Abstract
Background/Objectives:
Obesity-associated insulin resistance is a major risk factor for the development of type 2 diabetes, cardiovascular disease and non-alcoholic liver disease. Over-activation of the RhoA-Rho kinase (ROCK) pathway has been implicated in the development of obesity-induced insulin resistance, but the relative contribution of ROCK2 has not been elucidated. This was investigated in the present study.
Methods:
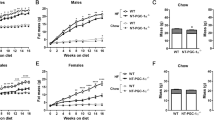
Male ROCK2+/− mice and their wild-type (WT) littermate controls were fed normal chow or a high fat diet (HFD) for 18 weeks. Glucose and insulin tolerance tests were conducted 8 and 16 weeks after the start of feeding. At termination, isoform-specific ROCK activity and insulin signaling were evaluated in epididymal adipose tissue. Adipocyte size was assessed morphometrically, while adipose tissue production of PPARγ was determined by western blotting, and inflammatory cytokines were evaluated by RT-PCR and immunofluorescence.
Results:
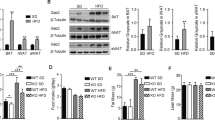
The decrease in systemic insulin sensitivity and glucose tolerance produced by high fat feeding was attenuated in ROCK2+/− mice. There was no reduction in food intake, body weight or epididymal fat pad weight in HFD-ROCK2+/− mice. However, the increase in adipocyte size detected in HFD-WT mice was attenuated in HFD-ROCK2+/− mice. The increase in adipose tissue ROCK2 activity produced by high fat feeding in WT mice was also prevented in ROCK2+/− mice, and this was accompanied by improved insulin-induced phosphorylation of Akt. The expression of both isoforms of PPARγ was increased in adipose tissue from HFD-ROCK2+/− mice, while adipocyte hypertrophy and production of inflammatory cytokines were reduced compared with HFD-WT mice.
Conclusions:
These data suggest that activation of ROCK2 in adipose tissue contributes to obesity-induced insulin resistance. This may result in part from suppression of PPARγ expression, leading to adipocyte hypertrophy and an increase in inflammatory cytokine production. ROCK2 may be a suitable target to improve insulin sensitivity in obesity.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Lau DC . Obesity Canada Clinical Practice Guidelines Steering Committee and Expert Panel. Synopsis of the 2006 Canadian clinical practice guidelines on the management and prevention of obesity in adults and children. CMAJ 2007; 176: 1103–1106.
Gastaldelli A, Kozakova M, Hojlund K, Flyvbjerg A, Favuzzi A, Mitrakou A et al. Fatty liver is associated with insulin resistance, risk of coronary heart disease, and early atherosclerosis in a large European population. Hepatology 2009; 49: 1537–1544.
Steinberger J, Daniels SR . American Heart Association Atherosclerosis, Hypertension, and Obesity in the Young Committee (Council on Cardiovascular Disease in the Young); American Heart Association Diabetes Committee (Council on Nutrition, Physical Activity, and Metabolism). Obesity, insulin resistance, diabetes, and cardiovascular risk in children: an American Heart Association scientific statement from the Atherosclerosis, Hypertension, and Obesity in the Young Committee (Council on Cardiovascular Disease in the Young) and the Diabetes Committee (Council on Nutrition, Physical Activity, and Metabolism). Circulation 2003; 107: 1448–1453.
Vella CA, Burgos X, Ellis CJ, Zubia RY, Ontiveros D, Reyes H et al. Associations of insulin resistance with cardiovascular risk factors and inflammatory cytokines in normal-weight Hispanic women. Diabetes Care 2013; 36: 1377–1383.
Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest 2003; 112: 1821–1830.
Bastard JP, Maachi M, Lagathu C, Kim MJ, Caron M, Vidal H et al. Recent advances in the relationship between obesity, inflammation, and insulin resistance. Eur Cytokine Netw 2006; 17: 4–12.
Pelosi M, Marampon F, Zani BM, Prudente S, Perlas E, Caputo V et al. ROCK2 and its alternatively spliced isoform ROCK2m positively control the maturation of the myogenic program. Mol Cell Biol 2007; 27: 6163–6176.
Julian L, Olson MF . Rho-associated coiled-coil containing kinases (ROCK): structure, regulation, and functions. Small GTPases 2014; 5: e29846.
Amano M, Nakayama M, Kaibuchi K . Rho-kinase/ROCK: A key regulator of the cytoskeleton and cell polarity. Cytoskeleton (Hoboken) 2010; 67: 545–554.
Begum N, Sandu OA, Ito M, Lohmann SM, Smolenski A . Active Rho kinase (ROK-alpha) associates with insulin receptor substrate-1 and inhibits insulin signaling in vascular smooth muscle cells. J Biol Chem 2002; 277: 6214–6222.
Furukawa N, Ongusaha P, Jahng WJ, Araki K, Choi CS, Kim HJ et al. Role of Rho-kinase in regulation of insulin action and glucose homeostasis. Cell Metab 2005; 2: 119–129.
Chun KH, Araki K, Jee Y, Lee DH, Oh BC, Huang H et al. Regulation of glucose transport by ROCK1 differs from that of ROCK2 and is controlled by actin polymerization. Endocrinology 2012; 153: 1649–1662.
Lee DH, Shi J, Jeoung NH, Kim MS, Zabolotny JM, Lee SW et al. Targeted disruption of ROCK1 causes insulin resistance in vivo. J Biol Chem 2009; 284: 11776–11780.
Lee SH, Huang H, Choi K, Lee DH, Shi J, Liu T et al. ROCK1 isoform-specific deletion reveals a role for diet-induced insulin resistance. Am J Physiol Endocrinol Metab 2014; 306: E332–E343.
Hara Y, Wakino S, Tanabe Y, Saito M, Tokuyama H, Washida N et al. Rho and Rho-kinase activity in adipocytes contributes to a vicious cycle in obesity that may involve mechanical stretch. Sci Signal 2011; 4: ra3.
Kanda T, Wakino S, Homma K, Yoshioka K, Tatematsu S, Hasegawa K et al. Rho-kinase as a molecular target for insulin resistance and hypertension. FASEB J 2006; 20: 169–171.
Liu PY, Chen JH, Lin LJ, Liao JK . Increased Rho kinase activity in a Taiwanese population with metabolic syndrome. J Am Coll Cardiol 2007; 49: 1619–1624.
Noguchi M, Hosoda K, Fujikura J, Fujimoto M, Iwakura H, Tomita T et al. Genetic and pharmacological inhibition of Rho-associated kinase II enhances adipogenesis. J Biol Chem 2007; 282: 29574–29583.
Zhou Q, Mei Y, Shoji T, Han X, Kaminski K, Oh GT et al. Rho-associated coiled-coil-containing kinase 2 deficiency in bone marrow-derived cells leads to increased cholesterol efflux and decreased atherosclerosis. Circulation 2012; 126: 2236–2247.
Zhou Z, Meng Y, Asrar S, Todorovski Z, Jia Z . A critical role of Rho-kinase ROCK2 in the regulation of spine and synaptic function. Neuropharmacology 2009; 56: 81–89.
Parlee SD, Lentz SI, Mori H, MacDougald OA . Quantifying size and number of adipocytes in adipose tissue. Methods Enzymol 2014; 537: 93–122.
Mong PY, Wang Q . Activation of Rho Kinase Isoforms in Lung Endothelial Cells during Inflammation. J Immunol 2009; 182: 2385–2394.
Sun K, Kusminski CM, Scherer PE . Adipose tissue remodeling and obesity. J Clin Invest 2011; 121: 2094–2101.
Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW Jr . Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest 2003; 112: 1796–1808.
Sugii S, Olson P, Sears DD, Saberi M, Atkins AR, Barish GD et al. PPARgamma activation in adipocytes is sufficient for systemic insulin sensitization. Proc Natl Acad Sci USA 2009; 106: 22504–22509.
Yin R, Fang L, Li Y, Xue P, Li Y, Guan Y et al. Pro-inflammatory Macrophages suppress PPARgamma activity in Adipocytes via S-nitrosylation. Free Radic Biol Med 2015; 89: 895–905.
Aguirre V, Werner ED, Giraud J, Lee YH, Shoelson SE, White MF . Phosphorylation of Ser307 in insulin receptor substrate-1 blocks interactions with the insulin receptor and inhibits insulin action. J Biol Chem 2002; 277: 1531–1537.
Aguirre V, Uchida T, Yenush L, Davis R, White MF . The c-Jun NH (2)-terminal kinase promotes insulin resistance during association with insulin receptor substrate-1 and phosphorylation of Ser(307). J Biol Chem 2000; 275: 9047–9054.
Soliman H, Nyamandi V, Garcia-Patino M, Varela JN, Bankar G, Lin G et al. Partial deletion of ROCK2 protects mice from high-fat diet-induced cardiac insulin resistance and contractile dysfunction. Am J Physiol Heart Circ Physiol 2015; 309: H70–H81.
Acknowledgements
This study was funded by operating grants from the Canadian Institutes of Health Research (MOP 97861) and Canadian Diabetes Association to KMM. VN is supported by a doctoral student research award from the Canadian Diabetes Association, MGP and JNV are supported by PhD scholarships from the Mexican National Council for Science and Technology (CONACyT), and CAPES foundation, Ministry of Education, Brazil, respectively.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on International Journal of Obesity website
Rights and permissions
About this article
Cite this article
Soliman, H., Varela, J., Nyamandi, V. et al. Attenuation of obesity-induced insulin resistance in mice with heterozygous deletion of ROCK2. Int J Obes 40, 1435–1443 (2016). https://doi.org/10.1038/ijo.2016.89
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ijo.2016.89
This article is cited by
-
Selectivity matters: selective ROCK2 inhibitor ameliorates established liver fibrosis via targeting inflammation, fibrosis, and metabolism
Communications Biology (2023)
-
Unraveling the rationale and conducting a comprehensive assessment of KD025 (Belumosudil) as a candidate drug for inhibiting adipogenic differentiation—a systematic review
Naunyn-Schmiedeberg's Archives of Pharmacology (2023)
-
Advanced glycation end-products regulate extracellular matrix-adipocyte metabolic crosstalk in diabetes
Scientific Reports (2019)
-
Anti-adipogenic effects of KD025 (SLx-2119), a ROCK2-specific inhibitor, in 3T3-L1 cells
Scientific Reports (2018)
-
Regulation of hepatic Na+/K+-ATPase in obese female and male rats: involvement of ERK1/2, AMPK, and Rho/ROCK
Molecular and Cellular Biochemistry (2018)