Abstract
Mutations in BRCA genes elevate risk for breast and ovarian cancer. These mutations are population specific. As there are no data on BRCA mutation screening on larger number of probands in Serbia to date, aim of this study was to determine types and frequencies of BRCA mutations in individuals from high-risk families from Serbia, as well as to determine which BRCA mutations may be considered as founder for Serbian population. We analyzed 94 probands and detected 9 frameshift mutations in 12 individuals, 1 benign BRCA2 nonsense mutation and numerous missense and synonymous mutations in both genes. Frequency of frameshift mutations is 12.77%. In addition to two novel mutations detected in our population we reported previously, we detected another novel mutation—c.7283delT in BRCA2 exon 14. None of the detected deleterious mutations may be considered as founder mutations for Serbian population, as each of them was found in no more than two high-risk families. This mutation diversity is most probably due to high migration rate in history of this part of Europe. Interpretation of genetic testing results with missense mutations of unknown clinical importance is very challenging and should be approached with caution, using all available data sources for results’ interpretation.
Similar content being viewed by others
Introduction
BRCA1 (breast cancer susceptibility gene 1) gene was the first gene associated with elevated breast/ovarian cancer risk.1 Its sequence is divided into 24 exons, 22 of which are coding exons for 1863 amino-acid (aa) protein product.2, 3 BRCA1 protein has four main structural domains: RING domain at N-terminus (aa 24–64),4 two nuclear localization signals (NLS; aa 503–508 and 606–615),5 DNA-binding domain (aa 452–1079)6 and two BRCA1 C-terminal domains7 (aa 1642–1736 and 1756–1855, http://www.uniprot.org/uniprot/P38398).
The second gene associated with hereditary breast cancer, BRCA2 (breast cancer susceptibility gene 2, also known as FANCD1), is divided into 27 exons, 26 of which are coding exons for 3418 aa protein product.8, 9 BRCA2 protein contains some distinctive domains: eight BRC motifs (aa 1009 and 2083),10 single strand DNA-binding domain11 and two NLSs in last 156 aa.12
After DNA damage occurs, the information about it needs to be transmitted via various molecules (signaling) in order to stop cell cycle, choose appropriate DNA repair mechanism depending on the type of DNA damage, to remodel chromatin so that proteins of repair machinery may approach DNA damage site and correct the mutation. If the damage cannot be repaired, cell activates pathways that lead to apoptosis.
BRCA1 and BRCA2 proteins are involved in almost all mentioned functions. BRCA1 has a role in signaling, DNA repair, chromatin remodeling, ubiquitination and regulation of cell cycle, apoptosis and transcription.13 BRCA2 is involved in cell cycle M-phase checkpoint regulation, homologous recombination and DNA repair.14 Mutations that compromise BRCA protein functions lead to decreased ability of DNA repair and regulation of cell cycle and apoptosis. This leads to accumulation of mutations (genomic instability) and uncontrolled proliferation, hallmarks of cancerous cells. That is why individuals who inherit mutation in one of BRCA genes are more prone to developing cancer.
Mutations in BRCA genes are scattered throughout numerous coding regions without clustering, complicating mutation detection. More than 1600 and 1900 mutations in BRCA1 and BRCA2, respectively have been identified.15 The majority of them are frameshift mutations (around 70%), while nonsense and missense mutations contribute with around 10% each. In addition to clinically significant mutations that impair protein function, numerous variants, especially missense ones, have been identified. These variants may have unknown impact on protein function and may low or moderately elevate cancer risk.
BRCA mutation spectra are population specific. Some mutation(s) may be frequent in one population and rare in the other. This is usually due to so-called founder effect. In populations that are geographically and/or reproductively isolated, or derived from limited number of individuals (founders), whole gene pool of that population is based on the founders’ gene pool. The consequence is high frequency of mutations that may be rather rare in other populations.16 Such are Ashkenazi Jew and Polish populations, which are characterized by three founder BRCA mutations each.17, 18 Testing for founder mutations covers majority of BRCA mutation carriers in these populations, making genetic testing faster, cheaper and easier. At the same time, large portion of BRCA mutations have been detected only once, and they are considered as family-specific mutations.
Apart from our previous results on new BRCA mutations detected in two families in Serbia19 and some earlier reports on mutation testing of small number of probands from Serbia,20, 21 to date there are no data on more extensive BRCA mutation screening in Serbia.
Aim of this study was to determine types and frequencies of BRCA mutations in larger number of probands from Serbia that meet criteria for BRCA testing, as well as to determine which BRCA mutations are the most frequent in Serbian high-risk families and may be considered as founder for Serbian population.
Materials and methods
We analyzed 94 (87 female and 7 male) probands from 71 families. The criteria for proband selection were:
-
More than one breast cancer case on the same side of pedigree,
-
Ovarian cancer with positive family history for breast or ovarian cancer,
-
Breast and ovarian cancer in same individual,
-
Bilateral breast cancer,
-
Male breast cancer, with or without positive family history,
-
Early breast cancer (before age 35), with or without family history,
-
Relative who is BRCA mutation carrier.
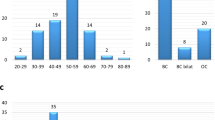
Of 94 probands, 52 had breast cancer (including early, bilateral and male breast cancer cases), 4 had ovarian cancer and 6 had both breast and ovarian cancer. Details on probands’ personal history are listed in Table 1.
Of 94 probands tested, 54 had family history of breast cancer (including early, bilateral and male breast cancer cases), 20 had family history of ovarian cancer or both breast and ovarian cancer, while 18 did not have positive family history for breast or ovarian cancer. Details on family histories of tested individuals are listed in Table 2.
Blood samples were collected from various parts of Serbia. All probands signed informed consent form approved by the Ethics Committee of Institute for Oncology and Radiology of Serbia.
DNA was isolated from blood samples on ABI Prism 6100 Nucleic Acid PrepStation using BloodPrep Kit (Applied Biosystems, Foster City, CA, USA) according to manufacturer’s instructions. BRCA1 and BRCA2 coding regions were amplified and dye-labeled using PCR and cycle sequencing conditions described previously.19 Samples were sequenced on ABI PRISM 310 or 3130 Genetic Analyzer. Obtained sequences were examined for the presence of mutations by alignment with wild-type complementary DNA sequence (GenBank U14680 for BRCA1; GenBank U43746 for BRCA2) using BLAST tool (http://www.ncbi.nlm.nih.gov/BLAST/). Observed mutations were then analyzed for their clinical significance and effect on protein product in Breast Cancer Information Core database (http://www.research.nhgri.nih.gov/bic/) and Leiden Open Variation Database (http://www.lovd.nl/2.0/), as well as using PolyPhen software (http://genetics.bwh.harvard.edu/pph/).
Results
We tested 94 individuals. Genes to be tested were determined according to the mutation carrier probability using BRCAPRO software for each individual. Twenty-four individuals were tested for the presence of family mutations only.
We detected 9 frameshift mutations (5 in BRCA1 and 4 in BRCA2 gene) in 12 individuals (Table 3). None of the detected frameshift mutations may be considered as founder mutations for Serbian population, as each of them was found in no more than two high-risk families.
Frequency of frameshift mutations is 12.77% (12/94) (Figure 1).
We detected one nonsense mutation in BRCA2 gene (10204A>T, K3326X, c.9976A>T, p.Lys3326*) in one individual.
We also detected numerous missense mutations, classified by PolyPhen software as ‘benign’, ‘possibly’ or ‘probably damaging’ (Table 4), as well as numerous synonymous mutations in BRCA1/2 genes (data not shown).
Discussion
Key event in tumorigenesis is losing control over genome stability and proliferation. To ensure that they do not proliferate after DNA damage, cells have developed crosslinked mechanisms of cell cycle checkpoints and DNA repair.22 If cell acquires damage in one of the genes involved in DNA repair and cell cycle control (such as BRCA genes), mutations that occur afterward remain unrepaired, leading to mutation accumulation and genome instability, uncontrolled proliferation and tumorigenesis. Therefore, individuals who inherit mutations in one of BRCA genes are more prone to develop cancers. Depending on position and type of mutation, protein function may be more or less impaired, more or less elevating cancer risk. Frameshift mutations impair protein function severely, it loses some of domains essential for its function, significantly elevating cancer risk. Missense mutations may have different impact on protein function depending on the position of altered aa: the more important role of altered amino acid in protein function, the more severe impact of the amino-acid alteration is.
In addition to BRCA1 mutation c.4646_4665del we previously reported and whose possible consequences to protein functions we described,19 we detected another four BRCA1 frameshift mutations (Table 3). All these mutations lead to synthesis of truncated protein products that lack BRCA1 C-terminal domains, disrupting interaction with other proteins, disturbing essential BRCA1 functions. In the presence of c.843_846delCTCA, c.844_850dupTCATTAC and c.2019delA mutations, BRCA1 protein is severely truncated and has only 296, 287 and 699 aa, respectively. These proteins lack numerous phosphorilation sites that activate BRCA1 to stop cell cycle in various cell cycle phases after DNA damage. These truncated proteins have only RING domain and one of the NLS signals, and it is highly unlikely that such short proteins have any function preserved. Cell with such truncated BRCA1 protein continues with cell cycle even after DNA damage, accumulating mutations, leading to genome instability and tumorigenesis.
BRCA2 mutation c.4139_4140dupTT was first reported by us19 and later by a group from Croatia.23 In addition, we detected another three frameshift BRCA2 mutations (Table 3). One of them is novel mutation that has not been reported previously in Breast Cancer Information Core or Leiden Open Variation databases: c.7283delT BRCA2 exon 14. Protein truncated by this mutation has intact BRC domains and is able to bind RAD51. However, each of frameshift mutations found in BRCA2 gene lead to syntheses of truncated proteins that lack NLSs at BRCA2 C-terminus. This leaves both BRCA2 and RAD51 proteins in cytoplasm, unable to have their roles in DNA repair in nucleus, leading to genomic instability and tumorigenesis. Findings that truncated BRCA2 proteins that lack NLSs are located in cytoplasm with majority of RAD51 protein support this conclusion.12, 24
BRCA2 nonsense mutation p.Lys3326* leads to truncated BRCA2 protein that lacks last 93 amino acids on C-terminus. In some studies this mutation has been found together with deleterious mutations,25, 26 whereas other studies showed no difference in frequency of this mutation in patients and in control group,27 so its neutral effect is assumed, as this mutation is classified in Breast Cancer Information Core and Leiden Open Variation databases. Functional analysis gave results similar to those for wild-type BRCA2.28 However, it was found that this nonsense mutation is five times more frequent in families with hereditary pancreatic cancer comparing with control group.29 In our study, BRCA2 mutation p.Lys3326* has been found in one healthy individual with positive family history for breast cancer (her sister, mother, maternal aunt and maternal grandmother developed breast cancers). It is possible that healthy proband did not inherit family deleterious mutation. As samples from cancer patients from this family were not available for testing, it was not possible to determine potential presence of family deleterious BRCA mutation that would eliminate suspicions about possible effect of detected nonsense mutation on cancer risk in this family.
Missense mutations that do not lead to complete disruption of protein function may slightly change structure of domains important for protein function. These mutations are marked as unclassified variants owing to their unknown clinical impact. The effect of these mutations is estimated according to their position and type of altered amino acid using computer softwares, and is described by probability of disrupting protein function and therefore elevating disease risk. PolyPhen software terms missense mutations as ‘benign’, ‘probably’ or ‘possibly damaging’. Missense mutation may increase cancer risk by altering gene penetrability in deleterious mutation carriers. In noncarriers, presence of more than one unclassified variants may have cumulative effect and increase cancer risk. Classification of missense mutations may change in time with acquiring more information on their impact, and some mutation first classified as possibly damaging may turn out to be benign, and vice versa.
On the basis of comparative analyses of BRCA1 protein sequences in different species, it was shown that majority of BRCA1 missense mutations found in our group are neutral or with small clinical significance.30 By combining several methods it was confirmed that p.Ser1512Ile, p.Met1652Ile and p.Ser1040Asn are neutral.31, 32, 33 Earlier studies showed that mutations p.Gln356Arg and p.Pro871Leu are associated with elevated ovarian cancer risk.34 However, analyzing larger number of probands it was shown that these mutations are not associated with elevated breast35, 36 or ovarian cancer risk.37 Risk contribution of BRCA1 missense mutations was estimated based on segregation with disease, absence in ethnically matched controls, amino-acid conservancies, and it was shown that majority of mutations we found are probably neutral.38, 39 BRCA1 mutation p.Arg841Trp, which was found together with known deleterious mutation, is considered to be neutral.40 However, possibility that this mutation moderately elevates cancer risk cannot be excluded,40 which is supported by PolyPhen analysis that classifies this mutation as ‘possibly damaging’. By combining various methods, several studies associated this mutation with elevated cancer risk.34, 41, 42, 43 Some data suggest that mutations p.Gln356Arg and p.Ser1512Ile found together elevate cancer risk.44 Among our probands, these two BRCA1 mutations were found together in one female with in situ breast cancer, which may suggest the confirmation of mentioned literature data.
Functional analysis of p.His372Asn BRCA2 mutation gave results similar to those for wild-type BRCA2 gene, so it is possible that this mutation is benign.28 However, it has been suggested that this mutation in homozygous His/His form elevates breast and ovarian cancer risk (relative risk=1.3–1.5).45, 46 Elevated breast cancer risk associated with this mutation was found only in a group of BRCA1/2-negative high-risk women.36 However, in large study that included 15 000 patients and 15 000 controls, this association has not been confirmed: for His/His homozygotes relative risk=1.12, whereas for heterozygotes relative risk=1.05.47 This mutation changes amino acid in region of BRCA2 protein (aa 290–453) that interacts with histone acetiltransferase P/CAF, modulating transcription by modifying chromatin.48 It is possible that this amino-acid change at position 372 has impact on this interaction, affecting chromatin remodeling. In our tested group, all three individuals with His/His genotype developed disease: first one developed early female breast cancer, second one ovarian cancer and third male breast cancer. These results may confirm that this mutation in His/His homozygous form contributes to cancer risk.
BRCA2 missense mutations p.Ser599Phe and p.Ser2536Pro have not been described previously. PolyPhen software classifies these mutations as ‘possibly damaging’, so their potential impact on BRCA2 protein function is suggested. However, unclassified variant p.Ser599Phe has been detected in 22 probands in our laboratory, almost all (20/22) as homozygous. Mutation p.Ser2536Pro is found in 12 probands so far, and all of them are homozygous. Although these are unclassified variants with potential impact on disease development, the fact that both of these mutations are common in our population, as well as the fact that they are most often found as homozygous, decreases the possibility that these mutations may be considered as deleterious or with high impact on cancer risk.
BRCA2 missense mutation p.Met927Val is detected in two individuals from family with breast cancer cases. This mutation is not described in literature, and PolyPhen analysis classifies this mutation as ‘probably damaging’, assuming that this mutation may increase cancer risk. The fact that both affected members of this family are mutation carriers suggests that this assumption may be correct.
Possible clinical significance of missense mutations changes in time, with acquiring more data regarding association of these mutations with increased cancer risk. Therefore, interpretation of results with missense mutations of unknown clinical impact is very challenging and should be approached with caution, using all available data sources (softwares, literature data, personal experience, and so on).
Overview of mutation carriers in our group, type of cancer, age of onset and number of cancers in their families are given in Table 5. In all individuals affected with ovarian cancer or in those who had family member(s) affected with ovarian cancer, BRCA1 mutation was detected. Among BRCA2 mutation carriers, there are neither ovarian cancer patients nor individuals with relative(s) affected with ovarian cancer. These findings are in accordance with literature data that ovarian cancer is more often associated with BRCA1 than with BRCA2 mutations.49 BRCA1 mutations were also found in individuals affected with both breast and ovarian cancer or in those who had family member affected with both types of cancer. This is in accordance with data found in literature that BRCA1 mutation carriers affected with breast cancer are at an increased risk for ovarian cancer.50 Among BRCA2 mutation carriers, there is one individual with one male relative affected with breast cancer, which is in accordance with the fact that male breast cancers are more often associated with BRCA2 mutations.51 Among 12 BRCA1/2 mutation carriers, three of them were not affected with cancer. One of them was BRCA1 mutation carrier (c.2019delA), age 33, from the family with ovarian cancer cases only. As BRCA1-related ovarian cancer is not associated with earlier onset of disease, it is possible that this mutation carrier will develop ovarian cancer later in life, as she did not opt for prophylactic surgery. In remaining two healthy mutation carriers BRCA2 frameshift mutations were detected. One of them was 43 (c.4139_4140dupTT), and the other was 35 years old (c.4987_4990delGTCA), both were from site-specific breast cancer families. Both of them opted for follow-up, refusing prophylactic surgery. As BRCA2 mutations are more often associated with later onset of breast cancer,52 these two individuals still may develop disease later in life. Furthermore, it is possible that not all of healthy mutation carriers will develop disease because of incomplete penetrability of BRCA genes.
According to literature data, in families with breast and/or ovarian cancers frequency of BRCA1 and BRCA2 mutations are 0.7–29% and 1.5–25%, respectively, and about 20% in total for both genes.53 In our sample this frequency is 12.77%, which is in accordance with data from other authors.
In early breast cancer cases, independent on family history, BRCA1 and BRCA2 mutations are found in 0.7–10% and 1–6%, respectively.53 In our test group, 19 individuals had early breast cancer (including one person who, in addition to early breast cancer, later developed contralateral breast cancer, and one individual who developed early breast cancer, ovarian cancer, colon cancer and lung cancer). Among these 19 early breast cancer patients, mutations were found in two individuals (10.53%). One of the mutations was found in BRCA1 gene, which is in accordance with data that associated BRCA1 mutations with early breast cancer.52 This mutation was found in individual whose mother (also a mutation carrier) first developed breast cancer and later ovarian cancer, which is supported by data that BRCA1 mutation carriers who developed breast cancer are at increased risk for developing ovarian cancer.50 Second mutation in early breast cancer group is found in BRCA2 gene, in individual who after early breast cancer developed contralateral breast cancer. BRCA2 mutations are rarely associated with early or bilateral breast cancers (more frequently they are associated with BRCA1 mutations), but this result is understandable when proband’s family history of male breast cancer is taken into account. In individuals who were affected with early breast cancer only (n=17), one BRCA1 mutation has been found (5.88%).
In eight probands who developed bilateral breast cancer, one mutation in BRCA2 gene was found. This is not in accordance with literature data that indicate BRCA1 mutations as more frequent in individuals with bilateral breast cancer,54 but this finding becomes understandable in the light of proband’s family history of male breast cancer. Mutation frequency in bilateral breast cancer group is 12.50%.
Literature data show that BRCA2 mutation frequency in male breast cancer cases, independently of family history and the age of onset, is 7–14%.53 So far, we tested four males affected with breast cancer and none of them were found to be BRCA2 mutation carriers. Among female individuals with family history of male breast cancer (n=4), who also developed breast cancer, we detected one BRCA2 mutation. In total, taking into account all individuals with male breast cancers in their families (eight individuals from seven families), one BRCA2 mutation was detected, frequency being 12.50%, which is in accordance with the given literature data.
In four probands with ovarian cancer, one BRCA1 mutation was found, frequency being 25%. In five probands with both breast and ovarian cancer (one of them developed early breast cancer, ovarian, colon and lung cancer), two BRCA1 mutations were found (40%; 2/5). This is in accordance with data in literature, suggesting that BRCA1 mutation carriers are at increased ovarian cancer risk after developing breast cancer.50 In addition, BRCA1 mutations increase risk for developing Fallopian tube cancer, which is supported by our finding that one proband with this type of cancer is BRCA1 mutation carrier. Individual who developed early breast cancer, ovarian, colon and lung cancer is not BRCA mutation carrier, although high probability of BRCA mutation presence may be supposed due to numerous various types of cancer this individual developed.
In conclusion, we detected nine deleterious BRCA mutations in 12 individuals. Frequency of BRCA mutations in our group is 12.77% (12/94). In addition to two novel mutations detected in our population and reported previously,19 we detected another novel mutation that was not previously reported in Breast Cancer Information Core or Leiden Open Variation databases by others—c.7283delT in BRCA2 exon 14. We did not identify BRCA mutation that would be characteristic for Serbian population, most probably because of high rate of migrations in history of this part of Europe. Possible clinical significance of missense mutations changes in time, with acquiring more data regarding association of these mutations with increased cancer risk. Therefore, interpretation of results showing missense mutations of unknown clinical importance is very challenging and should be approached with caution, using all available data sources.
References
Hall, J. M., Lee, M. K., Newman, B., Morrow, J. E., Anderson, L. A., Huey, B. et al. Linkage of early-onset familial breast cancer to chromosome 17q21. Science 250, 1684–1689 (1990).
Miki, Y., Swensen, J., Shattuck-Eidens, D., Futreal, P. A., Harshman, K., Tavtigian, S. et al. A strong candidate for the breast and ovarian cancer susceptibility gene BRCA1. Science 266, 66–71 (1994).
Scully, R., Ganesan, S., Brown, M., De Caprio, J. A., Cannistra, S. A., Feunteun, J. et al. Location of BRCA1 in human breast and ovarian cell lines. Science 272, 123–125 (1996).
Meza, J. E., Brzovic, P. S., King, M. C. & Klevit, R. E. Mapping the functional domains of BRCA1. Interaction of the ring finger domains of BRCA1 and BARD1. J. Biol. Chem. 274, 5659–5665 (1999).
Chen, C. F., Li, S., Chen, Y., Chen, P. L., Sharp, Z. D. & Lee, W. H. The nuclear localization sequences of the BRCA1 protein interact with the importin-α subunit of the nuclear transport signal receptor. J. Biol. Chem. 271, 32863–32868 (1996).
Paull, T. T., Cortez, D., Bowers, B., Elledge, S. J. & Gellert, M. From the cover: direct DNA binding by Brca1. Proc. Natl Acad. Sci. USA 98, 6086–6091 (2001).
Koonin, E. V., Altschul, S. F. & Bork, P. BRCA1 protein products… Functional motifs…. Nat. Genet. 13, 266–268 (1996).
Thorslund, T. & West, S. C. BRCA2: a universal recombinase regulator. Oncogene 26, 7720–7730 (2007).
Teng, L. S., Zheng, Y. & Wang, H. H. BRCA1/2 associated hereditary breast cancer. J. Zhejiang. Univ. Sci. B 9, 85–89 (2008).
Chen, C. F., Chen, P. L., Zhong, Q., Sharp, Z. D. & Lee, W. H. Expression of BRC repeats in breast cancer cells disrupts the BRCA2eRad51 complex and leads to radiation hypersensitivity and loss of G2/M checkpoint control. J. Biol. Chem. 274, 32931–32935 (1999).
Yang, H., Jeffrey, P. D., Miller, J., Kinnucan, E., Sun, Y., Thoma, N. H. et al. BRCA2 function in DNA binding and recombination from a BRCA2–DSS1–ssDNA structure. Science 297, 1837–1848 (2002).
Spain, B. H., Larson, C. J., Shihabuddin, L.S., Gage, F. H. & Verma, I. M. Truncated BRCA2 is cytoplasmic: implications for cancer-linked mutations. Proc. Natl Acad. Sci. USA 96, 13920–13925 (1999).
Huen, M. S. Y., Sy, S. M. H. & Chen, J. BRCA1 and its toolbox for the maintenance of genome integrity. Nat. Rev. Mol. Cell. Biol. 11, 138–148 (2010).
Gudmundsdottir, K. & Ashworth, A. The roles of BRCA1 and BRCA2 and associated proteins in the maintenance of genomic stability. Oncogene 25, 5864–5874 (2006).
Lindor, N. M., McMaster, M. L., Lindor, C. J. & Greene, M. H. National Cancer Institute, Division of Cancer Prevention, Community Oncology and Prevention Trials Research Group. Concise handbook of familial cancer susceptibility syndromes—second edition. J. Natl Cancer Inst. Monogr. 38, 1–93 (2008).
Ferla, R., Calo, V., Cascio, S., Rinaldi, G., Badalamenti, G., Carreca, I. et al. Founder mutations in BRCA1 and BRCA2 genes. Ann. Oncol. 18 (Suppl 6), vi93–vi98 (2007).
Abeliovich, D., Kaduri, L., Lerer, I., Weinberg, N., Amir, G., Sagi, M. et al. The founder mutations 185delAG and 5382insC in BRCA1 and 6174delT in BRCA2 appear in 60% of ovarian cancer and 30% of early-onset breast cancer patients among Ashkenazi women. Am. J. Hum. Genet 60, 505–514 (1997).
Gorski, B., Jakubowska, A., Huzarski, T., Byrski, T., Gronwald, J., Grzybowska, E. et al. A high proportion of founder BRCA1 mutations in Polish breast cancer families. Int. J. Cancer 110, 683–686 (2004).
Dobričić, J., Branković-Magić, M., Filipović, S. & Radulović, S. Novel BRCA1/2 mutations in Serbian breast and breast-ovarian cancer patients with hereditary predisposition. Cancer Genet. Cytogenet. 202, 27–32 (2010).
Papp, J., Raicevic, L., Milasin, J., Dimitrijevic, B., Radulovic, S. & Olah, E. Germline mutation analysis of BRCA1 and BRCA2 genes in Yugoslav breast-ovarian cancer families. Oncol. Rep. 6, 1435–1438 (1999).
Konstantopoulou, I., Janković, R., Raičević, L., Ladopoulou, A., Armaou, S., Nikolopoulos, G. et al. BRCA1 and BRCA2 genes mutation analysis in patients with a family history of breast and ovarian cancer. Jugoslav. Med. Biochem. 23, 271–277 (2004).
Jackson, S. P. & Bartek, J. The DNA-damage response in human biology and disease. Nature 461, 1071–1078 (2009).
Levanat, S., Musani, V., Cvok, M. L., Susac, I., Sabol, M., Ozretic, P. et al. Three novel BRCA1/BRCA2 mutations in breast/ovarian cancer families in Croatia. Gene 498, 169–176 (2012).
Davies, A. A., Masson, J. Y., McIlwraith, M. J., Stasiak, A. Z., Stasiak, A., Venkitaraman, A. R. et al. Role of BRCA2 in control of the RAD51 recombination and DNA repair protein. Mol. Cell. 7, 273–282 (2001).
Haraldsson, K., Loman, N., Zhang, Q. X., Johannsson, O., Olsson, H. & Borg, A. BRCA2 germ-line mutations are frequent in male breast cancer patients without a family history of the disease. Cancer Res. 58, 1367–1371 (1998).
Claes, K., Poppe, B., Machackova, E., Coene, I., Foretova, L., De Paepe, A. et al. Differentiating pathogenic mutations from polymorphic alterations in the splice sites of BRCA1 and BRCA2. Genes Chromosomes Cancer 37, 314–320 (2003).
Mazoyer, S., Dunning, A. M., Serova, O., Dearden, J., Puget, N., Healey, C. S. et al. A polymorphic stop codon in BRCA2. Nat. Genet. 14, 253–254 (1996).
Wu, K., Hinson, S. R., Ohashi, A., Farrugia, D., Wendt, P., Tavtigian, S. V. et al. Functional evaluation and cancer risk assessment of BRCA2 unclassified variants. Cancer Res. 65, 417–426 (2005).
Martin, S. T., Matsubayashi, H., Rogers, C. D., Philips, J., Couch, F. J., Brune, K. et al. Increased prevalence of the BRCA2 polymorphic stop codon K3326X among individuals with familial pancreatic cancer. Oncogene 24, 3652–3656 (2005).
Abkevich, V., Zharkikh, A., Deffenbaugh, A. M., Frank, D., Chen, Y., Shattuck, D. et al. Analysis of missense variation in human BRCA1 in the context of interspecific sequence variation. J. Med. Genet. 41, 492–507 (2004).
Arnold, N., Peper, H., Bandick, K., Kreikemeier, M., Karow, D., Teegen, B. et al. Establishing a control population to screen for the occurrence of nineteen unclassified variants in the BRCA1 gene by denaturing high-performance liquid chromatography. J. Chromatogr. B. Analyt. Technol. Biomed. Life. Sci. 782, 99–104 (2002).
Mirkovic, N., Marti-Renom, M. A., Weber, B. L., Sali, A. & Monteiro, A. N. Structure-based assessment of missense mutations in human BRCA1: implications for breast and ovarian cancer predisposition. Cancer Res. 64, 3790–3797 (2004).
Phelan, C. M., Đapić, V., Tice, B., Favis, R., Kwan, E., Barany, F. et al. Classification of BRCA1 missense variants of unknown clinical significance. J. Med. Genet. 42, 138–146 (2005).
Janezic, S. A., Ziogas, A., Krumroy, L. M., Krasner, M., Plummer, S. J., Cohen, P. et al. Germline BRCA1 alterations in a population-based series of ovarian cancer cases. Hum. Mol. Genet. 8, 889–897 (1999).
Dunning, A. M., Chiano, M., Smith, N. R., Dearden, J., Gore, M., Oakes, S. et al. Common BRCA1 variants and susceptibility to breast and ovarian cancer in the general population. Hum. Mol. Genet. 6, 285–289 (1997).
Seymour, I. J., Casadei, S., Zampiga, V., Rosato, S., Danesi, R., Falcini, F. et al. Disease family history and modification of breast cancer risk in common BRCA2 variants. Oncol. Rep. 19, 783–786 (2008).
Wenham, R. M., Schildkraut, J. M., McLean, K., Calingaert, B., Bentley, R. C., Marks, J. et al. Polymorphisms in BRCA1 and BRCA2 and risk of epithelial ovarian cancer. Clin. Cancer Res. 9, 4396–4403 (2003).
Couch, F. J., Farid, L. M., Deshano, M. L., Tavtigian, S. V., Calzone, K., Campeau, L. et al. BRCA2 germline mutations in male breast cancer cases and breast cancer families. Nat. Genet. 13, 123–125 (1996).
Greenman, J., Mohammed, S., Ellis, D., Watts, S., Scott, G., Izatt, L. et al. Identification of missense and truncating mutations in the BRCA1 gene in sporadic and familial breast and ovarian cancer. Genes Chromosomes Cancer 21, 244–249 (1998).
Goldgar, D. E., Easton, D. F., Deffenbaugh, A. M., Monteiro, A. N., Tavtigian, S. V., Couch, F. J. et al. Integrated evaluation of DNA sequence variants of unknown clinical significance: application to BRCA1 and BRCA2. Am. J. Hum. Genet. 75, 535–544 (2004).
Petersen, G. M., Parmigiani, G. & Thomas, D. Missense mutations in disease genes: a Bayesian approach to evaluate causality. Am. J. Hum. Genet. 62, 1516–1524 (1998).
Fleming, M. A., Potter, J. D., Ramirez, C. J., Ostrander, G. K. & Ostrander, E. A. Understanding missense mutations in the BRCA1 gene: an evolutionary approach. Proc. Natl Acad. Sci. USA 100, 1151–1156 (2003).
Lee, T. C., Lee, A. S. & Li, K. B. Incorporating the amino acid properties to predict the significance of missense mutations. Amino Acids 35, 615–626 (2008).
Hadjisavvas, A., Adamou, A., Kitsios, P., Phanis, C., Kyriacou, K. & Christodoulou, C. G. Q356R and S151RI are BRCA1 variants that may be associated with breast cancer in a Cypriot family. Oncol. Rep. 9, 383–386 (2002).
Healey, C. S., Dunning, A. M., Teare, M. D., Chase, D., Parker, L., Burn, J. et al. A common variant in BRCA2 is associated with both breast cancer risk and prenatal viability. Nat. Genet. 26, 362–364 (2000).
Spurdle, A. B., Hopper, J. L., Chen, X., Dite, G. S., Cui, J., McCredie, M. R. et al. The BRCA2 372 HH genotype is associated with risk of breast cancer in Australian women under age 60 years. Cancer Epidemiol. Biomarkers Prev. 11, 413–416 (2002).
The Breast Cancer Association Consortium. Commonly studied single-nucleotide polymorphisms and breast cancer: results from the breast cancer association consortium. J. Natl Cancer Inst. 98, 1382–1396 (2006).
Fuks, F., Milner, J. & Kouzarides, T. BRCA2 associates with acetyltransferase activity when bound to P/CAF. Oncogene 17, 2351–2354 (1998).
Elit, L. Familial ovarian cancer. Can. Fam. Physician 47, 778–784 (2001).
Finch, A., Beiner, M., Lubinski, J., Lynch, H. T., Moller, P., Rosen, B. et alHereditary Ovarian Cancer Clinical Study Group Salpingo-oophorectomy and the risk of ovarian, fallopian tube, and peritoneal cancers in women with a BRCA1 or BRCA2 Mutation. JAMA 296, 185–192 (2006).
The Breast Cancer Linkage Consortium. Cancer risks in BRCA2 mutation carriers. J. Natl Cancer Inst. 91, 1310–1316 (1999).
Loman, N., Johannsson, O., Kristoffersson, U., Olsson, H. & Borg, A. Family history of breast and ovarian cancers and BRCA1 and BRCA2 mutations in a population-based series of early-onset breast cancer. J. Natl Cancer Inst. 93, 1215–1223 (2001).
Fackenthal, J. D. & Olopade, O. I. Breast cancer risk associated with BRCA1 and BRCA2 in diverse populations. Nat. Rev. Cancer 7, 937–948 (2007).
Metcalfe, K., Lynch, H. T., Ghadirian, P., Tung, N., Olivotto, I., Warner, E. et al. Contralateral breast cancer in BRCA1 and BRCA2 mutation carriers. J. Clin. Oncol. 22, 2328–2335 (2004).
Acknowledgements
This study was supported by a grant of the Ministry of Education, Science and Technological Development of the Republic of Serbia (Grant number 41026). We thank Professor Dr Zvonko Magic and his co-workers on their permanent support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Dobričić, J., Krivokuća, A., Brotto, K. et al. Serbian high-risk families: extensive results on BRCA mutation spectra and frequency. J Hum Genet 58, 501–507 (2013). https://doi.org/10.1038/jhg.2013.30
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/jhg.2013.30
Keywords
This article is cited by
-
BRCA1 and BRCA2 germline variants in breast cancer patients from the Republic of Macedonia
Breast Cancer Research and Treatment (2018)
-
Prevalence and Penetrance of BRCA1 and BRCA2 Germline Mutations in Colombian Breast Cancer Patients
Scientific Reports (2017)
-
Spectrum and frequencies of BRCA1/2 mutations in Bulgarian high risk breast cancer patients
BMC Cancer (2015)