Abstract
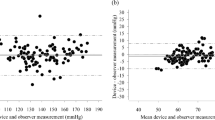
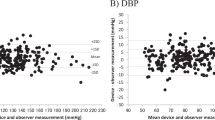
The aim of this study was to assess the blood pressure (BP) measurement accuracy of the Kinetik Blood Pressure Monitor—Series 1 (BPM-1) for use in home or clinical settings according to the 2002 European Society of Hypertension International Protocol (ESH-IP). Forty-two participants were recruited to fulfil the required number of systolic and diastolic BP measurements according to the ESH-IP. Nine sequential same-arm BP readings were measured and analysed for each participant using the test device and observer mercury standard readings according to the 2002 ESH-IP. Forty one participants were used to obtain 33 sets of systolic and diastolic BP readings and were included in the analysis. Mean difference between the device measurements and the observer (mercury standard) measurements was 1.1 ± 7.2/1.1 ± 6.8 mmHg (mean ± standard deviation; systolic/diastolic). The number of systolic BP differences between the test and observer measurements that fell within 5, 10 and 15 mmHg was 65, 86 and 92. For diastolic readings, the number of test—observer measurement differences within 5, 10 and 15 mmHg was 77, 91 and 94. The number of participants with at least two out of three differences within 5 mmHg was 28 for systolic and 40 for diastolic BP readings. Three participants had no differences between the test and observer measurements within 5 mmHg in both the systolic and diastolic measurement categories. The Kinetik BPM-1 device fulfilled the requirements of the ESH-IP validation procedure and can be recommended for clinical use and self-measurement within the home.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
McManus RJ, Wood S, Bray EP, Glasziou P, Hayen A, Heneghan C, et al. Self-monitoring in hypertension: a web-based survey of primary care physicians. J Hum Hypertens. 2014;28:123–7.
Tucker KL, Sheppard JP, Stevens R, Bosworth HB, Bove A, Bray EP, et al. Self-monitoring of blood pressure in hypertension: a systematic review and individual patient data meta-analysis. PLoS Med. 2017;14:e1002389.
Medicines, Healthcare Products Regulatory A. Blood Pressure Measurement Devices London: Medicines and Healthcare Products Regulatory Agency 2013. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/403448/Blood_pressure_measurement_devices.pdf.
O’Brien E, Pickering T, Asmar C, Myers M, Parati G, Staessen J, et al. Working Group on Blood Pressure Monitoring of the European Society of Hypertension International Protocol for validation of blood pressure measuring devices in adults. Blood Press Monit. 2002;7:3–17.
Lewis PS, Chapman N, Chowienczyk P, Clark C, Denver E, Lacy P, et al. Oscillometric measurement of blood pressure: a simplified explanation. A technical note on behalf of the British and Irish Hypertension Society. J Hum Hypertens. 2019;33:349–51.
Acknowledgements
This work was supported by the British and Irish Hypertension Society, Blood Pressure Measurement Working Party.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Schwartz, C.L., Edwards, K., Gamble, W. et al. Validation of the Kinetik Blood Pressure Monitor—Series 1 for use in adults at home and in clinical settings, according to the 2002 European Society of Hypertension International Protocol on the validation of blood pressure devices. J Hum Hypertens 35, 1046–1050 (2021). https://doi.org/10.1038/s41371-020-00445-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41371-020-00445-9