Abstract
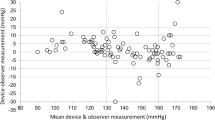
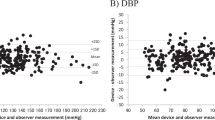
Both automated and auscultatory blood pressure (BP) devices have their strengths and accuracy limitations. Hybrid devices, such as the Nissei DM-3000, are mercury free and provide both automated and auscultatory measurement modes. The aim of this study was to validate all measurement modes of the Nissei DM-3000 device according to the European Society of Hypertension (ESH) protocol, as well as to develop and validate a ‘blinded’ auscultatory measurement mode. Different measurement modes were developed and evaluated in separate studies. Nine sequential same-arm BP measurements were taken alternating between simultaneous mercury sphygmomanometer readings and the device. The latter seven measurements were analysed according to the requirements of the ESH protocol. All measurement modes of the device passed the ESH protocol. The blinded mode achieved the best results with a mean difference±s.d. of −0.1±2.6 and 0.04±2.4 mm Hg for systolic BP (SBP) and diastolic BP (DBP), respectively. The most accurate auscultatory measurement results were obtained with a deflation rate of 2.5 mm Hg s−1 achieving a mean difference±s.d. of −0.6±4.4 (for SBP) and −1.4±2.8 mm Hg (for DBP). The automated mode achieved a mean difference±s.d. of −0.8±6.0 (SBP) and 0.8±4.8 mm Hg (DBP). The Nissei DM-3000 device is a suitable replacement for the mercury sphygmomanometer.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Pickering T, Hall J, Appel L, Falkner B, Graves J, Hill M et al. Recommendations for blood pressure measurement in humans and experimental animals: part 1: blood pressure measurement in humans: a statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Hypertension 2005; 45: 142–161.
Waugh J, Gupta M, Rushbrook J, Halligan A, Shennan AH . Hidden errors of aneroid sphygmomanometers. Blood Press Monit 2002; 7: 309–312.
Langford N, Ferner R . Toxicity of mercury. J Hum Hypertens 1999; 10: 651–656.
O'Brien E . Demise of the mercury sphygmomanometer and the dawning of a new era in blood pressure measurement. Blood Press Monit 2003; 8: 19–21.
van Ittersum FJ, Wijering RM, Lambert J, Donker AJ, Stehouwer CD . Determinants of the limits of agreement between the sphygmomanometer and the SpaceLabs 90207 device for blood pressure measurement in health volunteers and insulin-dependent diabetic patients. J Hypertens 1998; 16: 1125–1130.
Anastas ZM, Jimerson E, Garolis S . Comparison of non-invasive blood pressure measurements in patients with atrial fibrillation. J Cardiovasc Nurs 2008; 23: 519–524.
Shennan AH, de Greeff A . Measuring blood pressure in pregnancy and pre-eclampsia. In: Lyall F, Belfort M (eds). Pre-eclampsia, Etiology and Clinical Practice, Vol. 18 Cambridge University Press: Cambridge, 2007 pp 258–275.
Omboni S, Riva I, Giglio A, Caldara G, Groppelli A, Parati G . Validation of the Omron M5-I, R5-I and HEM-907 automated blood pressure monitors in elderly individuals according to the International Protocol of the European Society of Hypertension. Blood Press Monit 2007; 12: 233–242.
Pickering TG . What will replace the mercury sphygmomanometer? Blood Press Monit 2003; 8: 23–25.
Pickering T . The case for a hybrid sphygmomanometer. Blood Press Monit 2001; 6: 177–179.
Graves J, Tibor M, Murtagh B, Klein L, Sheps S . The Accuson Greenlight 300, the first non-automated mercury-free blood pressure measurement device to pass the International Protocol for blood pressure measuring devices in adults. Blood Press Monit 2004; 9: 13–17.
Stergiou GS, Giovas PP, Gkinos CP, Tzamouranis DG . Validation of the A&D UM-101 professional hybrid device for office blood pressure measurement according to the International Protocol. Blood Press Monit 2008; 13: 37–42.
Tasker F, de Greeff A, Liu B, Shennan AH . Validation of a non-mercury digital auscultatory device according to the European Hypertension Society protocol: Rossmax Mandaus II. Blood Press Monit 2009; 14: 121–124.
Wilton A, Shabeeh H, Cuckson C, Shennan A . Validation of a non-mercury digital auscultatory device with manual pressure registration (PMS Mandaus). Blood Press Monit 2006; 11: 161–164.
O'Brien E, Pickering T, Asmar R, Myers M, Parati G, Staessen J et al. Working group on blood pressure monitoring of the European Society of Hypertension International Protocol for validation of blood pressure measuring devices in adults. Blood Press Monit 2002; 7: 3–17.
British Hypertension Society Blood Pressure Measurement Tutorial. http://www.abdn.ac.uk/medical/bhs/, 1999.
Association for the Advancement of Medical Instrumentation. American National Standard for Electronic or Automated Sphygmomanometers ANSI/AAMI SP10-1992. AAMI: Arlington, VA, 1993.
Bland JM, Altman DG . Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986; 1: 307–310.
Thulin T, Schersten B, Anderson G . Measurement of blood pressure––a routine test in need of standardization. Postgrad Med J 1975; 51: 390–395.
Yong PG, Geddes LA . The effect of cuff pressure deflation rate on accuracy in indirect measurement of blood pressure with the auscultatory method. J Clin Monit 1987; 3: 155–159.
Fozard J, Vercryssen M, Reynolds S, Hancock P, Quilter R . Age differences and changes in reaction time: the Baltimore Longitudinal Study of Aging. J Gerontol 1994; 49: 179–189.
Acknowledgements
We thank the staff and patients who kindly helped us with this research. This study was approved by the Local Research Ethics Committee of Guy's and St Thomas' Hospital NHS Foundation Trust (Ref. EC99/016). All participants provided written informed consent. Partial data were presented at the British Hypertension Society meeting in Cambridge (UK). Individuals performing the study were employed by King's College London. This study was partly funded by Japan Precision Instruments.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Tasker, F., De Greeff, A. & Shennan, A. Development and validation of a blinded hybrid device according to the European Hypertension Society protocol: Nissei DM-3000. J Hum Hypertens 24, 609–616 (2010). https://doi.org/10.1038/jhh.2009.113
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/jhh.2009.113
Keywords
This article is cited by
-
Factors influencing the accuracy of non-invasive blood pressure measurements in patients admitted for cardiogenic shock
BMC Cardiovascular Disorders (2019)
-
From mercury sphygmomanometer to electric device on blood pressure measurement: correspondence of Minamata Convention on Mercury
Hypertension Research (2016)
-
Automated-auscultatory (Hybrid) sphygmomanometers for clinic blood pressure measurement: a suitable substitute to mercury sphygmomanometer as reference standard?
Journal of Human Hypertension (2012)
-
A perfect replacement for the mercury sphygmomanometer: the case of the hybrid blood pressure monitor
Journal of Human Hypertension (2012)
-
Replacing the mercury manometer with an oscillometric device in a hypertension clinic: implications for clinical decision making
Journal of Human Hypertension (2011)