Abstract
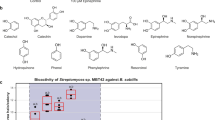
Autoregulators are low-molecular-weight signaling compounds that control the production of many secondary metabolites in actinomycetes and have been referred to as ‘Streptomyces hormones’. Here, potential producers of Streptomyces hormones were investigated in 40 Streptomyces and 11 endophytic actinomycetes. Production of γ-butyrolactone-type (IM-2, VB) and butenolide-type (avenolide) Streptomyces hormones was screened using Streptomyces lavendulae FRI-5 (ΔfarX), Streptomyces virginiae (ΔbarX) and Streptomyces avermitilis (Δaco), respectively. In these strains, essential biosynthetic genes for Streptomyces hormones were disrupted, enabling them to respond solely to the externally added hormones. The results showed that 20% of each of the investigated strains produced IM-2 and VB, confirming that γ-butyrolactone-type Streptomyces hormones are the most common in actinomycetes. Unlike the γ-butyrolactone type, butenolide-type Streptomyces hormones have been discovered in recent years, but their distribution has been unclear. Our finding that 24% of actinomycetes (12 of 51 strains) showed avenolide activity revealed for the first time that the butenolide-type Streptomyces hormone is also common in actinomycetes.
Similar content being viewed by others
Introduction
Actinomycetes are Gram-positive, high-GC-content bacteria that can produce a variety of secondary metabolites, many of which are used as anti-bacterial, anti-fungal and anti-cancer medicines. Production of these compounds in actinomycetes is influenced by a variety of environmental and physiological factors, including autoregulators.1 Although they are produced at very low concentrations, these autoregulators have important roles in regulating secondary metabolism and sometimes morphological differentiation in actinomycetes.2 Hence, they are considered ‘Streptomyces hormones.’ The first studied Streptomyces hormone was A-factor, which was identified in 1967 as a signal controlling streptomycin production in Streptomyces griseus;3 followed by the discovery of VB in 1987, which regulates virginiamycin M1 and virginiamycin S production in S. virginiae.4 In 1989, IM-2, which triggers the production of several nucleoside antibiotics, as well as the blue pigment indigoidine in S. lavendulae were also discovered.5 The structures of these autoregulators are shown in Figure 1.
The distribution of the γ-butyrolactone-type and butenolide-type Streptomyces hormones is of interest to us, because an understanding of it will help us evaluate their significance as controlling tools of secondary metabolite production in the genus actinomycetes. However, a distribution study of Streptomyces hormones is hindered by the fact that they are produced only in extremely small amounts in culture medium, ~0.5 , 3.3 and 0.6 pg ml−1 for IM-2, VB and avenolide, respectively.4, 5, 6 This complicates the detection, quantification and characterization processes by chemical (colorimetric or enzymatic) or physical (GC or HPLC) methods. On the other hand, Streptomyces hormones can induce their cognate secondary metabolites even at nanomolar concentrations,7 making bioassay with the responsive Streptomyces strains the most sensitive and convenient method.
In the distribution study of A-factor carried out by Hara and Beppu,8 a mutant S. griseus strain that lost the ability to produce A-factor and streptomycin was obtained by UV-irradiation and was used as an indicator strain in the bioassay. Actinomycetes strains capable of restoring streptomycin production in the A-factor-deficient S. griseus were supposed to produce A-factor. Unlike the A-factor study, in the previous distribution studies of VB and IM-2, wild-type strains were used as the indicators in bioassay due to the lack of VB-deficient and IM-2-deficient strains;7, 9, 10 thus, interference by endogenous hormones could not be completely avoided. In the course of clarifying the regulatory cascades by Streptomyces hormones in actinomycetes, our laboratory successfully constructed VB-, IM-2- and avenolide-deficient strains by disrupting essential genes in the biosynthetic pathway.6, 11, 12 In the present study, the VB- and IM-2-deficient strains were used as indicators in bioassay to investigate VB and IM-2 distribution. The avenolide distribution was also investigated for the first time with the avenolide-deficient strain as an indicator.
Materials and methods
Strains and cultivation conditions
The 51 actinomycetes strains used in this study are listed in Supplementary Table 1 and 2. Among the 40 Streptomyces strains listed in Supplementary Table 1, 38 strains were obtained from the Biological Resource Center, NITE, Tokyo, Japan. Streptomyces fradiae C373.1D and Streptomyces albus J1074 were provided by Professor Eric Cundliffe (University of Leicester, Leicester, England) and Professor Mervyn Bibb FRS (John Innes Centre, Norwich, England), respectively. Eleven actinomycetes listed in Supplementary Table 2 were isolated form leaf samples collected in Izu Peninsula, Japan. f-medium (containing (g l−1) bacto casitone, 7.5; yeast extract, 7.5; glycerol, 15; NaCl, 2.5) was used for cultivation.4 To prepare seed cultures, 20 ml of f-medium in a 100-ml Erlenmeyer flask was inoculated with spores and incubated on a reciprocating shaker (120 s.p.m) at 28 oC for 3 days. The main cultivation was prepared by inoculating 2.4 ml of the seed culture into 80 ml of f-medium in a 500 ml baffled flask, followed by incubation for 3 days under the same conditions as above.
Preparation of ethyl acetate extract
The main culture (60 ml) of each strain was adjusted to pH 3.0 with 3 M HCl and was extracted three times with 60 ml of ethyl acetate. The solvent layer was evaporated to dryness and the residue re-dissolved in 600 μl of ethyl acetate. The dissolved samples were then assayed for IM-2, VB and avenolide activities.
Assays of Streptomyces hormones
IM-2 activity was assayed in a liquid culture of S. lavendulae FRI-5 (ΔfarX) by observing the IM-2-dependent production of blue pigment as described previously.5 In this study, instead of the wild-type strain in the previous work,7 we used the farX-disrupted strain.12 As farX is the key biosynthetic gene of IM-2, the farX disruptant lost completely the ability to produce IM-2 and the influence of endogenous IM-2 that the wild-type strain produces was avoided.
VB activity was assayed in liquid culture of S. virginiae (ΔbarX) by measuring the VB-dependent production of virginiamycin as described previously.13 Similar to the case in the IM-2 assay system, the key biosynthetic gene (barX) for VB was disrupted and the barX-disruptant was used in this study to avoid the influence of endogenous VB.11
Avenolide activity was assayed in solid culture of S. avermitilis (Δaco) by measuring the avenolide-dependent production of avermectin.6 As aco is one of the essential biosynthetic genes of avenolide, the aco disruptant lost the ability to produce endogenous avenolide. Spores (1.0 × 105 CFU) of S. avermitilis (Δaco) were spread onto 2 ml YMS-MC medium pre-mixed with an ethyl acetate extract of each strain in a 16-well plate, followed by incubation at 28 oC for 8 days.14 The agar culture was diced and extracted with an equal volume of methanol for 2 h. The methanol extract was collected by centrifugation and analyzed by high-pressure liquid chromatography to evaluate avermectin production.15
Quantification of Streptomyces hormone activity
One unit of Streptomyces hormones was defined as the minimum amount of hormones in each bioassay needed to trigger the production of blue pigment, virginiamycin and avermectin, respectively. The unit corresponds to 5 nM for synthetic IM-2-C5, 79 nM for synthetic VB-C7 and 8 nM for synthetic avenolide. We tested various amounts of ethyl acetate extract of each strain on three Streptomyces hormone assays. The minimal amount of ethyl acetate extract that could trigger the production of secondary metabolites in each assay was assumed to contain one unit per milliliter of Streptomyces hormones. Authentic IM-2-C5, VB-C7 and avenolide were chemically synthesized as described, respectively.5, 13, 16
Results
Specificity of the IM-2, VB and avenolide assay system
In order to confirm that our assay systems can discriminate among IM-2, VB and avenolide, the specificity of each assay was checked by using synthetic Streptomyces hormones at various concentrations. The results of the specificity check are shown in Figure 2.
In the IM-2 assay, IM-2-C5 was effective as low as 5 nM, whereas VB-C7 and avenolide did not show any ability to trigger blue-pigment production even at 20 μM. This indicated that S. lavendulae FRI-5 (ΔfarX) responded 4,000-fold more specifically toward IM-2-C5 than VB-C7 or avenolide.
In the VB assay, the minimum effective concentration of VB-C7 was 79 nM. On the other hand, IM-2-C5 and avenolide did not show any activity at 15 or 20 μM, indicating that S. virginiae (ΔbarX) could respond 190 and 253-fold more specifically toward VB-C7 than IM-2-C5 and avenolide.
In the avenolide assay, avenolide was effective as low as 8 nM, while IM-2-C5 and VB-C7 did not induce any avermectin production even at 20 μM, confirming that S. avermitilis (Δaco) could discriminate avenolide 2,500-fold more specifically from IM-2 or VB.
Distribution of IM-2, VB and avenolide in 40 strains of Streptomyces
To know the general distribution of Streptomyces hormones in Streptomyces species, 40 Streptomyces strains available in our laboratory were selected for this study (Supplementary Table S1). Among the strains tested, 15 of them (37.5%) showed the ability to produce at least one kind of active Streptomyces hormone (Table 1).
Among the 40 strains, 22 were investigated in our previous study for production of IM-2 and VB using wild-type strains as indicator strains.7 Under our current assay systems, these strains showed similar production abilities to those in our previous study, except for Streptomyces longwoodensis NBRC14251 and Streptomyces sioyaensis NBRC 12820. IM-2 activity from S. longwoodensis NBRC 14251 and VB activity from S. sioyaensis NBRC 12820 were detected in our current assay system for the first time. These results indicated that our assay system was suitable for the study of Streptomyces hormone production.
Avenolide was first discovered in S. avermitilis in 2011 as a new class of Streptomyces hormones.6 Until now, no other strains were found to have an avenolide signaling pathway. With our avenolide assay system, we detected nine strains (22.5%) that had avenolide activity. Among these nine strains, Streptomyces albidoflavus NBRC 12790, S. albus J1074 and Streptomyces bambergiensis NBRC 13479 showed very high avenolide activity (≥ 500 units per ml) compared with S. avermitilis, which produced ~33 units per ml of avenolide. On the other hand, Streptomyces cyaneofuscatus NBRC 13190, S. griseus NBRC 13350 and Streptomyces sahachiroi NBRC 13928 showed quite low avenolide activity (< 10 units per ml).
Regarding the biosynthesis of avenolide, an aco/cyp17 cluster was identified in S. avermitilis as the essential genes and two Streptomyces species (S. fradiae and Streptomyces ghanaensis) were found to have homologous genes and gene arrangements similar to that of the aco/cyp17 cluster,6 suggesting that these two species might also use avenolide as their hormone to control the secondary metabolism. However, neither of them shows any avenolide production nor IM-2/VB production in f-medium. Although MM-1 medium (tylosin production medium) for S. fradiae and TSB medium (moenomycin production medium) for S. ghanaensis were also tested,17, 18 no production was detected, suggesting that the aco/cyp17 homolog in the genomes of these two strains might not function as biosynthetic genes of avenolide.
Distribution of IM-2, VB and avenolide in endophytic actinomycetes
In recent years, endophytic actinomycetes have emerged as a promising source of bioactive compounds.19 Whether bioactive compounds in endophytic actinomycetes are controlled by Streptomyces hormones is also our concern. In order to address this question, as well as to have a broader picture about the distribution of Streptomyces hormones in actinomycetes, 11 strains with different morphologies and 16S DNA sequence were selected from our in-house library of endophytic actinomycetes (Supplementary Table S2) and tested for the ability to produce IM-2, VB and avenolide active compounds. As a result, a high ratio (7 of 11) of strains showed the ability to produce at least one kind of Streptomyces hormone (Table 2). Among them, five strains had IM-2 or VB activity and three showed avenolide activity. Especially, Streptomyces sp. HN5 and Streptomyces sp. HN70 showed high avenolide activity of 500 and 333 units per ml, respectively, indicating their potential as avenolide producers.
Discussion
To the best of our knowledge, the signaling cascade involving γ-butyrolactone autoregulators (A-factor-type, VB-type and IM-2-type autoregulators) is the most studied in the regulation of secondary metabolite production in actinomycetes. A-factor, VB and IM-2 were first discovered in S. griseus, S. virginiae and S. lavendulae FRI-5 in 1967, 1987 and 1989, respectively, as low-molecular-weight signaling compounds that trigger the production of streptomycin, virginiamycin and blue pigment, respectively, resulting in the general term ‘Streptomyces hormones.’3, 4, 5
Distribution studies of Streptomyces hormones are often hindered by the fact that they are usually effective and present only at nano molar concentrations; thus, chemical (colorimetric or enzymatic) or physical (GC or HPLC) means of detection can not be employed to detect and quantify the Streptomyces hormones in actinomycetes. However, as Streptomyces hormones usually have high host specificity and the cognate host can respond to the presence of specific types of hormones at extremely low concentrations, bioassay becomes the most suitable approach to clarify their presence in actinomycetes.
In this study, we used S. lavendulae FRI-5 (ΔfarX) to detect the IM-2-type Streptomyces hormones, S. virginiae (ΔbarX) for the VB type and S. avermitilis (Δaco) for the avenolide type. These strains are disruptants of essential biosynthetic genes for the cognate Streptomyces hormones, so that no endogenous Streptomyces hormone is produced, enabling them to respond solely to the exogenously added Streptomyces hormones; they are therefore considered ideal bioassay hosts. According to our results, 10 among the 51 tested actinomycete strains produce IM-2 and the same number of strains produce VB-active compounds, indicating that IM-2 activity and VB activity are each distributed in about 20% of actinomycetes. These values match with those in our previous study (13.2% for IM-2 and 15.2% for VB) using wild-type strains as the indicator strains.7, 9, 10 The distribution of A-factor, another group of γ-butyrolactone autoregulators, was not investigated in this study, because such studies with A-factor-deficient strains of S. griseus was already reported by Hara and Beppu,8 and by Eritt et al.,20 indicating that 14.8 and 24.1% of actinomycetes seem to produce A-factor-active compounds. A sum of these distributions (20% for IM-2, 20% for VB, and 24.1% for A-factor) indicates that ~64.1% of actinomycetes are capable of producing at least one kind of Streptomyces hormone of the γ-butyrolactone type, affirming that γ-butyrolactone is the most common type of Streptomyces hormone in actinomycetes.
Regarding the remaining 35.9% of actinomycetes that produce neither A-factor, VB, nor IM-2, it has been a mystery whether they have different types of Streptomyces hormones or do not use any signaling compounds to regulate their secondary metabolism. Although addressing this question using S. avermitilis as a representative non-producer of the γ-butyrolactone Streptomyces hormones, we identified avenolide as a novel class of autoregulator, butenolide-type Streptomyces hormones,6 suggesting that the butenolide-type Streptomyces hormones (including SRBs from S. rochei) might function as signaling compounds in the remaining 35.9% of actinomycetes.21 Our bioassay data in this study revealed that 12 of the 51 strains showed the production of an avenolide-active compound. Furthermore, the presence of essential biosynthetic gene aco homolog in the genome was detected in 11 of the 12 strains by Southern blot analysis (data not shown), suggesting strongly that the 12 strains are producing avenolide-like compounds. This, in turn, implies the 24% distribution of the butenolide-type Streptomyces hormones in actinomycetes. Taken together, the results allow us to conclude that most actinomycetes probably use either γ-butyrolactone Streptomyces hormones or butenolide Streptomyces hormones to control their secondary metabolism; γ-butyrolactone Streptomyces hormones in 64.1% of actinomycetes and butenolide Streptomyces hormones in 24% of actinomycetes. The remaining 11.9% of actinomycetes might not use Streptomyces hormones to regulate the production of secondary metabolites, or they might use another type of Streptomyces hormone that has not been discovered yet.
Among all the strains tested, five showed high avenolide activity (>200 units per ml). In the case of S. albus J1074 (producer of antimycins, candicidins and paulomycins), avenolide activity reached as high as 1,000 units per ml, whereas in the cases of S. bambergiensis (the producer of moenomycin) and Streptomyces sp. HN5 (producing antimicrobial compounds against Candida albicans and Saccharomyces cerevisiae, Table 2), avenolide activity reached as high as 500 units per ml. It is not yet clear whether the avenolide-like compounds in these strains might control the production of any of the secondary metabolites. Our investigation of this is underway in our laboratory.
References
Bibb, M. J. Regulation of secondary metabolism in streptomycetes. Curr. Opin. Microbiol. 8, 208–215 (2005).
Takano, E. γ-Butyrolactones Streptomyces signalling molecules regulating antibiotic production and differentiation: Curr. Opin. Microbiol. 9, 287–294 (2006).
Khokhlov, A. S. et al. A-factor responsible for the biosynthesis of streptomycin by a mutant strain of Actinomyces streptomycini. Dokl. Akad. Nauk. SSSR 177, 232–235 (1967).
Yamada, Y., Sugamura, K., Kondo, K., Yanagimoto, M. & Okada, H. The structure of inducing factors for virginiamycin production in Streptomyces virginiae. J. Antibiot. 40, 496–504 (1987).
Sato, K., Nihira, T., Sakuda, S., Yanagimoto, M. & Yamada, Y. Isolation and structure of a new butyrolactone autoregulator from Streptomyces sp. FRI-5. J. Ferment. Bioeng. 68, 170–173 (1989).
Kitani, S. et al. Avenolide, a Streptomyces hormone controlling antibiotic production in Streptomyces avermitilis. Proc. Natl Acad. Sci. USA 108, 16410–16415 (2011).
Hashimoto, K., Nihira, T. & Yamada, Y. Distribution of virginiae butanolides and IM-2 in the genus Streptomyces. J. Ferment. Bioeng. 73, 61–65 (1992).
Hara, O. & Beppu, T. Mutants blocked in streptomycin production in Streptomyces griseus-the role of A-factor. J. Antibiot. 35, 349–358 (1982).
Ohashi, H., Zheng, Y., Nihira, T. & Yamada, Y. Distribution of virginiae butanolides in antibiotic-producing actinomycetes, and identification of the inducing factor from Streptomyces antibioticus as virginiae butanolide A. J. Antibiot. 42, 1191–1195 (1989).
Choi, S. U., Lee, C. K., Hwang, Y. I., Kinoshita, H. & Nihira, T. γ-Butyrolactone autoregulators and receptor proteins in non-Streptomyces actinomycetes producing commercially important secondary metabolites. Arch. Microbiol. 180, 303–307 (2003).
Lee, Y. J., Kitani, S. & Nihira, T. Null mutation analysis of an afsA-family gene, barX, that is involved in biosynthesis of the γ-butyrolactone autoregulator in Streptomyces virginiae. Microbiology 156, 206–210 (2010).
Kitani, S., Doi, M., Shimizu, T., Maeda, A. & Nihira, T. Control of secondary metabolism by farX, which is involved in the γ-butyrolactone biosynthesis of Streptomyces lavendulae FRI-5. Arch. Microbiol. 192, 211–220 (2010).
Nihira, T., Shimizu, Y., Kim, H. S. & Yamada, Y. Structure-activity relationships of virginiae butanolide C, an inducer of virginiamycin production in Streptomyces virginiae. J. Antibiot. 41, 1828–1837 (1988).
Miyamoto, K. T., Kitani, S., Komatsu, M., Ikeda, H. & Nihira, T. The autoregulator receptor homologue AvaR3 plays a regulatory role in antibiotic production, mycelial aggregation and colony development of Streptomyces avermitilis. Microbiology 157, 2266–2275 (2011).
Kitani, S., Ikeda, H., Sakamoto, T., Noguchi, S. & Nihira, T. Characterization of a regulatory gene, aveR, for the biosynthesis of avermectin in Streptomyces avermitilis. Appl. Microbiol. Biotechnol. 82, 1089–1096 (2009).
Uchida, M. et al. Total synthesis and absolute configuration of avenolide, extracellular factor in Streptomyces avermitilis. J. Antibiot. 64, 781–787 (2011).
Butler, R., Bate, N. & Cundliffe, E. Impact of thioesterase Streptomyces fradiae. Chem. Biol. 6, 287–292 (1999).
Makitrynskyy, R. et al. Genetic factors that influence moenomycin production in streptomycetes. J. Ind. Microbiol. Biotechnol. 37, 559–566 (2011).
Matsumoto, A. & Takahashi, Y. Endophytic actinomycetes: promising source of novel bioactive compounds. J. Antibiot. 70, 514–519 (2017).
Eritt, I., Gräfe, U. & Fleck, W. T. Inducers of both cytodifferentiation and anthracycline biosynthesis of Streptomyces griseus and their occurrence in actinomycetes and other microorganisms. Z. Allg. Mikrobiol. 24, 3–12 (1984).
Arakawa, K., Tsuda, N., Taniguchi, A. & Kinashi, H. The butenolide signaling molecules SRB1 and SRB2 induce lankacidin and lankamycin production in Streptomyces rochei. ChemBioChem 13, 1447–1457 (2012).
Olano, C. et al. Activation and identification of five clusters for secondary metabolites in Streptomyces albus J1074. Microb. Biotechnol. 7, 242–256 (2014).
Schacht, U. & Huber, G. Isolation and properties of further components of the antibiotic moenomycin. J. Antibiot. 22, 597–602 (1969).
Johnstone, D. B. & Selman, A. The production of streptomycin by Streptomyces bikiniensis. J. Bacteriol. 55, 317–326 (1948).
Acknowledgements
We thank Professor Tohru Nagamitsu (School of Pharmacy, Kitasato University, Japan) for providing chemically synthesized avenolide. This work was supported by a Grant-in-Aid for Scientific Research (C) (Grant Number JP15K07358) from the Japan Society for the Promotion of Science to SK, by the New Chemical Technology Research Encouragement Award from the Japan Association for Chemical Innovation to SK, by a Research Grant for Plant Science from the New Technology Development Foundation to SK and by a scholarship from the Ministry of Education, Culture, Sports, Science and Technology of Japan to TNB.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on The Journal of Antibiotics website
Supplementary information
Rights and permissions
About this article
Cite this article
Thao, N., Kitani, S., Nitta, H. et al. Discovering potential Streptomyces hormone producers by using disruptants of essential biosynthetic genes as indicator strains. J Antibiot 70, 1004–1008 (2017). https://doi.org/10.1038/ja.2017.85
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ja.2017.85
This article is cited by
-
The regulatory cascades of antibiotic production in Streptomyces
World Journal of Microbiology and Biotechnology (2020)
-
Manipulation of metabolic pathways controlled by signaling molecules, inducers of antibiotic production, for genome mining in Streptomyces spp.
Antonie van Leeuwenhoek (2018)