Abstract
Most guidelines for the management of hypertension define it as a home blood pressure (HBP) value >135/85 mm Hg. However, there is no reference HBP value to diagnose hypertension in pregnant women. Therefore, in this study, we analyzed HBP measurements of pregnant women to determine whether it is appropriate to use the criteria for non-pregnant subjects for pregnant women. The participants of this study were 100 singleton pregnant women who visited our hospital between September 2013 and September 2016. We lent sphygmomanometers to the patients so they could measure their BP at home twice daily, and we measured their clinical BP when they visited the hospital. Six patients developed hypertensive disorders in pregnancy, whereas there were 63 women without hypertension or other complications that may affect BP. In the normotensive pregnant women, HBP values significantly correlated with the clinical BP values. HBP values equivalent to a clinical BP of 140/90 mm Hg, determined using the standard major axis method, were 120.8/83.5 mm Hg, 126.0/85.2 mm Hg and 136.3/89.3 mm Hg in the first, second and third trimesters, respectively. In normotensive pregnant women, HBP levels that indicate a risk of hypertensive disorder in pregnancy may be lower than 135/85 mm Hg before 28 weeks of gestation.
Similar content being viewed by others
Introduction
Blood pressure (BP) measurements are routinely monitored in the management of pregnancy, and the assessment of hypertension is based on BP values measured when the women visit the hospital (clinical BP (CBP)). International guidelines for treatment of hypertension, including those employed in Japan, have defined hypertension as a CBP>140/90 mm Hg. However, many studies have examined ambulatory BP and home BP (HBP) and determined that these measurements have a stronger correlation than CBP with potential organ damage and long-term prognosis.1, 2, 3 Consequently, BP management using HBP or ambulatory BP has been recommended in some guidelines for the management and treatment of hypertension.4, 5
HBP fluctuates during the day and varies according to the season and the patient’s age, sex and body mass index,6, 7 as well as gestational age in pregnant patients. Specifically, the CBP values decrease gradually with advancing gestational age, and the lowest CBP values are typically observed at 18 weeks of gestation, with a gradual increase noted in later gestational weeks.8 An almost identical phenomenon has been reported with HBP measurements.9, 10, 11, 12 Although some studies have proposed that the hypertension criteria for non-pregnant subjects are unsuitable for pregnant women,13 there are currently few reports detailing the ideal diagnostic criteria for hypertension using HBP measurements for pregnant women. Therefore, in this study, we examined HBP variations during the pregnancy and postpartum (6 months after delivery) periods and evaluated the appropriateness of using the existing criteria for hypertension in pregnant women.
methods
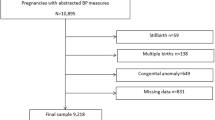
A prospective study was performed in pregnant women who visited and were planning to deliver at Saitama Medical Center of Saitama Medical University between September 2013 and September 2016. We explained the present study to all women during the early gestational age and enrolled women who provided written informed consent to participate in the study. Finally, we included 100 singleton pregnant women who could perform HBP measurements for >3months and who delivered infants within the described study period. The flowchart of the study subjects is shown in Figure 1.
The participants measured the HBP twice daily from early gestation to 6 months postpartum using an automatic sphygmomanometer (Omron HEM-7251G, OMRON HEALTHCARE, Kyoto, Japan). This sphygmomanometer has been demonstrated to have an adequately high precision based on the mean differences between the device and mercury readings for systolic and diastolic BPs (−0.6±4.7 and −0.2±4.4 mm Hg, respectively).14 The sphygmomanometers were purchased by our department and lent to the participants for home use. Each sphygmomanometer was returned upon completion of the study.
HBP measurements were performed according to the Japanese Society of Hypertension 2014 guideline.4 BP was measured at the upper arm by maintaining the arm-cuff position at heart level after a 1–2 min rest period with the patient in a seated position. The morning time was defined as between 0400 and 1000 hours, and HBP measurements were obtained within 1 h of awakening, after urination and before breakfast. The evening time was defined as between 1500 and 0300 hours, and HBP measurements were obtained before going to bed. These definitions were used because the acrophase of the BP was approximately at 1500 hours.15 Because some participants measured the HBP twice or more, while others measured it only once, the HBP values of the first measurement were used for the calculations. Data revealing unfitness of the arm-cuff or movement during the measurement were excluded.
The measured data (identification number, date, time, systolic BP, diastolic BP, pulse rate, room temperature, measuring times, fitness of the arm-cuff and any movement during the measurement) were automatically transferred and stored on a dedicated server managed by OMRON HEALTHCARE using a 3G universal mobile telecommunications system (Medical LINK, OMRON HEALTHCARE). We accessed the server through the internet and downloaded the data for analysis. The identification numbers of the participants were anonymized. The list of identifying codes and personal information was protected with a lock, and only the principal investigator had access to it.
In the present study, we divided the second trimester into two periods, defined as the early second trimester (between 14 weeks, 0 days and 20 weeks, 6 days) and late second trimester (between 21 weeks, 0 days and 27 weeks, 6 days). This was carried out because the HBP values are affected by gestational age, and the nadir of HBP was found to be around 20 weeks of gestation in many previous reports.8, 11, 12 As BP values fluctuate with the circadian rhythm and vary as a function of the day, week and season of the year,11, 15, 16 some guidelines for the management of hypertension recommend the use of the mean BP level over several days.4, 5, 17 Thus we used the mean value of 1 gestational week for analysis.
We collected the CBP data (systolic and diastolic BP) from the patients’ medical records. The CBP values were measured using an automated sphygmomanometer (BR-203RVIIB, OMRON HEALTHCARE, Kyoto, Japan) at every visit. This sphygmomanometer has been shown to have an adequately high precision based on the mean and s.d. of the errors (2.5 mm Hg±5.2 mm Hg) when compared with a mercury sphygmomanometer.18 All participants measured their CBP after a resting period, in sitting position, with their right arm at the heart level in a quiet room. The attending nurse instructed the participants in the outpatient department the measurement methods at the beginning of the study. Usually, the CBP was measured once per occasion; if two recordings were entered in the medical record, the mean value was used in our analysis. The CBP records of every visit were used for the analysis.
Hypertensive disorders in pregnancy (HDP) were defined using the definitions and classification proposed by the Japan Society of Obstetrics and Gynecology.19 Preeclampsia was defined as a BP of ⩾140/90 mm Hg with proteinuria (⩾300 mg of protein in a 24-h urine specimen) after 20 weeks of gestation in a patient with neither hypertension nor proteinuria prior to pregnancy, gestational hypertension was defined as a BP of ⩾140/90 mm Hg without proteinuria after 20 weeks of gestation in a patient with neither hypertension nor proteinuria prior to pregnancy and superimposed preeclampsia was defined as patients in whom chronic hypertension existed before 20 weeks of gestation and was accompanied by proteinuria. Early-onset type was defined as the presentation of hypertension before 32 weeks of gestation. Patients who did not have diseases that may affect BP, such as chronic hypertension, thyroid disease, impaired glucose tolerance abnormality, kidney disease and collagen disease, and who did not have HDP were defined as normotensive patients.
The relationship between the HBP and CBP was analyzed with Pearson’s product–moment correlation analysis. The statistical analysis was performed with the JMP 10 software (SAS Institute, Cary, NC, USA), and P<0.05 was considered statistically significant.
The study protocol was approved by the ethical committee of Saitama Medical Center of Saitama Medical University and was performed in accordance with the principles of the Declaration of Helsinki.
Results
The clinical characteristics and gestational complications of the study participants are shown in Table 1. Seven patients developed HDP (three with superimposed preeclampsia, two with preeclampsia and two with gestational hypertension).
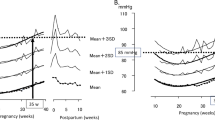
The changes in HBP and pulse rate measurements throughout pregnancy and the postpartum period in the normotensive patients are shown in Figure 2. The HBP values decreased gradually in early pregnancy; the lowest morning HBP (102.3/63.6 mm Hg) was seen at 15 weeks of gestation, while the lowest evening pressure (102.4/63.2 mm Hg) was measured at 17 weeks of gestation. The HBP values then increased gradually in later gestational weeks and returned to early pregnancy levels at 25–36 weeks after delivery. The changes in HBP throughout pregnancy and the postpartum period in HDP patients are shown in Figure 3. Because in this study there were only seven HDP patients, Figure 3 data are not separated in morning or evening BP values. The change of the HBP was similar to the normotensive patients, but BP values were higher in HDP patients. The comparison of changes of CBP values in normotensive and HDP patients are shown in Figure 4. The J-shaped change that was found for HBP data was not seen clearly for the CBP values. The median and range of BP values and the numbers of BP records that we collected in each trimester are shown in Table 2.
Change of home blood pressure (HBP) values and pulse rate during pregnancy and the postpartum period in normotensive patients (without hypertensive disorder and without complications that may affect the blood pressure). Straight lines show the morning values, and the dotted lines show the evening values.
Change of home blood pressure (HBP) values during pregnancy and the postpartum period in HDP patients. Gray broken lines show the data from the normotensive patients, the black broken lines show preeclampsia (PE) patients, the black straight lines show gestational hypertension (GH) patients and the black dotted lines show superimposed preeclampsia (sPE) patients.
The pulse rate increased gradually from early to middle pregnancy; the highest pulse rates (83.0 beats min−1 in the morning and 87.9 beats min−1 in the evening) were measured at 31 weeks of gestation, which subsequently decreased gradually in the later gestational weeks and postpartum period.
Previous studies have reported that the nadir of HBP occurs around 20 weeks of gestation, and our results were consistent with these reports.8, 11, 12 For the normotensive patients, we calculated the mean HBP values of each gestational week in each patient and compared them with the CBP values measured at the same gestational week. There were significantly positive correlations between the HBP and CBP values in every trimester (Table 3). In the normotensive patients, the percentage of white coat hypertension and masked hypertension was low. White coat hypertension was found in 0.7% of the systolic HBP measurements in the first trimester, 0.9% of the systolic HBP measurements in early second trimester, 0.6% of the systolic and 0.1% of the diastolic HBP measurements in the third trimester. Masked hypertension was found in 0.3% of the diastolic HBP values in late second trimester, 0.7% of the systolic and 3.4% of the diastolic HBP in third trimester. The strengths of the correlation of CBP and HBP values were consistent for both the morning and evening HBPs. Further, we calculated the mean HBP values at each week after delivery and compared them with the CBP values measured at the same week postpartum. Because there were relatively few CBP measurements in the postpartum period, we combined the data of the morning and evening HBPs for the analysis. As a result, there were significant, positive correlations between the HBP and CBP values in the postpartum period as well, but it was seen only in the systolic BP.
Next we obtained the HBP levels equivalent to 140/90 mm Hg of CBP (the diagnostic criteria of HDP), using the regression line from standardized major axis methods in each trimester. The results are shown in Table 4; the HBPs for the first, early second, late second and third trimester periods were 120.8/83.5, 124.1/84.2, 127.4/86.1 and 136.3/89.3 mm Hg, respectively. In the postpartum period, the HBP was 126.9/88.7 mm Hg in the first 8 weeks.
Discussion
BP has systolic and diastolic components, fluctuates throughout the day and is affected by various factors. The American Heart Association stated in 2008 that ‘HBP monitoring is theoretically ideal for monitoring changes in BP during pregnancy because it is the best technique for providing multiple readings recorded at the same time of day over prolonged periods of time’.20 It is clinically useful to measure HBP during pregnancy and the postpartum period when hemodynamics show great changes. The most important reasons for measuring BP are to detect pregnancy-associated hypertensive disease, that is, gestational hypertension and preeclampsia, and to lower perinatal mortality. For that goal, it is necessary to set the standard value of HBP during pregnancy.
Currently, HBP values >135/85 mm Hg during pregnancy are defined as hypertension following the diagnostic criteria using HBP readings in non-pregnant subjects4, 5; however, some reports have pointed out that this standard may be unsuitable.13 Actually, in the third trimester, when BP will rise even in normotensive pregnant women, the +2.0 s.d. value of HBP has been reported as 121/8012 or 126/80 mm Hg16; thus, the true standard value of HBP for pregnant women may be lower than that in non-pregnant subjects.
In this report, we analyzed a large data set of HBP measurements from early pregnancy to the postpartum period. One of the unique characteristics of this study was the use of automatic sphygmomanometers (Omron HEM-7251G) that automatically sent measured data to a dedicated internet site when the patients measured their BP. By using these sphygmomanometers, researchers can obtain the data without bias, such as recording errors or the patients reporting only the lowest BP value. Additionally, because the data included body movement and cuff-fitting errors, we could remove failed measurements from the analysis. One reason for why studies measuring long-term HBP in pregnant women are limited is the difficulty of maintaining the compliance of the participants. In other words, in young women without hypertensive disease, it is difficult to ensure continuous and daily recordings of HBP measurements. This task is especially difficult after delivery because of changes in their daily routine due to having a new baby in their lives. Thus many women stop measuring BP at this time. With the automatic sphygmomanometers and Medical LINK system, the study participants only had to measure the HBP and did not need to write it down. Hence, we consider that this system might be helpful for the maintenance of participant compliance.
Because some studies have reported that the BP levels were significantly decreased when the BP was measured multiple times on one occasion,21 we instructed the participants to measure their BP two or three times at each occasion. However, some participants measured the BP only once, while others measured it multiple times on one occasion. Accordingly, we used only the first measurement value of all patients in the present study. In future, we plan to analyze and report whether the width of BP variation of multiple measurements and circadian variations affect the diagnosis of hypertensive disorder.
The variations in the HBP and pulse rate during pregnancy in this study were almost the same as in some prior studies.8, 12, 22 Herein, we continued measuring the HBP during the postpartum period and discovered that the pulse rate returned to the level of early pregnancy at 25–36 weeks after delivery. Further, the change of the pulse rate during pregnancy in this study corresponded to the reported changes in plasma volume during pregnancy, that is, the plasma volume started to increase at 6–8 weeks of gestation and rises progressively until 28–30 weeks of gestation.22 Additionally, the evening pulse rate was significantly higher than that of the morning pulse rate from 11 weeks of gestation until 8 weeks after delivery, as determined by Student’s t-test. It is known that the acrophase occurs during the afternoon in terms of the circadian variations of the BP and pulse rate in non-pregnant people,15, 23 and the same phenomenon was seen in the pulse rate of pregnant women in the present study. Regarding HBP, there were no significant differences between the morning and evening measurements in the present study. As for why the morning and evening pulse rates did not show significant differences in the early stage of pregnancy, we speculate that the small number of data points may have influenced the analysis. Circadian variation was not observed at 9 weeks after delivery in the present study, although there were sufficient data at this period. This result may have been affected by sleep loss or changes in the time to fall asleep due to breast-feeding.
The HBP and CBP values showed mild-to-moderate positive correlations, independent of whether we used the morning or evening HBP. Furthermore, this phenomenon was seen through the entire pregnancy to the postpartum period in the systolic BP. As there are variations in the BP week by week, the differences in the results between HBP and CBP should ideally be evaluated at each gestational week rather than at each trimester. However, the CBP data were few in the first place, because normotensive pregnant women come to the hospital approximately 20 times at the most for health checkups in Japan. Hence, we analyzed the correlations between HBP and CBP at each trimester in the present study. Of note, we used different monitors for HBP (Omron HEM-7251G) and CBP (BR-203RVIIB) in this study; the performances of these two monitors were validated by comparison with a standard mercury sphygmomanometer.14, 18 Device performance was examined strictly according to the criteria for controlled medical class II devices stated in the Pharmaceutical Affairs Act. Therefore, we judged that the measurements obtained using these devices correlated with each other.
We calculated the HBP value equivalent to 140/90 mm Hg of CBP from regression analysis by using the standardized major axis method and the BP records from 63 normotensive patients. By using this method, we found that the HBP level in pregnant women equivalent to the CBP value that was defined as hypertension was lower in the first and second trimesters than the HBP value that was defined as hypertension in non-pregnant people (135/85 mm Hg; Table 4).
In conclusion, from our results, the HBP level in pregnant women to detect a hypertensive disorder early during pregnancy was determined to be 135/85 mm Hg in the third trimester and postpartum period. However, in the earlier trimesters, the HBP levels that indicate a higher risk of hypertensive disorder may be lower than 135/85 mm Hg. However, these results were not conclusive as there were only seven patients who developed hypertensive disorders and as the HBP data in the first trimester were limited in this study. The HBP levels to predict the pregnancy-associated hypertensive disorders may be examined by multiple regression analysis using a BP expect index, such as infant body weight,24 or angiogenesis factor associated with HDP (for example, soluble fms-like tyrosine kinase-1 or placental growth factor or renin–angiotensin system19) as criterion variable. In future, we plan to perform further studies and collect more data regarding HBP beginning in the early gestational weeks.
References
Ohkubo T, Imai Y, Tsuji I, Nagai K, Kato J, Kikuchi N, Nishiyama A, Aihara A, Sekino M, Kikuya M, Ito S . Home blood pressure measurement has a stronger predictive power for mortality than does screening blood pressure measurement: a population-based observation in Ohasama, Japan. J Hypertens 1998; 16: 971–975.
Her AY, Kim YH, Rim SJ, Kim JY, Choi EY, Min PK, Lee BK, Hong BK, Kwon HM . Home blood pressure is the predictor of subclinical target organ damage like ambulatory blood pressure monitoring in untreated hypertensive patients. Anadolu Kardiyol Derg 2014; 14: 711–718.
Karpettas N, Destounis A, Kollias A, Nasothimiou E, Moyssakis I, Stergiou GS . Prediction of treatment-induced changes in target-organ damage using changes in clinic, home and ambulatory blood pressure. Hypertens Res 2014; 37: 543–547.
Shimamoto K, Ando K, Fujita T, Hasebe N, Higaki J, Horiuchi M, Imai Y, Imaizumi T, Ishimitsu T, Ito M, Ito S, Itoh H, Iwao H, Kai H, Kario K, Kashihara N, Kawano Y, Kim-Mitsuyama S, Kimura G, Kohara K, Komuro I, Kumagai H, Matsuura H, Miura K, Morishita R, Naruse M, Node K, Ohya Y, Rakugi H, Saito I, Saitoh S, Shimada K, Shimosawa T, Suzuki H, Tamura K, Tanahashi N, Tsuchihashi T, Uchiyama M, Ueda S, Umemura S . Japanese Society of Hypertension Committee for Guidelines for the Management of Hypertension. The Japanese Society of Hypertension guidelines for the management of hypertension (JSH2014). Hypertens Res 2014; 37: 253–392.
Imai Y, Kario K, Shimada K, Kawano Y, Hasebe N, Matsuura H, Tsuchihashi T, Ohkubo T, Kuwajima I, Miyakawa M . The Japanese Society of Hypertension guidelines for self-monitoring of blood pressure at home (second edition). Hypertens Res 2012; 35: 777–795.
Kato T, Kikuya M, Ohkubo T, Satoh M, Hara A, Obara T, Metoki H, Asayama K, Hirose T, Inoue R, Kanno A . Factors associated with day-by-day variability of self-measured blood pressure at home: the Ohasama study. Am J Hypertens 2010; 23: 980–986.
Hosseini M, Baikpour M, Yousefifard M, Fayaz M, Koohpayehzadeh J, Ghelichkhani P, Asady H, Asgari F, Etemad K, Rafei A, Gouya MM . Blood pressure percentiles by age and body mass index for adults. EXCLI J 2015; 14: 465–477.
Macdonald-Wallis C, Tilling K, Fraser A, Nelson SM, Lawlor DA . Established preeclampsia risk factors are related to patterns of blood pressure change in normal term pregnancy: findings from the Avon Longitudinal Study of Parents and Children. J Hypertens 2011; 29: 1703–1711.
Imai Y, Munakata M, Tsuji I, Ohkubo T, Satoh H, Yoshino H, Watanabe N, Nishiyama A, Onodera N, Kato J, Sekino M . Seasonal variation in blood pressure in normotensive women studied by home measurements. Clin Sci (Lond) 1996; 90: 55–60.
Ochsenbein-Kölble N, Roos M, Gasser T, Huch R, Zimmermann R . Cross sectional study of automated blood pressure measurement throughout pregnancy. BJOG 2004; 111: 319–325.
Metoki H, Ohkubo T, Watanabe Y, Nishimura M, Sato Y, Kawaguchi M, Hara A, Hirose T, Obara T, Asayama K, Kikuya M . Seasonal trends of blood pressure during pregnancy in Japan: the babies and their parents' longitudinal observation in Suzuki Memorial Hospital in Intrauterine Period study. J Hypertens 2008; 26: 2406–2413.
Denolle T, Daniel JC, Calvez C, Ottavioli JN, Esnault V, Herpin D . Home blood pressure during normal pregnancy. Am J Hypertens 2005; 18: 1178–1180.
Ray E, Pilon F, Boudreault J . Home blood pressure levels in pregnant women with chronic hypertension. Hypertens Pregnancy 2007; 26: 403–414.
Takahashi H, Yoshika M, Yokoi T . Validation of two automatic devices: Omron HEM-7252G-HP and Omron HEM-7251G for self-measurement of blood pressure according to the European Society of Hypertension International Protocol revision 2010. Blood Press Monit 2015; 20: 286–290.
Tsuchihashi T, Uezono K, Abe I, Matsuo M, Kawasaki T . Seasonal variation in 24-h blood pressure pattern of young normotensive women. Hypertens Res 1995; 18: 209–214.
Metoki H, Ohkubo T, Obara T, Akutsu K, Yamamoto M, Ishikuro M, Sakurai K, Iwama N, Katagiri M, Sugawara J, Hirose T, Sato M, Kikuya M, Yagihashi K, Matsubara Y, Yaegashi N, Mori S, Suzuki M, Imai Y, BOSHI Study Group. Daily serial hemodynamic data during pregnancy and seasonal variation: the BOSHI study. Clin Exp Hypertens 2012; 34: 290–296.
Pickering TG, Hall JE, Appel LJ, Falkner BE, Graves JW, Hill MN, Jones DW, Kurtz T, Sheps SG, Roccella EJ . Recommendations for blood pressure measurement in humans: an AHA Scientific Statement from the Council on High Blood Pressure Research Professional and Public Education Subcommittee. J Clin Hypertens (Greenwich) 2005; 7: 102–109.
Sekine M, Goto E, Ochiai H, Umezu M, Ishii T . Examination of the white coat effect at the time of the blood pressure measurement in the hospital. Ther Res 1997; 18: 118–121 (in Japanese).
Seki H . The role of the renin–angiotensin system in the pathogenesis of preeclampsia – new insights into the renin–angiotensin system in preeclampsia. Med Hypotheses 2014; 82: 362–367.
Pickering TG, Miller NH, Ogedegbe G, Krakoff LR, Artinian NT, Goff D . Call to action on use and reimbursement for home blood pressure monitoring: a joint scientific statement from the American Heart Association, American Society of Hypertension, and Preventive Cardiovascular Nurses Association. Hypertension 2008; 52: 10–29.
Poon LC, Zymeri NA, Zamprakou A, Syngelaki A, Nicolaides KH . Protocol for measurement of mean arterial pressure at 11–13 weeks gestation. Fetal Diagn Ther 2012; 31: 42–48.
Sanghave M, Rutherford JD . Cardiovascular physiology of pregnancy. Circulation 2014; 130: 1003–1008.
Koroboki E, Manios EF, Psaltopoulou T, Vemmos KO, Michas FO, Alexaki EL, Zakopoulos N . Circadian variation of blood pressure and heart rate in normotensives, white-coat, masked, treated and untreated hypertensives. Hellenic J Cardiol 2012; 53: 432–438.
Iwama N, Metoki H, Ohkubo T, Ishikuro M, Obara T, Kikuya M, Yagihashi K, Nishigori H, Sugiyama T, Sugawara J, Yaegashi N, Hoshi K, Suzuki M, Kuriyama S, Imai Y, BOSHI Study Group. Maternal clinic and home blood pressure measurements during pregnancy and infant birth weight: the BOSHI study. Hypertens Res 2016; 39: 151–157.
Acknowledgements
We would like to thank Editage (http://www.editage.jp/) for English language editing.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/4.0/
About this article
Cite this article
Mikami, Y., Takai, Y., Era, S. et al. Provisional criteria for the diagnosis of hypertension in pregnancy using home blood pressure measurements. Hypertens Res 40, 679–684 (2017). https://doi.org/10.1038/hr.2017.6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/hr.2017.6
Keywords
This article is cited by
-
A multicenter prospective study of home blood pressure measurement (HBPM) during pregnancy in Japanese women
Hypertension Research (2022)
-
Hypertensive disorders of pregnancy: definition, management, and out-of-office blood pressure measurement
Hypertension Research (2022)
-
Short-term prediction of preeclampsia using the sFlt-1/PlGF ratio: a subanalysis of pregnant Japanese women from the PROGNOSIS Asia study
Hypertension Research (2021)
-
Hypertension in Women Across the Lifespan
Current Atherosclerosis Reports (2021)
-
Association of maternal home blood pressure trajectory during pregnancy with infant birth weight: the BOSHI study
Hypertension Research (2020)