and type 2 diabetic kidney disease.30 A similar finding has been observed in UUO kidneys.22 In support of this finding, we demonstrated that TβRII expression is significantly increased by UUO and that the treatment with 17AAG effectively prevents the enhancement of TβRII abundance.
Recent progress has shown that TGF-β receptors are subject to post-translational modifications such as ubiquitination, phosphorylation, and sumoylation.31 Phosphorylation of TβRI by TβRII enables the interaction with signaling molecules including Smad and non-Smad pathways, leading to the activation of TGF-β responses.6, 7 Sumoylation results in the covalent attachment of a SUMO polypeptide to a lysine residue in the cytoplasmic domain of the TβRI.32 Sumoylation of TβRI facilitates recruitment of Smad2/3 and enhances Smad signaling.32 In contrast, the role of ubiquitination is to mediate degradation of the TGF-β receptor complex, leading to downregulation of TGF-β signaling.33 Smurf1 and Smurf2 proteins are known E3 ligases for TGF-β receptors.16, 34 As it has been reported that Smurf1 deficiency did not alter the degradation or availability of TGF-β receptors,35 we focused on Smurf2 in our study. Our observations showing the ability of 17AAG to inhibit TGF-β signaling was reversed by Smurf2 siRNA along with restoration of TβRII clearly indicate that the Hsp90 inhibitor promotes Smurf2-mediated degradation of TβRII.
In this study, the levels of phospho-Akt, phospho-GSK-3β, and snail1 of cells treated with 17AAG (but not with TGF-β1) were significantly increased by co-treatment with MG132, as shown in Figures 6a and b. We speculate that this is because of the effect of the Hsp90 inhibitor on the upstream Akt kinase, 3-phosphoinositide-dependent protein kinase-1 (PDK1). PDK1 is a kinase responsible for phosphorylation of Akt36 and is one of the Hsp90 client proteins.37 Therefore, in addition to the effect on the expression of TβRII, a presumed 17AAG-induced degradation of PDK1 might be effectively restored by co-treatment with MG132. It is known that increased activation of Akt induces GSK-3β phosphorylation,9 leading to the stabilization of snail1.38
The toxic effects of 17AAG have not been addressed in this study. However, it has been documented that 25-week-old mice treated with 25 mg/kg 17AAG three times a week for 20 weeks were free from obvious side effects.20 There are now many drug candidates that target Hsp90 as both intravenous and oral therapeutics.14 The most advanced product is 17AAG in a phase 3 clinical trial in combination therapy for the treatment of multiple myeloma.14 Therefore, once the safety issue is cleared, this Hsp90 inhibitor would be a good candidate as an anti-fibrotic therapeutic.
In conclusion, this study demonstrates that an Hsp90 inhibitor prevents the development of renal fibrosis induced by TGF-β1 via a mechanism dependent on Smurf2-mediated degradation of TβRII. These data suggest that a new therapeutic strategy based on Hsp90 inhibition may prove beneficial in chronic kidney diseases.
Change history
30 November 2021
A Correction to this paper has been published: https://doi.org/10.1038/s41374-021-00705-3
References
Boor P, Ostendorf T, Floege J . Renal fibrosis: novel insights into mechanisms and therapeutic targets. Nat Rev Nephrol 2010;6:643–656.
Kriz W, Kaissling B, Le Hir M . Epithelial-mesenchymal transition (EMT) in kidney fibrosis: fact or fantasy? J Clin Invest 2011;121:468–474.
Iwano M, Plieth D, Danoff TM et al. Evidence that fibroblasts derive from epithelium during tissue fibrosis. J Clin Invest 2002;110:341–350.
Bottinger EP, Bitzer M . TGF-beta signaling in renal disease. J Am Soc Nephrol 2002;13:2600–2610.
Liu Y . Renal fibrosis: new insights into the pathogenesis and therapeutics. Kidney Int 2006;69:213–217.
Massague J, Seoane J, Wotton D . Smad transcription factors. Genes Dev 2005;19:2783–2810.
Feng X-H, Derynck R . Specificity and versatility in TGF-beta signaling through Smads. Ann Rev Cell Dev Biol 2005;21:659–693.
Derynck R, Zhang YE . Smad-dependent and Smad-independent pathways in TGF-beta family signalling. Nature 2003;425:577–584.
Kattla JJ, Carew RM, Heljic M et al. Protein kinase B/Akt activity is involved in renal TGF-beta1-driven epithelial-mesenchymal transition in vitro and in vivo. Am J Physiol Renal Physiol 2008;295:F215–F225.
Rhyu DY, Yang Y, Ha H et al. Role of reactive oxygen species in TGF-beta1-induced mitogen-activated protein kinase activation and epithelial-mesenchymal transition in renal tubular epithelial cells. J Am Soc Nephrol 2005;16:667–675.
Pratt WB, Toft DO . Regulation of signaling protein function and trafficking by the hsp90/hsp70-based chaperone machinery. Exp Biol Med 2003;228:111–133.
Isaacs JS, Xu W, Neckers L . Heat shock protein 90 as a molecular target for cancer therapeutics. Cancer Cell 2003;3:213–217.
Kamal A, Thao L, Sensintaffar J et al. A high-affinity conformation of Hsp90 confers tumour selectivity on Hsp90 inhibitors. Nature 2003;425:407–410.
Porter JR, Fritz CC, Depew KM . Discovery and development of Hsp90 inhibitors: a promising pathway for cancer therapy. Curr Opin Chem Biol 2010;14:412–420.
Wrighton KH, Lin X, Feng X-H . Critical regulation of TGFbeta signaling by Hsp90. Proc Natl Acad Sci 2008;105:9244–9249.
Kavsak P, Rasmussen RK, Causing CG et al. Smad7 binds to Smurf2 to form an E3 ubiquitin ligase that targets the TGF-beta receptor for degradation. Mol Cell 2000;6:1365–1375.
Auluck PK, Bonini NM . Pharmacological prevention of Parkinson disease in drosophila. Nat Med 2002;8:1185–1186.
Lu A, Ran R, Parmentier-Batteur S et al. Geldanamycin induces heat shock proteins in brain and protects against focal cerebral ischemia. J Neurochem 2002;81:355–364.
Murphy P, Sharp A, Shin J et al. Suppressive effects of ansamycins on inducible nitric oxide synthase expression and the development of experimental autoimmune encephalomyelitis. J Neurosci Res 2002;67:461–470.
Waza M, Adachi H, Katsuno M et al. 17-AAG, an Hsp90 inhibitor, ameliorates polyglutamine-mediated motor neuron degeneration. Nat Med 2005;11:1088–1095.
Schnaper HW, Hayashida T, Hubchak SC et al. TGF-beta signal transduction and mesangial cell fibrogenesis. Am J Physiol Renal Physiol 2003;284:F243–F252.
Liu N, Tolbert E, Pang M et al. Suramin inhibits renal fibrosis in chronic kidney disease. J Am Soc Nephrol 2011;22:1064–1075.
Wang S, Wilkes MC, Leof EB et al. Imatinib mesylate blocks a non-Smad TGF-beta pathway and reduces renal fibrogenesis in vivo. FASEB J 2005;19:1–11.
Li Y, Tan X, Dai C et al. Inhibition of integrin-linked kinase attenuates renal interstitial fibrosis. J Am Soc Nephrol 2009;20:1907–1918.
Sharma K, Jin Y, Guo J et al. Neutralization of TGF-beta by anti-TGF-beta antibody attenuates kidney hypertrophy and the enhanced extracellular matrix gene expression in STZ-induced diabetic mice. Diabetes 1996;45:522–530.
Border WA, Okuda S, Languino LR et al. Suppression of experimental glomerulonephritis by antiserum against transforming growth factor beta1. Nature 1990;346:371–374.
Han DC, Hoffman BB, Hong SW et al. Therapy with antisense TGF-beta1 oligodeoxynucleotides reduces kidney weight and matrix mRNAs in diabetic mice. Am J Physiol Renal Physiol 2000;278:F628–F634.
Wang W, Huang XR, Li AG et al. Signaling mechanism of TGF-beta1 in prevention of renal inflammation: role of Smad7. J Am Soc Nephrol 2005;16:1371–1383.
Isono M, Mogyorosi A, Han DC et al. Stimulation of TGF-beta type II receptor by high glucose in mouse mesangial cells and in diabetic kidney. Am J Physiol Renal Physiol 2000;278:F830–F838.
Hong SW, Isono M, Chen S et al. Increased glomerular and tubular expression of transforming growth factor-beta1, its type II receptor, and activation of the Smad signaling pathway in the db/db mouse. Am J Pathol 2001;158:1653–1663.
Kang JS, Liu C, Derynck R . New regulatory mechanisms of TGF-beta receptor function. Trends Cell Biol 2009;19:385–394.
Kang JS, Saunier EF, Akhurst RJ et al. The type I TGF-beta receptor is covalently modified and regulated by sumoylation. Nat Cell Biol 2008;10:654–664.
D'Azzo A, Bongiovanni A, Nastasi T . E3 ubiquitin ligases as regulators of membrane protein trafficking and degradation. Traffic 2005;6:429–441.
Ebisawa T, Fukuchi M, Murakami G et al. Smurf1 Interacts with transforming growth factor-beta type I receptor through Smad7 and induces receptor degradation. J Biol Chem 2001;276:12477–12480.
Yamashita M, Ying S-X, Zhang G-m et al. Ubiquitin ligase Smurf1 controls osteoblast activity and bone homeostasis by targeting MEKK2 for degradation. Cell 2005;121:101–113.
Balendran A, Casamayor A, Deak M et al. PDK1 acquires PDK2 activity in the presence of a synthetic peptide derived from the carboxyl terminus of PRK2. Curr Biol 1999;9:393–404.
Fujita N, Sato S, Ishida A et al. Involvement of Hsp90 in signaling and stability of 3-phosphoinositide-dependent kinase-1. J Biol Chem 2002;277:10346–10353.
Zhou BP, Deng J, Xia W et al. Dual regulation of Snail by GSK-3beta-mediated phosphorylation in control of epithelial-mesenchymal transition. Nat Cell Biol 2004;6:931–940.
Acknowledgements
This work was supported by the National Research Foundation of Korea Grant funded by the Korean Government (2010-0017163 to HN).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
An Hsp90 inhibitor prevents development of renal fibrosis induced by TGF-β1 via a mechanism dependent on Smurf2-mediated degradation of the TGF-β type II receptor. The data suggest that a new therapeutic strategy based on Hsp90 inhibition may prove beneficial in chronic kidney diseases.
Rights and permissions
About this article
Cite this article
Noh, H., J Kim, H., R Yu, M. et al. Heat shock protein 90 inhibitor attenuates renal fibrosis through degradation of transforming growth factor-β type II receptor. Lab Invest 92, 1583–1596 (2012). https://doi.org/10.1038/labinvest.2012.127
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/labinvest.2012.127
Keywords
This article is cited by
-
PROGRESS STUDY: Progression of chronic kidney disease in children and heat shock proteins
Cell Stress and Chaperones (2021)
-
DsbA-L mediated renal tubulointerstitial fibrosis in UUO mice
Nature Communications (2020)
-
Molecular functional analyses revealed essential roles of HSP90 and lamin A/C in growth, migration, and self-aggregation of dermal papilla cells
Cell Death Discovery (2018)
-
Effects of chronic alcohol exposure on ischemia–reperfusion-induced acute kidney injury in mice: the role of β-arrestin 2 and glycogen synthase kinase 3
Experimental & Molecular Medicine (2017)
-
Acetylated α-Tubulin Regulated by N-Acetyl-Seryl-Aspartyl-Lysyl-Proline(Ac-SDKP) Exerts the Anti-fibrotic Effect in Rat Lung Fibrosis Induced by Silica
Scientific Reports (2016)