Abstract
Early exercise engagement elicits meaningful changes in peripheral blood pressure in patients diagnosed with transient ischaemic attack (TIA) or minor stroke. However, central hemodynamic markers may provide clinicians with important diagnostic and prognostic information beyond that provided by peripheral blood pressure readings. The purpose of this single-centre, randomized, parallel-group clinical trial was to determine the effect of a 12-week aerobic exercise intervention on central and peripheral hemodynamic variables in patients with TIA or minor stroke. In this study, 47 participants (66±10 years) completed a baseline assessment, which involved the measurement of central and peripheral hemodynamic parameters, undertaken in the morning, in a fasted state. Participants were randomized to either a 12-week exercise or control group on completion of the baseline assessment. An identical follow-up assessment was completed post intervention. Central hemodynamic variables were assessed using an oscillometric device at both assessments. Analysis of covariance demonstrated a significant interaction for central and peripheral blood pressure and augmentation index (all P<0.05; ηp2.09–.11), with the exercise group presenting lower values than the control group post intervention (118±17 vs 132±28 mm Hg for central blood pressure; 125±19 vs 138±28 mm Hg for peripheral blood pressure; 104±49 vs 115±67% for augmentation index). The present study demonstrates that participation in an exercise program soon after stroke/TIA diagnosis may elicit significant beneficial changes to a patient’s central systolic blood pressure and augmentation index. This may positively impact upon the treatment strategies implemented by clinicians in the care of patients with TIA and minor stroke.
Similar content being viewed by others
Introduction
Blood pressure is an important risk factor for stroke,1 and is widely cited as a marker that needs to be controlled post stroke by pharmacological and lifestyle management.2 A recent meta-analysis with stroke and transient ischaemic attack (TIA) patients has shown that significant reductions in peripheral blood pressure responses may be evident following participation in behavioural interventions (that is, exercise, lifestyle management programs).3 However, the measurement of central hemodynamic parameters, including central systolic blood pressure (cSBP) and arterial wave reflection (that is, augmentation index, AIx), hold the potential to provide stroke clinicians with important diagnostic and prognostic information beyond that of peripheral blood pressure readings.
Peripheral blood pressures may not accurately reflect the effects of peak arterial blood pressure on centrally located organs such as the heart and brain.4 This is important because individuals who experience a stroke or TIA are at heightened risk of experiencing subsequent vascular events, whether from a cerebrovascular (that is, stroke/TIA) or cardiovascular (that is, myocardial infarction) origin.5, 6 Thus, the assessment of cSBP is particularly important as it reflects the stress and loading on the left ventricle and coronary arteries.7, 8 Yet to date, the effects of secondary prevention behavioural interventions on central blood pressure responses in stroke or TIA patients have not been assessed. As behavioural interventions which have incorporated exercise training, counseling and lifestyle education reduce the risk of subsequent cardiac events in patients with stroke,3 further research is necessary to elucidate the underlying central hemodynamic response to these types of intervention for patients with stroke or TIA.
The purpose of the current randomized control study was to determine the effect of implementing an exercise program soon after minor stroke/TIA diagnosis on cSBP and AIx. It was hypothesised that those individuals who engaged in the exercise program would demonstrate greater changes in central hemodynamic responses compared with those individuals randomized to a usual care control group.
Methods
Participants
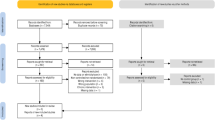
Forty-seven participants (Male, n=35; 66±10 years; 90±19 kg; 173±7 cm; Female, n=12; 64±12 years; 80±20 kg; 159±4 cm) diagnosed with either TIA or minor stroke completed the baseline assessment, of which 43 took part part in a post-intervention assessment (Figure 1). Recruitment commenced in September 2013 and lasted 12 months with participants being randomized to either an exercise or usual care control group (exercise group, n=25; 66±12 years; 88±19 kg; 168±10 cm; control group, n=22; 68±10 years; 90±21 kg; 171±8 cm). Follow-up (post-intervention) assessments were completed between December 2013 and December 2014.
TIA and minor stroke diagnosis was based upon criteria from the New Zealand assessment and management guidelines.9 At the time of hospital presentation within the inpatient setting, stroke severity was assessed using the National Institutes of Health Stroke Scale, functional severity was assessed using the Barthel Index, and stroke risk of TIA patients was assessed using the ABCD2 score. Participants were eligible if they had been diagnosed with their first high-risk TIA (ABCD2 score of ⩾2) or minor stroke (defined as an National Institutes of Health Stroke Scale of ⩽5), lived within the local district health board catchment, and if they did not meet exclusion criteria (Figure 1). Exclusion criteria were: unstable cardiac conditions, uncontrolled diabetes mellitus, severe claudication, oxygen dependence, significant dementia, inability to communicate in English or unable to take part in exercise. All participants complied with drug treatment and standardised therapy in accordance with stroke physician recommendations. The trial was approved by New Zealand’s Central Regional Health and Disabilities Ethics Committee and registered with the Australian and New Zealand Clinical Trials Registry (Trial Registration Number: ACTRN12613000869774), and conformed to the guidelines established in the Declaration of Helsinki. Written informed consent was obtained before participation.
Sample size
On the basis of the findings of Woolley et al.,10 and when examining changes in peripheral systolic blood pressure (pSBP) in TIA patients following participation in an exercise intervention, and when using a two-sided 5% significance level and a power of 80%, a minimum sample size of 19 participants per group was calculated.
Study design
The study was a single-centre, randomized, parallel-group design. TIA or minor stroke was confirmed by a specialist stroke physician at Wellington Hospital within 7 days of symptom onset. Eligible participants were invited to attend a baseline assessment conducted within a thermoneutral laboratory (21–22 °C) typically between 0700 and 1000 hours All participants were fasted (overnight fast), euhydrated, had abstained from caffeine and supplement intake during the morning, and from strenuous physical activity and alcohol consumption for 24 h before assessment. Following completion of a health history questionnaire, and 15 min of quiet supine rest, central blood pressure responses were obtained with the participant in the supine position. Identical assessments were completed post-intervention.
On completion of the baseline assessment, participants were randomized to either an experimental group (usual care plus participation in a 12-week aerobic exercise intervention) or to a control group (usual care, only) using computerized random numbers. Details pertaining to each allocated group were provided on a piece of paper contained within sequentially numbered, opaque sealed envelopes. The randomization procedures were prepared by an investigator with no clinical involvement in the trial. Although participants and the health and exercise practitioner were aware of the allocated treatment condition, data analysts were kept blinded to the allocation.
Subjects randomized to the exercise program completed twice weekly, group-based (three to five participants) exercise sessions for 12 weeks. Exercise was prescribed on a one-to-one basis by health and exercise practitioners. In accordance with recommendations for moderate physical activity participation, participants completed 30–60 min of aerobic exercise at each exercise session.11 This included up to 30 min of continuous walking and up to 30 mins of continuous cycling, with an interim period of ~5 min of passive recovery between bouts. Blood pressure, heart rate and ratings of perceived exertion were measured prior to, during and following each bout of aerobic exercise. Participants commenced their exercise program at 50% of age-predicted maximal heart rate during all aerobic exercise tasks. The exercise intensity and/or volume typically increased by ~5% each week, up to a maximal heart rate training intensity of 90% of age-predicted maximal heart rate. The rate of progression was dependent upon how the subject felt during each session. Subjects were instructed not to exercise beyond an ratings of perceived exertion of 15 (‘hard’ feeling of exertion) during both walking and cycling exercise.12 Exercise practitioners ensured that subjects did not exercise above 90% of their age-predicted maximal heart rate.
The control group (usual care) received standard secondary prevention and educational information from the hospital on discharge and were requested to adhere to their prescribed medication for the duration of the study.
Pulse wave analysis
An oscillometric device (BP+, Uscom Lts, Sydney, NSW, Australia) was used to conduct pulse wave analysis, from which central and peripheral blood pressures and AIx were derived. The AIx% is an independent predictor of cardiovascular risk and mortality.13 Pressure waveforms were recorded on the upper arm, following standard manufacturer guidelines.14 Briefly, the BP+ incorporates an oscillometric blood pressure module which complies with the Association for the Advancement of Medical Instrumentation (AAMI SP10) requirements and receives an A/A rating from the British Hypertension Society evaluation protocol.15 Each measurement cycle (~40 s) records brachial blood pressures and then one set of suprasystolic (~30 mm Hg>systolic) recordings for 10 s. The suprasystolic pressure signals were recorded by a high-fidelity pressure transducer, and the central pressure waveform was derived in the time-domain from the relationship between the total oscillatory pressure in the aorta and the total oscillatory pressure under the occlusion cuff.15, 16 This estimation was scaling-independent (that is, the estimated aortic wave shape did not depend on brachial blood pressure). AIx% was calculated using the formula: AIx%=(P3 – P0)/(P1 – P0), where P0 is pressure at the onset of the pulse (diastolic), P1 is peak pressure of the incident wave (systolic), P3 is peak pressure of the reflected wave. This index describes the relative height of the reflected pressure wave when compared with the incident waveform. Only recordings with a high signal quality were accepted. Signal quality was assessed using the signal to noise (S/N) ratio (in decibels), where S/N values >3 dB were considered acceptable. Two measurements were taken, with 5-min interval. If AIx varied by >4% or blood pressures by >5 mm Hg, a third recording was made and the two closest recordings were averaged.
Statistical analysis
Independent samples t-tests (age, certain lifestyle factors) and Pearson Chi-squared tests (descent, family history of cardiovascular disease (CVD), personal history of CVD, signs and symptoms of CVD, medication) were used to compare baseline data between conditions (exercise, control). A similar analysis was used to assess central and peripheral hemodynamic properties (that is, cSBP, pSBP, peripheral diastolic blood pressure, central pulse pressure, peripheral pulse pressure, AIx) at baseline, and to assess medication use post-intervention. To assess the effect of the exercise intervention on the aforementioned central and peripheral hemodynamic properties, and following checks for normality to determine that these outcome variables were parametric, a series of a-priori repeated-measures analysis of covariance (Test [baseline, post intervention] by Condition [exercise, control], with baseline inserted as a covariate) were conducted. An intention-to-treat analysis was used on all repeated-measures statistical procedures, whereby the last recorded data from a participant’s subsequent assessment was carried forward and used in place of any missing assessments thereafter. Partial eta squared (ηp2) was used to demonstrate the strength of the effect of exercise on the various outcome measures with. 0099,. 0588 and. 1379 representing a small, medium and large effect, respectively.17 Partial eta squared was calculated using the following formula:

Whereby, SSEffect is the estimated variance for a given outcome measure, and SSError is the error variance that is attributable to the effect. Alpha was set at 0.05. Statistical analyses were performed using Statistical Package for Social Sciences version 22 (SPSS, Inc., Chicago, IL, USA). All data are reported as means (s.d.), unless otherwise specified.
Results
Participant recruitment, adherence and recurrent TIA risk
There were no differences in subject characteristics, medication use or CVD risk factors at baseline between groups (P>0.05; Tables 1 and 2 and Supplementary Table). Baseline assessments were undertaken 8±3 days post diagnosis. Mean medication use post-intervention was approaching significance (P=0.09) with participants in the exercise group typically taking less medication compared to those in the control group (Mean difference (95% CI); 0.67 (−1.48 to 0.13); Table 2). Participants randomized to the exercise program attended, on average, 96% of the available sessions. There were no adverse events from participation in the exercise program.
Central and peripheral hemodynamic variables
Table 3 summarises the mean values for the central and peripheral hemodynamic properties measured at baseline and post intervention for both exercise and control conditions. There were no statistical differences in any of the peripheral or central hemodynamic variables reported at baseline between the exercise and control group (all P>0.05). Analysis of covariance demonstrated a significant interaction for cSBP, pSBP and AIx (all P<0.05), with the exercise group presenting lower values than the control group post intervention. A significant interaction was also observed for peripheral pulse pressure and central pulse pressure (both P<0.05). An increase in pulse pressure was observed for the control group between baseline and post intervention, but a decrease was observed with the exercise group.
Discussion
This is the first study to demonstrate that cSBP and AIx are significantly reduced in participants with minor stroke and high-risk TIA following participation in an exercise intervention, soon after diagnosis. In the present study, despite similarities in baseline demographics, the 7% improvement in cSBP following participation in a 12-week exercise program equated to a large effect size (ηp2=0.11). The significance of this finding is further enhanced when considering that there was a large reduction in medication use for those participants randomized to the exercise group, and when considering that there were no changes in cSBP for those individuals who received usual care.
Of the various CVD risk factors that are often assessed in patients with minor stroke and TIA following engagement in an exercise program, peripheral blood pressure responses have received the greatest attention.3, 18, 19, 20, 21 However, it has been suggested that peripheral blood pressures may not accurately reflect the effects of peak arterial blood pressure on centrally located organs,4 nor the stress and loading on the left ventricle and coronary arteries.7, 8 Accordingly, in the present study, the significant reduction in cSBP is of pragmatic importance for this population group, as individuals diagnosed with stroke and TIA are at a heightened risk of experiencing further cardiovascular complications.5, 6 It is interesting to note that the 9 mm Hg mean reduction in cSBP is greater than that reported in a recent meta-analysis, whereby, on average, a 4 mm Hg reduction in peripheral blood pressure is suggested to occur following lifestyle and behavioural interventions.3 The prescription of exercise at a moderate- to high-intensity in the current study may be an underpinning reason for this difference. Nevertheless, as the reduction in pSBP was identical to that reported for cSBP for those in the exercise group, the ecological benefit of monitoring cSBP may be somewhat reduced. In the current study, the exercise group also demonstrated a significant reduction in central and peripheral pulse pressure in comparison to the control group (ηp2=0.10 to 0.14). Because the statistically similar diastolic blood pressures reported between conditions (exercise and control) and assessment sessions (baseline, post-intervention), the ~5 mm Hg reduction in pulse pressure for the exercise group is largely derived by the aforementioned changes in systolic blood pressure (Table 3).
A further novelty of the present study relates to the change in AIx; an indicator of arterial wave reflection.22 In this study, participants who took part in the exercise program demonstrated a 15% reduction in AIx (Table 3). This finding is of significant importance for those with stroke/TIA as AIx is an indicator of systemic arterial stiffness23 and demonstrates the contribution made by the reflected pressure wave to the ascending aortic pressure waveform.22 Conversely, and unexpectedly, AIx increased for the control group. Increased arterial wave reflections have been reported to have adverse effects on ventricular afterload and coronary perfusion, have been significantly correlated to the degree of coronary artery disease,24 and independently predict cardiovascular risk and mortality.25 When considering that stroke and TIA patients have an elevated risk of experiencing cardiovascular and cerebrovascular events post stroke/TIA,1 the measurement of central hemodynamic parameters, including cSBP and AIx, could provide clinicians with important diagnostic and prognostic information beyond that provided by traditional peripheral blood pressure readings. From a pragmatic standpoint, oscillometric devices, like that used in the current study, can provide clinicians with a quick, non-invasive and valid assessment of such hemodynamic parameters.25, 26, 27, 28 Despite this study being sufficiently powered, it is plausible that the observed differences in AIx between the experimental and control groups may be associated with the relatively small sample size and thus, future research should assess such hemodynamic parameters in a larger TIA/minor stroke population.
A recent study reported that the monitoring of central blood pressure, compared with peripheral blood pressure, aided in the management of hypertension and led to decreased medication use without adverse effects of left ventricular mass.29 Therefore, future research should assess whether the monitoring of central hemodynamic variables in those with minor stroke or TIA may lead to similar, positive clinical outcome changes, which may lead to more targeted treatment strategies for these population groups. However, it is important to recognize that in the current study there are some important findings with regards to medication use (Table 2). At the time of follow-up, participants who engaged in the exercise program were shown to be taking significantly fewer anti-thrombotic medications, and there was a trend for taking fewer medications overall, compared to those randomized to the usual care control group. When considering that medicinal management is primarily carried out to have an impact on the cardiovascular system, this finding is pertinent, and reflects the value of engaging patients in an exercise program soon after diagnosis.
In conclusion, the present study has demonstrated that participation in an exercise program soon after stroke/TIA diagnosis may elicit significant changes in cSBP and AIx. It may be important for stroke clinicians to consider central hemodynamic parameters at diagnosis and during follow-up assessments as these parameters may provide important information beyond that provided by traditional peripheral blood pressure readings. As automated oscillometric devices can provide a valid, quick and non-invasive assessment of central hemodynamic parameters, clinicians could consider the use of such measurements in providing more targeted treatment strategies for those with TIA or minor stroke.

References
Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Borden WB et al. Heart disease and stroke statistics-2013 update: A report from the American Heart Association. Circulation 2013; 127: e6–e245.
Adams HJ, Adams R, Brott T, del Zoppo G, Furlan A, Goldstein L et al. Guidelines for the early management of patients with ischemic stroke: a scientific statement from the stroke council of the American Stroke Association. Stroke 2003; 324: 1056–1083.
Lawrence M, Pringle J, Kerr S, Booth S, Govan L, Roberts NJ . Multimodal secondary prevention behavioural interentions for TIA and stroke: a systematic review and meta analysis. PLos ONE 2015; 10: e0120902.
Protogerou AD, Papaioannou TG, Blacher J, Papamichael CM, Lekakis JP, Safar ME . Central blood pressures: do we need them in the management of cardiovascular disease? Is it a feasible therapeutic target? J Hypertens 2007; 25: 265–272.
Touze E, Varenne O, Chatellier G, Peyrard S, Rothwell P, Mas J . Risk of myocardial infarction and vascular death after transient ischemic attack and ischemic stroke: a systematic review and meta-analysis. Stroke 2005; 36: 2748–2755.
Albers GW, Caplan LR, Easton JD, Fayad PB, Mohr JP, Saver JL et al. Transient ischemic attack-proposal for a new definition. N Engl J Med 2002; 347: 1713–1716.
Roman MJ, Devereux RB, Kizer JR, Lee ET, Galloway JM, Ali T et al. Central pressure more strongly relates to vascular disease and outcome than does brachial pressure: the strong heart study. Hypertens 2007; 50: 197–203.
Young Y, Abdolhosseini P, Brown F, Faulkner J, Lambrick D, Williams MA et al. Reliability of oscillometric central blood pressure and wave reflection readings: effects of posture and fasting. J Hypertens 2015; 33: 1588–1593.
Stroke-Foundation. New Zealand Guidelines for the Assessment and Management of People with Recent Transient Ischaemic Attack (TIA). 2008.
Woolley B, Stoner L, Lark S, Wong L, Lanford J, Faulkner J . Effect of early exercise engagement on arterial stiffness in patients diagnosed with a transient ischaemic attack. J Human Hypertens 2015; 29: 87–91.
ACSM's Guidelines for Exercise Testing and Prescription. 8th edn. Lippincott, Williams and Wilkins: Philadelphia, 2010.
Borg G . Borg's Perceived Exertion and Pain Scales. Human Kinetics: Leeds, UK, 1998.
Vlachopoulos C, Aznaouridis K, Stefanadis C . Prediction of cardiovascular events and all-cause mortality with arterial stiffness: a systematic review and meta-analysis. J Am Coll Cardiol 2010; 55: 1318–1327.
Stoner L, Lambrick DM, Faulkner J, Young J . Guidelines for the use of pulse wave analysis in adults and children. J Atheroscler Thromb 2013; 20: 404–406.
Lowe A, Harrison W, El-Aklouk E, Ruygrok P, Al-Jumaily AM . Non-invasive model-based estimation of aortic pulse pressure using suprasystolic brachial pressure waveforms. J Biomech 2009; 42: 2111–2115.
Park CM, March K, Ghosh AK, Tillin T, Mayet J, Lowe A et al. A method comparsion of central blood pressure measurements by pulsecor and sphygmocor devices. Artery Res 2010; 4: 171.
Cohen J . A power primer. Psychol Bull 1992; 112: 155–159.
Faulkner J, Lambrick D, Woolley B, Stoner L, Wong L, McGonigal G . The long-term effect of exercise on vascular risk factors and aerobic fitness in those with TIA; a randomized controlled trial. J Hypertens 2014; 32: 2064–2070.
Faulkner J, Lambrick D, Woolley B, Stoner L, Wong L-K, McGonigal G . Effects of early exercise engagement on vascular risk in patients with transient ischemic attack and nondisabling stroke. J Stroke Cerebrovasc Dis 2013; 22: e388–e398.
Lennon O, Carey A, Gaffney N, Stephenson J, Blake C . A pilot randomized controlled trial to evaluate the benefit of the cardiac rehabilitation paradigm for the non-acute ischaemic stroke population. Clin Rehabil 2008; 22: 125–133.
Prior PL, Hachinski V, Unsworth K, Chan R, Mytka S, O'Callaghan C et al. Comprehensive cardiac rehabilitation for secondary prevention after transient ischemic attack or mild stroke: I: feasibility and risk factors. Stroke 2011; 42: 3207–3213.
Oliver JJ, Webb DJ . Noninvasive assessment of arterial stiffness and risk of atherosclerotic events. Arterioscl Throm Vas 2003; 23: 554–566.
Stoner L, Faulkner J, Lowe A, Lambrick D, Young M, Love R et al. Should augmentation index be normalized to heart rate? J Atheroscl Throm 2014; 21: 11–16.
Weber T, Auer J, O'Rourke MF, Kvasm E, Lassnig E, Lamm G et al. Increased arterial wave reflections predict severe cardiovascular events in patients undergoing percutaneous coronary interventions. Eur Heart J 2005; 26: 2657–2663.
Weber T, O’Rourke MF, Lassnig E, Porodko M, Ammer M, Rammer M et al. Pulse waveform characteristics predict cardiovascular events and mortality in patients undergoing coronary angiography. J Hypertens 2010; 28: 797–805.
Lowe A, Harrison W, Wl-Aklouk E, Ruygrok P, Al-Jumaily AM . Noninvasive model-based estimation of aortic pulse pressure using suprasystolic brachial pressure waveforms. J Biomech 2009; 42: 2111–2115.
Hwang MH, Yoo JK, Kim HK, Hwang CL, Mackay K, Hemstreet O et al. Validity and reliability of aortic pulse wave velocity and augmentation index determined by the new cuff-based SphygmoCor Xcel. J Hum Hypertens 2014; 28: 475–481.
Papaioannou T, Karatzis EN, Karatzi KN, Gialafos EJ, Protogerou AD, Stamatelopoulos KS et al. Hour-to-hour and week-to-week variability and reproducibility of wave reflection indices derived by aortic pulse wave analysis: implications for studies with repeated measurements. J Hypertens 2007; 25: 1678–1686.
Sharman JE, Marwick TH, Gilroy D, Otahal P, Abhayaratna WP, Stowasser M . Randomized trial of guiding hypertension management using central aortic blood pressure compared with best-practice care: principal findings of the BP GUIDE study. Hypertens 2013; 62: 1138–1145.
Acknowledgements
Funding was provided by the Massey University Research Fund. Funding was used to support consumable purchase and participant travel expenses.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on the Journal of Human Hypertension website
Supplementary information
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Faulkner, J., Tzeng, YC., Lambrick, D. et al. A randomized controlled trial to assess the central hemodynamic response to exercise in patients with transient ischaemic attack and minor stroke. J Hum Hypertens 31, 172–177 (2017). https://doi.org/10.1038/jhh.2016.72
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/jhh.2016.72