Abstract
Neurofibromatosis type 1 (NF1) is an hereditary disorder characterized by abnormal proliferation of multiple tissues of neural crest origin, and presents mainly with multiple café-au-lait macules, axillary freckling and neurofibromas. Choroidal involvement in NF1 patients has been studied, thanks to the development of non-invasive tools such as infrared monochromatic light during fundus examination, which showed bright patchy lesions consistent with choroidal nodules. Choroidal abnormalities identified with near-infrared reflectance have reported with a frequency of up to 100% in NF1, and have been recently been proposed as a novel diagnostic criterion for NF1. Legius syndrome can be clinically indistinguishable from NF1 and results in a small percentage of individuals being misdiagnosed. We investigated the presence of choroidal abnormalities in Legius syndrome to determine their specificity to NF1 and their potential usefulness as a novel diagnostic criterion for NF1. We examined the fundus of 16 eyes by confocal scanning laser ophthalmoscopy with infrared monochromatic light in eight patients with molecularly confirmed Legius syndrome. No abnormalities were observed, confirming the diagnostic value of choroidal abnormalities for the diagnosis of NF1.
Similar content being viewed by others
Introduction
Café-au-lait macules (CALM) are frequently observed in children, and can pose a diagnostic challenge, especially if isolated. Neurofibromatosis type 1 (NF1) (OMIM #162200) is the most common condition associated with multiple CALM.1 The diagnosis of NF1 is based on the National Institute of Health diagnostic criteria,2 which have been the mainstay for the diagnosis of NF1 over the last 30 years, and include the presence of multiple CALM, intertriginous freckling, neurofibromas and plexiform neurofibromas, iris Lisch nodules, osseous dysplasia, optic pathway glioma, and/or a first-degree relative with NF1. The diagnosis of NF1 is especially challenging in children who have multiple CALM but no other signs of NF1, because most clinical findings develop with age.3 Other clinical manifestations in NF1, such as T2-hyperintensities on brain magnetic resonance imaging (unidentified bright objects, focal areas of signal intensities) and anemic naevi, have been suggested as additional diagnostic criteria to improve diagnosis of NF1.4
In the last 15 years, the availability of novel tools to examine the fundus of the eye during an ophthalmological examination (confocal scanning laser ophthalmoscope, with near-infrared reflectance imaging (NIR)) provide an opportunity to identify abnormalities of the choroid both in pediatric and adult NF1 patients.5, 6
Recently Parrozzani et al.7 conducted a study on choroidal abnormalities in a large cohort of NF1 pediatric patients (0–16 years old) (140 patients affected by NF1, fulfilling atleast two diagnostic criteria, and 59 suspected, fulfilling a single diagnostic criterion). NF1-related choroidal abnormalities were detected in 60.5% of the affected and 2.4% of the suspected patients. No choroidal abnormalities were observed in the healthy subjects (n=42, age matched). Compared with standard National Institute of Health criteria for the diagnosis of NF1, the presence of choroidal abnormalities was the third parameter for positive predictive value, and the fourth for sensitivity, specificity and negative predictive value. On the basis of these findings, the authors suggested choroidal abnormalities as a diagnostic criterion for NF1. The application of this sign as a diagnostic criterion could help in anticipating the age of NF1 diagnosis, allowing an appropriate follow-up.
NF1 is caused by mutations in the NF1 gene, ultimately resulting in the loss of growth control and increased cell proliferation, through an alteration of the RAS/MAPK pathway.8 Because of this, individuals with NF1 are at higher risk for developing tumors. Individuals with NF1 have a lifetime cancer risk of ~60%. Specifically, NF1 patients have high risks for malignant brain tumors including high-grade gliomas, adrenal cancer and malignant peripheral nerve sheath tumor. Among benign tumors, optic pathway gliomas are found in ~15% of patients. Moreover, children with NF1 have an increased risk of leukemia.9
While NF1 is the most common syndrome seen in children with multiple CALM, other syndromes caused by mutations in genes involved in the RAS-MAPK pathway, including Legius syndrome, Noonan syndrome and other Noonan syndrome with multiple lentigines, cardio-facio-cutaneous syndrome and Costello syndrome, present with overlapping phenotypic features and need to be considered during the diagnostic process.8
Individuals with Legius syndrome can be misdiagnosed as NF1 based on pigmentary manifestations of CALMs, distinctive freckling patterns and a family history of multiple CALMs.10 The overlapping phenotype between NF1 and Legius syndrome can potentially complicate diagnostic evaluations and lead to medically inappropriate screening protocols.
The present study was designed to investigate the presence of choroidal abnormalities detected by NIR with confocal scanning laser ophthalmoscope in patients with Legius syndrome.
Materials and methods
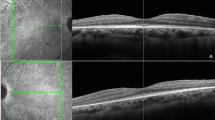
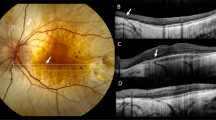
Patients were clinically and molecularly diagnosed at the Medical Genetic Unit, at the Pediatric Clinic of Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico of Milan, Italy, at Developmental Neurology Unit of IRCCS Foundation C. Besta Neurological Institute of Milan, Italy, and at the Genomic and post-genomic Center IRCCS Mondino National Neurological Institute of Pavia, Italy. The patients were referred to the Ophthalmological Unit of Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico of Milan, Italy, and underwent a general routine ophthalmological evaluation of best-corrected visual acuity, assessment for Lisch nodules, mydriatic fundus biomicroscopy with a 90-diopter lens and with NIR at 815 nm by means of a confocal scanning laser ophthalmoscope (Spectralis HRA, Heidelberg Engineering, Heidelberg, Germany) of the posterior pole and mid-periphery of the retina, as previously described in detail.6 The study was approved by Ethics Committee of the participating Centers. Written informed consent was obtained from the patients ⩾18 years; in case of patients <18 years, parents’ written consent was obtained and patient’s written assent was obtained if ⩾8 years.
Results
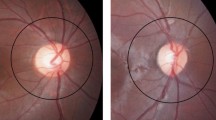
Between November 2010 and May 2016, a total of eight patients with Legius syndrome were evaluated. Demographic and clinical characteristics of the patients are shown in Table 1. The cohort includes four males and four females, with an age ranging from 3 to 66 years. In all cases, Legius syndrome was suspected based on the presence of multiple CALMS along with a positive family history and a negative molecular genetic testing for the NF1 gene. The diagnosis of Legius syndrome was confirmed by the molecular analysis of the SPRED1 gene. The fundus of 16 eyes was examined using NIR, failing to identify any choroidal abnormality (Figure 1).
Discussion
Choroidal nodules consist of proliferating Schwann cells arranged concentrically around an axon.11 They have histological similarities to cutaneous neurofibromas and Lisch nodules of the iris,12 in line with the characteristic aberrant proliferation of multiple tissues of neural crest origin in NF1.13
Firstly, observed using ophthalmoscopy by Huson et al.,14 choroidal nodules were thought to be a rare manifestation of NF1,15 but this was an underestimate due to the limitations of conventional methods used for fundus examination. Following the availability of indocyanine green angiography first, and then of the development of infrared light examination with a confocal scanning laser ophthalmoscope for detecting the choroidal structure, a number of studies have investigated choroidal involvement in NF1 patients.
The overall prevalence of choroidal abnormalities in NF1 patients varies from 60.5 to 100%, depending on the age group examined and on the number of patients involved. In the first study using NIR imaging to examine choroidal structure, Yasunari et al.5 reported a prevalence of choroidal nodules of 100% in 17 adult patients; Viola et al.6 in 2006 studied 95 NF1 patients and 100 controls, reporting a prevalence of choriodal nodules of 86% in the adult group and of 71% in the pediatric one (⩽12y). Later, Goktas et al.16 reported a prevalence of 78.9% in a pediatric population of 19 patients. In the largest study involving a pediatric population (140 patients), Parrozzani et al.7 reported a prevalence of choroidal nodules of 60.5%; likewise Vagge et al.17 identified choroidal nodules in 69.2% of 78 pediatric patients. Conversely, choroidal abnormalities have been rarely detected in controls (0 to 7%). The number of nodules seen in NF1 patients varies, but it was calculated that highest diagnostic accuracy (90% in the adult group) can be achieved with a cutoff value between 1 and 2 choroidal nodules (sensitivity 83% and specificity 96%).6, 18
However, given the phenotypic overlap between NF1 and the syndromes associated with CALMs, and whose causative genes encode for proteins within the RAS-MAPK signaling pathway, it is important to investigate whether choriodal nodules are specific to NF1. The presence of choroidal abnormalities in Legius syndrome has been recently investigated in one study involving 11 patients. Choroidal nodules were observed in two patients, presenting as one isolated nodule in each patient.18 Our preliminary result, coupled with this study, seems to confirm that multiple choroidal abnormalities are specific to neurofibromatosis 1 and that a cutoff value of 2 nodule can accurately diagnose NF1. To date, no patient has been described with choroidal nodules out of 19 individuals with Legius syndrome, using 2 nodules as cutoff value. Thus, we have a 95% confidence intervals equal to 0–9.49% computed with the Bayes method, considering one single eye but 0–4.9% if we consider the two eyes. Future studies are needed to assess the prevalence of choroidal abnormalities in other rasopathies, to consider this sign as an additional diagnostic criterion for NF1.5, 6, 7, 16 Given the high prevalence of choroidal nodules in NF1 both in the pediatric and the adult population, their absence or low frequency in Legius syndrome and controls, and the ease of infrared light examination, our study confirms the usefulness of choroidal nodules in the differential diagnosis between NF1 and Legius syndrome.
References
Whitehouse, D. Diagnostic value of the café-au-lait spot in children. Arch. Dis. Child 41, 316–319 (1966).
Neurofibromatosis. Conference statement. National Institutes of Health Consensus Development Conference. Arch Neurol 45, 575–578 (1998).
DeBella, K., Szudek, J. & Friedman, J. M. Use of the National Institutes of Health criteria for diagnosis of neurofibromatosis 1 in children. Pediatrics 105 (3 Pt 1), 608–614 (2000).
Tadini, G., Milani, D., Menni, F., Pezzani, L., Sabatini, C. & Esposito, S. Is it time to change the neurofibromatosis 1 diagnostic criteria? Eur. J. Intern. Med. 25, 506–510 (2014).
Yasunari, T., Shiraki, K., Hattori, H. & Miki, T. Frequency of choroidal abnormalities in neurofibromatosis type 1. Lancet 356, 988–992 (2000).
Viola, F., Villani, E., Natacci, F., Selicorni, A., Melloni, G., Vezzola, D. et al. Choroidal abnormalities detected by near-infrared reflectance imaging as a new diagnostic criterion for neurofibromatosis 1. Ophthalmology 119, 369–375 (2012).
Parrozzani, R., Clementi, M., Frizziero, L., Miglionico, G., Perrini, P., Cavarzeran, F. et al. In vivo detection of choroidal abnormalities related to NF1: feasibility and comparison with standard NIH diagnostic criteria in pediatric patients. Invest. Ophthalmol. Vis. Sci. 56, 6036–6042 (2015).
Tidyman, W. E. & Rauen, K. A. The RASopathies: developmental syndromes of Ras/MAPK pathway dysregulation. Curr. Opin. Genet. Dev. 19, 230–236 (2009).
Gutmann, D. H., Ferner, R. E., Listernick, R. H., Korf, B. R., Wolters, P. L. & Johnson, K. J. Neurofibromatosis type 1. Nat. Rev. Dis. Primers 3, 17004 (2017).
Brems, H., Chmara, M., Sahbatou, M., Denayer, E., Taniguchi, K., Kato, R. et al. Germline loss-of-function mutations in SPRED1 cause a neurofibromatosis 1-like phenotype. Nat. Genet. 39, 1120–1126 (2007).
Kurosawa, A. & Kurosawa, H. Ovoid bodies in choroidal neurofibromatosis. Arch. Ophthalmol. 100, 1939–1941 (1982).
Richetta, A., Giustini, S., Recupero, S. M., Pezza, M., Carlomagno, V., Amoruso, G. et al. Lisch nodules of the iris in neurofibromatosis type 1. J. Eur. Acad. Dermatol. Venereol. 18, 342–344 (2004).
Kissel, P., Andre, J. M. & Jacquire, A. The Neurocristopathies (Masson, New York, 1981).
Huson, S., Jones, D. & Beck, L. Ophthalmic manifestations of neurofibromatosis. Br. J. Ophthalmol. 71, 235–238 (1987).
Destro, M., D’Amico, D. J., Gragoudas, E., Brockhurst, R. J., Pinnolis, M. K., Albert, D. et al. Retinal manifestation of neurofibromatosis. Diagnosis and management. Arch. Ophthalmol. 109, 662–666 (1991).
Goktas, S., Sakarya, Y., Ozcimen, M., Alpfidan, I., Uzun, M., Sakarya, R. et al. Frequency of choroidal abnormalities in pediatric patients with neurofibromatosis type 1. J. Pediatr. Ophthalmol. Strabismus 51, 204–208 (2014).
Vagge, A., Camicione, P., Capris, C., Sburlati, C., Panarello, S., Calevo, M. G. et al. Choroidal abnormalities in neurofibromatosis type 1 detected by near-infrared reflectance imaging in paediatric population. Acta Ophthalmol. 93, e667–e671 (2015).
Cassiman, C., Casteels, I., Jacob, J., Plasschaert, E., Brems, H., Dubron, K. et al. Choroidal abnormalities in café-au-lait syndromes: a new differential diagnostic tool? Clin. Genet. 91, 529–535 (2017).
Acknowledgements
We thank the patients and their parents for their kind cooperation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Tucci, A., Saletti, V., Menni, F. et al. The absence that makes the difference: choroidal abnormalities in Legius syndrome. J Hum Genet 62, 1001–1004 (2017). https://doi.org/10.1038/jhg.2017.78
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/jhg.2017.78
This article is cited by
-
Histologic correlates of “Choroidal abnormalities” in Neurofibromatosis type 1 (NF1)
Acta Neuropathologica (2024)
-
An update on choroidal abnormalities and retinal microvascular changes in neurofibromatosis type 1
Orphanet Journal of Rare Diseases (2022)
-
Challenges in the diagnosis of neurofibromatosis type 1 (NF1) in young children facilitated by means of revised diagnostic criteria including genetic testing for pathogenic NF1 gene variants
Human Genetics (2022)
-
Congenital grouped albinotic spots of the retinal pigment epithelium in a patient with hemihypertrophy and café au lait spots
Documenta Ophthalmologica (2018)