Abstract
KB425796-C is a novel antifungal metabolite produced by the newly isolated bacterial strain Paenibacillus sp. No. 530603. This compound is a 40-membered macrocyclic lipopeptidolactone consisting of 12 amino acids and a 3-hydroxy-15-methylpalmitoyl moiety. KB425796-C displayed antifungal activity against micafungin-resistant fungi and was fungicidal to Trichosporon asahii in vitro. In a murine systemic infection model of T. asahii, KB425796-C showed excellent efficacy upon i.p. administration at 32 mg kg−1. In addition, KB425796-C induced morphological changes in the hyphae of Aspergillus fumigatus and had fungicidal effects in combination with micafungin. In a mouse model of septic A. fumigatus infection, although non-treated mice survived for a maximum of only 6 days, the survival rate of micafungin-treated mice (0.1 mg kg−1) increased to 20%, while the survival rate of mice treated with a combination of micafungin (0.1 mg kg−1) and KB425796-C (32 mg kg−1) increased to 100% during the 31-day post-infection period. Our findings suggest that KB425796-C is a good candidate for the treatment of aspergillosis in combination with micafungin.
Similar content being viewed by others
Introduction
The number of reported infections caused by Aspergillus species is increasing each year in most developed countries.1 Disseminated trichosporonosis, which is most commonly caused by Trichosporon asahii, is another severe fungal infection found predominantly in immunocompromised patients. To date, efforts to treat systemic trichosporonosis using a single drug have not yielded completely satisfactory results.2, 3 In addition, as antifungal therapies are currently limited to relatively few compounds, a need exists for the development of novel, safe and effective therapeutic agents against fungal diseases, particularly life-threatening infections caused by A. fumigatus.
The cell wall structure of fungi is distinct from that of mammalian cells,4, 5 and therefore consists of several unique components, such as chitin, α- and β-linked glucans, and various types of mannoproteins, which may be suitable antifungal targets. For example, the biosynthesis of 1,3-β-glucan, a major and specific component of the fungal cell wall, is inhibited by the echinocandin-like lipopeptide micafungin (Figure 1), which has potent antifungal activity against pathogenic Candida and Aspergillus species. In addition, among the fungal cell wall biosynthesis enzymes, chitin synthase is competitively inactivated by nikkomycins, which are a group of nucleoside peptides. It has been demonstrated that micafungin and nikkomycin Z act synergistically in vitro and in vivo against A. fumigatus.6, 7 Although micafungin alone does not exhibit in vitro activity against T. asahii,8 the combination of micafungin and fluconazole is effective in experimental infection models.9 These facts suggest that the combination of two drugs with different mechanisms can be an effective treatment against fungal infection, which is not satisfied with current clinical practices.
In a previous report, we described a novel antifungal antibiotic, KB425796-C (Figure 1), which is a potent derivative of a bacterial metabolite KB425796-A. This compound is a macrocyclic lipodepsipeptide (C81H119N19O18) that induces morphological changes, such as the swelling and bulging of the hyphae, in A. fumigatus, similar to the activity of nikkomycins. Here, we examined the antifungal activity of KB425796-C against several micafungin-resistant fungal species and the activity in a disseminated murine model of trichosporonosis. We further sought to elucidate the synergistic activity of KB425796-C in combination with micafungin in a murine model of disseminated aspergillosis.
Results
Checkerboard assay
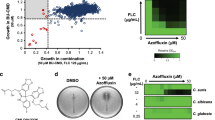
KB425796-C induced morphological changes in A. fumigatus hyphae similar to those observed with KB425796-A.10 In microscopic observations, both micafungin and KB425796-C displayed fungistatic effects on hyphae when used alone. Micafungin induced truncation, branching and shortening of hyphae, whereas KB425796-C treatment of hyphae caused swelling and the formation of protuberances. When the two drugs were used in combination, A. fumigatus cells exhibited spherical dilatation. The isobolograms for the interactions between KB425796-C and micafungin are presented in Figure 2, and the representative MICs, minimum effective concentrations (MECs) and fractional inhibitory concentration (FIC) indices against A. fumigatus are presented in Table 1. Notably, a marked synergistic effect was observed in MICs, which revealed that none of the treated cells were viable.
Time-kill curve study
Figure 3 shows a plot of cell viability over time for A. fumigatus cells treated with micafungin and KB425796-C alone or in combination for 17, 24, 42 and 48 h. A synergistic antifungal effect was observed when the two drugs were used in combination after 24 h. The combination of micafungin and KB425796-C was fungicidal and markedly reduced the number of CFU, in contrast to the fungistatic effects (no reduction in CFU) observed at all examined time points when each drug was used alone. In addition, the fungicidal effects of the combined treatment were more pronounced than those of voriconazole, which is a commercially available fungicidal drug.
In vivo antifungal activities of KB425796-C against T. asahii and A. fumigatus in murine infection models
In the previous report, we have demonstrated that KB425796-C had antifungal activities against T. asahii (MEC=1.56 μg ml−1, MIC=3.13 μg ml−1) and A. fumigatus (MEC=3.13 μg ml−1, MIC>50 μg ml−1). Here the protective efficacy of KB425796-C administered intraperitoneally against murine systemic infection with T. asahii and A. fumigatus was examined. Survival curves for the in vivo trichosporonosis model are shown in Figure 4. The ED50 of KB425796-C on day 14 after challenge was 12.5 mg kg−1. In this infection model, we found that a dose of 32 mg kg−1 KB425796-C was superior to 5 mg kg−1 micafungin (P<0.01) and equal to the combination of 5 mg kg−1 micafungin and 1 mg kg−1 of amphotericin B (AMPH) (data not shown).
Survival curves for the in vivo aspergillosis model treated with KB425796-C, micafungin and nikkomycin X are shown in Figure 5. All mice administered micafungin (0.1 mg kg−1) survived. In comparison with the control group, mice administered KB425796-C (32 mg kg−1) were significantly protected from infection with A. fumigatus (P<0.05).
In vivo antifungal effects of KB425796-C in combination
Long-term survival curves for the murine aspergillosis model in which mice were treated for 2 days with KB425796-C and micafungin in combination are shown in Figure 6. The first deaths occurred on day 4 post infection, with 100% of the control mice succumbing to infection by day 6. Administration of KB425796-C (32 mg kg−1) alone did not show protective efficacy under the same conditions in a separate experiment (data not shown). Fewer mice treated with micafungin died of infection than the control mice, and all mice treated with AMPH (1 mg kg−1) survived. Using the log-rank test, micafungin at a dose of 0.1 mg kg−1 significantly prolonged survival compared with mice administered saline (P<0.01). Animals given a combination of micafungin (0.1 mg kg−1) and KB425796-C (32 mg kg−1) showed a trend towards prolonged survival in comparison with those treated with micafungin (0.1 mg kg−1) alone (P=0.085; Figure 6).
Tissue burden studies
The number of A. fumigatus CFU recovered from the kidneys and livers of saline-treated DBA/2 mice on day 3 post infection were similar (Figure 7). The CFUs in the kidneys were significantly decreased in mice treated with 1 mg kg−1 micafungin (P<0.01) or AMPH (P<0.01) in comparison with saline-treated mice. Compared with micafungin (0.32 mg kg−1) alone, combination treatment with micafungin and KB425796-C had a significant additional effect on the reduction of CFU, but only at a dose of 0.32 and 32 mg kg−1, respectively (P=0.016). In the livers of micafungin-treated mice, the number of CFUs also decreased, although the clearance effect was less than that found in the kidneys. Combination treatment with micafungin and KB425796-C resulted in a significant decrease in the number of CFUs compared with the treatment with micafungin alone at all examined doses (P<0.01). The clearance effect associated with KB425796-C in combination with micafungin was greater than that observed in AMPH-treated animals.
Tissue distribution study
Drug concentrations in the liver, kidneys, lungs, spleen and brain of ICR mice after administration of KB425796-C are shown in Figure 8. The concentrations of KB425796-C in the brain were first detected 48 h after the first drug administration. The KB425796-C concentration in the other organs was similar at 30 min. The concentration of the drug in the liver increased at 4 and 48 h, and was approximately 2.5-fold higher at 4 h than those in the kidneys, lungs and spleen, and 10-fold higher at 48 h than those in the kidneys and lungs.
Discussion
In the present study, the in vitro and in vivo antifungal activities and efficacies of KB425796-C, both alone and in combination with micafungin, were evaluated. KB425796-C displayed potent anti-Aspergillus and anti-Trichosporon activities, suggesting that it has potential as a chemotherapeutic drug in treating disseminated mycoses.
Disseminated trichosporonosis is a life-threatening infection that mainly occurs in immunocompromised patients.2, 3 As Trichosporon spp. are generally less susceptible to AMPH, micafungin and fluconazole,11 attempts to treat systemic trichosporonosis have not been completely satisfactory to date. In our study of a murine model of systemic infection with clinically relevant T. asahii, micafungin treatment did not improve the survival rate of mice, even at much higher doses than are recommended clinically, while AMPH at the highest dose did not completely protect from infection. In contrast, KB425796-C protected mice from T. asahii infection in a dose-dependent manner and resulted in 100% survival at a dose of 32 mg kg−1. Together, these findings suggest that KB425796-C is a promising chemotherapeutic agent against disseminated trichosporonosis.
In vitro treatment of A. fumigatus with KB425796-C induced the swelling and bulging of hyphae, which are morphological changes that are similar to those induced by nikkomycin X. Nikkomycin derivatives, including nikkomycin X, function as competitive inhibitors of chitin synthase, which is involved in the synthesis of the fungal cell wall component chitin, a linear polymer of β-1,4-linked N-acetylglucosamine residues. Nikkomycin Z also has strong synergistic antifungal activity against A. fumigatus in combination with micafungin,6, 7 which inhibits 1,3-β-glucan synthase, a key enzyme necessary for the synthesis of β-glucan, a major structural component of the cell walls of Aspergillus spp.12 These facts suggested a possibility that KB425796-C inhibits a component of cell wall synthesis and has synergistic activity in combination with micafungin, although further study will be needed to determine the specific target.
Here, we demonstrated by serial two-dimensional checkerboard inhibitory assays that the treatment of A. fumigatus with KB425796-C in combination with micafungin resulted in synergistic hyphal damage. Each single drug exhibited fungistatic effects on A. fumigatus, with micafungin having greater activity than KB425796-C. After 48 h of treatment, micafungin at a dose of 0.5 μg ml−1 had inhibited 80% of A. fumigatus growth, whereas KB425796-C at 6.1 μg ml−1 inhibited growth only by 20% in the Alamar blue assay. However, the combination of the two drugs completely inhibited the growth of A. fumigatus at a wide range of drug concentrations. Under this treatment condition, the fungal cells exhibited spherical dilatation similar to spheroplasts, and with longer culture time, often ruptured. The FIC indices were 0.38 and 0.064 when MEC and MIC, respectively, were used as end points. The former value was markedly higher because each drug has only growth-reducing activity when used alone, and the MIC was greater than the tested concentrations, whereas the combination of KB425796-C and micafungin completely inhibited fungal growth. These findings confirm that KB425796-C and micafungin have synergistic antifungal activity against A. fumigatus.
To determine whether the combined effect of KB425796-C and micafungin was fungicidal or fungistatic, we performed a time-kill curve study. Micafungin is fungicidal to Candida albicans, but is only fungistatic to A. fumigatus.8, 13 Here, micafungin resulted in a 70% reduction in the growth of A. fumigatus at a dose of 0.05 μg ml−1, whereas 2 μg ml−1 KB425796-C reduced growth only by 40% in 48 h. In contrast, the combination of 0.05 μg ml−1 micafungin and 4.0 μg ml−1 KB425796-C achieved a >99% growth reduction after mere 24 h. This finding demonstrates that KB425796-C in combination with micafungin is fungicidal to A. fumigatus.
We also evaluated whether KB425796-C could protect against A. fumigatus infection in vivo. KB425796-C at a dose of 32 mg kg−1 had detectable effects with respect to prolonging survival in an A. fumigatus systemic infection murine model. In this model, KB425796-C treatment had greater efficacy than that of nikkomycin X (32 mg kg−1), but the efficacy was less than that provided by micafungin (0.1 mg kg−1) in this model. As described above, KB425796-C also had lower in vitro fungistatic efficacy than micafungin; thus, the protective effect of KB425796-C in vivo appears to reflect its efficacy in vitro.
The combination of micafungin and KB425796-C had good in vitro fungicidal activity against A. fumigatus; therefore, we also evaluated the efficacy of this drug combination against Aspergillus infection in vivo. Specifically, we attempted to determine whether the combination therapy would have long-term therapeutic efficacy using a short-term administration schedule based on the respective fungicidal activities of the two compounds. The doses of KB425796-C and micafungin were determined according to the doses that prolonged survival in the systemic infection models. We found that 100% of challenged DBA/2 mice that were given AMPH or the combination of micafungin (1 mg kg−1) and KB425796-C (32 mg kg−1) survived (P=0.002 versus controls) during the 32-day post-infection observation period. AMPH is a representative fungicidal antibiotic; therefore, fungicidal activity seems to be important for the long-term survival of mice challenged with A. fumigatus.
Micafungin and AMPH reduce the number of fungal cells recovered from the kidneys in mouse models of disseminated candidiasis14 and from the lungs in mouse models of pulmonary and systemic aspergillosis.15, 16 Here, we performed tissue burden studies and found that the number of CFUs of A. fumigatus recovered from the livers and kidneys of mouse models of systemic aspergillosis were significantly reduced in the mice treated with AMPH or a combination of micafungin and KB425796-C. Interestingly, the addition of KB425796-C at any dose to micafungin treatment significantly lowered fungal burden in the livers. In addition, the tissue distribution study revealed that the concentration of KB425796-C was higher in the livers than in other organs. This finding suggests that KB425796-C is more efficacious at prolonging survival if its concentration in all tissues is sufficiently high to kill the fungi.
Several limitations of the study warrant mention. First, the mechanism of action of KB425796-C is unclear, though the synergistic effect with micafungin and morphological change of hyphae suggest a possibility that cell wall synthesis inhibition will be involved. Second, combination treatment with KB425796-C and micafungin in the A. fumigatus infection model decreased the CFU in the kidneys only at a dose of 0.32 and 32 mg kg−1, respectively, probably because of low concentration of KB425796-C. While the main target organs attacked by Aspergillus are the lungs in patients, the concentration of KB425796-C in the lungs was similar to that in the kidneys. Hence, some more improvements of concentration in the spleens may be required to achieve significant effect in clinical use.
In conclusion, we report the in vitro synergistic activity of KB425796-C and micafungin, and in vivo efficacy against T. asahii and A. fumigatus infection models. These findings suggest that KB425796-C is a good lead compound for a drug against fungal infection in combination with micafungin. Further studies to improve the efficacy and tissue distribution are needed in the future.
Methods
Compounds
Micafungin and voriconazole were synthesized by Astellas Pharma, Inc. (Ibaraki, Japan). Nikkomycin X was purified at Astellas Pharma, Inc. AMPH-B was purchased from Bristol-Myers Squibb (Tokyo, Japan).
Antifungal activity
Seven clinical isolates stored in our laboratory, A. fumigatus FP1305, Candida albicans FP633, T. asahii FP2044, Rhizopus oryzae FP1988, Fusarium solani FP1930, Pseudallescheria boydii FP1987 and Trichophyton mentagrophytes FP2103, were used to test the antifungal activity of KB425796-C. Each fungal isolate was incubated statically in yeast–maltose (YM) agar broth for 24 h at 30 °C. Cryptococcus neoformans YC203 was grown in YM broth medium for 20 h at 30 °C with shaking at 200 r.p.m. A cell suspension was prepared by washing the cultured cells once with sterile saline. A. fumigatus FP1305 was cultured on a potato dextrose agar (PDA) slant for 4 days, and spores were then harvested in sterile saline and collected by filtering through gauze. Antifungal activity against all isolates, with the exception of C. neoformans, was measured by the micro-broth dilution method in 96-well culture plates using RPMI 1640 medium (Invitrogen Japan, Tokyo, Japan) supplemented with L-glutamine, but without sodium bicarbonate, and buffered to pH 7.0 with 0.165 M MOPS. For C. neoformans, yeast nitrogen base-glucose (YNBD) medium was used. For the assay, the test microorganism was inoculated into each well to yield 1 × 105 CFU/well, and the plates were then incubated for 20 h (C. albicans FP633, T. asahii FP2044, F. solani FP1930, R. oryzae FP1988, P. boydii FP1987 and A. fumigatus FP1305) or 48 h (C. neoformans YC203 and T. mentagrophytes FP2103) at 37 °C. Two end points were determined by microscopic observation: MEC, which was defined as a substantial reduction in fungal growth, and MIC, which was defined as a complete inhibition of growth.
Checkerboard broth microdilution method to evaluate synergistic drug effects
Drug interactions were assessed using a checkerboard titration method according to the recommendations of the National Committee for Clinical Laboratory Standards (NCCLS) for in vitro susceptibility testing.17 Briefly, 25-μl aliquots of each drug (KB425796-C and micafungin) at 4 times the final target concentration were dispensed into the wells of a microtiter plate to give a total of 77 drug combinations. Each agent alone (25 μl drug mixed with 25 μl medium control) was also tested. A. fumigatus FP1305 was prepared as described above, and a 50-μl suspension in RPMI 1640 medium (Invitrogen) supplemented with L-glutamine (buffered to pH 7.0 with 0.165 M MOPS) was added to each well. The microtiter plates were incubated for 48 h at 37 °C without shaking, and antifungal activity against A. fumigatus was then determined by microscopic observation using Alamar Blue (Iwaki Glass Co., Ltd.) as a vital stain.
The MIC of each drug, either alone or in combination, was defined as the lowest drug concentration that produced a decrease in absorbance of ⩾90% compared with control wells (drug-free conditions). The MEC was defined as the concentration that produced a substantial reduction in fungal growth based on microscopic observation of the hyphae. Drug interactions were classified as synergistic, additive, autonomous or antagonistic on the basis of the FIC index, which was defined as the sum of the FIC for each drug. The FIC was defined as the MIC (MEC) of each drug when used in combination divided by the MIC (MEC) of the drug when used alone. The interaction of drugs was defined as synergistic if the FIC index was <1.0, additive if the FIC index was 1.0, autonomous if the FIC index was between 1.0 and 2.0, and antagonistic if the FIC index was >2.0.
Time-kill curve assay
Drugs were diluted at twice the target final concentration with RPMI 1640 medium (Invitrogen) supplemented with L-glutamine and (buffered to pH 7.0 with 0.165 M MOPS) and were then added to wells of a microtiter plate in 50-μl aliquots. 1 × 104 spores of A. fumigatus in the same RPMI 1640 medium (50 μl) were added to each well, and the microtiter plate was then incubated for 48 h at 37 °C without shaking. At several time points, colony formation assays were performed. Briefly, the entire contents of the test well were transferred to a 10-cm dish, into which 20 ml PDA medium was melted and cooled to 40 °C before being added. The dish was incubated for 24 h at 37 °C, and the CFU per dish was counted.
In vivo antifungal activities of KB425796-C against T. asahii and A. fumigatus in murine infection models
All animal experimental procedures were approved by the Committee for Animal Experiments of Astellas Pharma Inc. In vivo anti-Trichosporon and anti-Aspergillus activities were evaluated in a murine model of systemic infection. To test anti-Trichosporon activity, the inoculum was prepared from 1-day-old cultures of T. asahii FP2044 grown on a PDA slant. The cells were harvested in sterile saline (not filtered). Seven-week-old female DBA/2 mice were intravenously injected with 1.0 × 107 yeast cells. For anti-Aspergillus activity, the inoculum was prepared from a 4-day-old culture of A. fumigatus FP1305 grown on a PDA slant. The spores were harvested in sterile saline and then filtered through gauze. DBA/2 mice were intravenously injected with 1.6 × 106 spores. Test compounds were prepared in sterile saline (micafungin), 5% glucose/saline (AMPH) or 10% HCO-60 (polyoxyethylene (60) hydrogenated castor oil)/saline (KB425796-C and nikkomycinX), and were subcutaneously (micafungin and nikkomycinX) or intraperitoneally (KB425796-C and AMPH) administered to mice (n=5, each test group). Drugs were given 1 h after challenge and then twice a day for three consecutive days for the anti-Trichosporon model, and 1 h after challenge and then once a day for five consecutive days for the anti-Aspergillus model. Outcome was determined by survival analysis by using Kaplan–Meier plots and the log rank test.
In vivo antifungal effects of drugs in combination and tissue burden studies
Eight groups of ten female DBA/2 mice (7 weeks old) were intravenously injected with 2.0 × 106 A. fumigatus FP1305 spores, which were prepared as described above. The test groups received the following treatments: AMPH at 1 mg kg−1 of body weight/dose given intraperitoneally (i.p.) once daily (q.d.); micafungin at 0.1, 0.32 or 1 mg kg−1 of body weight/dose given subcutaneously (s.c.) (q.d.); micafungin given s.c. (0.1, 0.32 or 1 mg kg−1 q.d.) plus KB425796-C given i.p. (32 mg kg−1) twice daily (b.i.d.); and saline (b.i.d.). Drugs were administered on days 1 and 2. Five mice in each group were killed 1 day after the completion of treatment. The livers and kidneys were aseptically removed, and each organ was then homogenized in 5 ml sterile saline. Serial 10-fold dilutions of the homogenates were plated on PDA and incubated for 48 h at 37 °C, and the numbers of CFU per gram of tissue were then calculated. The survival rate of remaining five mice of each group were examined daily for 31 days after the challenge. The ED50 value was determined on the day when all control mice died. Survival curves were analyzed using the Kaplan–Meier plots and the log rank test. Differences in the CFU number in the kidney and liver were determined according to the Student’s t-test.
Tissue distribution study
For the determination of KB425796-C concentration in tissues, 4-week-old female ICR mice were administered KB425796-C (32 mg kg−1 b.i.d.) for 2 days. The liver, kidneys, lungs, spleen and brain were removed at 30 min, 4 h and 48 h from the time of the first administration. The organs were weighed and homogenized with 5 (v/w) volumes of methanol. The concentrations of KB425796-C were determined by HPLC methods as described in the previous paper.10 The lower detection limit of KB425796-C was 10 μg ml−1 using a 5 μl sample.
References
Wade, J. C. Treatment of fungal and other opportunistic infections in immunocompromised patients. Leukemia 11 (Suppl 4), S38–S39 (1997).
Itoh, T., Hosokawa, H., Kohdera, U., Toyazaki, N. & Asada, Y. Disseminated infection with Trichosporon asahii. Mycoses 39, 195–199 (1996).
Moretti-Branchini, M. L. et al. Trichosporon species infection in bone marrow transplanted patients. Diagn. Microbiol. Infect. Dis. 39, 161–164 (2001).
Shepherd, M. G. Cell envelope of Candida albicans. CRC Crit. Rev. Microbiol. 15, 7–25 (1987).
Debono, M. & Gordee, R. S. Antibiotics that inhibit fungal cell wall development. Annu. Rev. Microbiol. 48, 471–497 (1994).
Chiou, C. C., Mavrogiorgos, N., Tillem, E., Hector, R. & Walsh, T. J. Synergy, pharmacodynamics, and time-sequenced ultrastructural changes of the interaction between nikkomycin Z and the echinocandin FK463 against Aspergillus fumigatus. Antimicrob. Agents Chemother. 45, 3310–3321 (2001).
Clemons, K. V. & Stevens, D. A. Efficacy of micafungin alone or in combination against experimental pulmonary aspergillosis. Med. Mycol. 44, 69–73 (2006).
Tawara, S. et al. In vitro activities of a new lipopeptide antifungal agent, FK463, against a variety of clinically important fungi. Antimicrob. Agents Chemother. 44, 57–62 (2000).
Serena, C., Pastor, F. J., Gilgado, F., Mayayo, E. & Guarro, J. Efficacy of micafungin in combination with other drugs in a murine model of disseminated trichosporonosis. Antimicrob. Agents Chemother. 49, 497–502 (2005).
Kai, H. et al. Identification of ten KB425796-A congeners from Paenbacillus sp. 530603 using an antifungal assay against Aspergillus fumigatus in combination with micafungin. J. Antibiot. (doi:10.1038/ja.2013.64).
Steinbach, W. J. & Perfect, J. R. Newer antifungal therapy for emerging fungal pathogens. Int. J. Infect. Dis. 7, 5–20 (2003).
Hatano, K., Morishita, Y., Nakai, T. & Ikeda, F. Antifungal mechanism of FK463 against Candida albicans and Aspergillus fumigatus. J. Antibiot. (Tokyo) 55, 219–222 (2002).
Watabe, E., Nakai, T., Matsumoto, S., Ikeda, F. & Hatano, K. Killing activity of micafungin against Aspergillus fumigatus hyphae assessed by specific fluorescent staining for cell viability. Antimicrob. Agents Chemother. 47, 1995–1998 (2003).
Ikeda, F. et al. Efficacy of FK463, a new lipopeptide antifungal agent, in mouse models of disseminated candidiasis and aspergillosis. Antimicrob. Agents Chemother. 44, 614–618 (2000).
Matsumoto, S. et al. Efficacy of FK463, a new lipopeptide antifungal agent, in mouse models of pulmonary aspergillosis. Antimicrob. Agents Chemother. 44, 619–621 (2000).
Graybill, J. R., Bocanegra, R., Gonzalez, G. M. & Najvar, L. K. Combination antifungal therapy of murine aspergillosis: liposomal amphotericin B and micafungin. J. Antimicrob. Chemother. 52, 656–662 (2003).
Standards., N. C. f. C. L.. Reference method for broth dilution antifungal susceptibility testing of yeasts; approved standard. NCCLS document M27-A, National Committee for Clinical Laboratory Standards: Wayne, PA, USA, (1997).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kai, H., Yamashita, M., Nakamura, I. et al. Synergistic antifungal activity of KB425796-C in combination with micafungin against Aspergillus fumigatus and its efficacy in murine infection models. J Antibiot 66, 479–484 (2013). https://doi.org/10.1038/ja.2013.57
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ja.2013.57
Keywords
This article is cited by
-
Synthesis and Antifungal Activity Evaluation of 1-(2-Benzyloxy-2-Phenylethyl)-1,2,3-Triazole Miconazole Analogs
Pharmaceutical Chemistry Journal (2021)