Abstract
Antenatal malnutrition could be linked to hypertension and vascular diseases in fetal origins. This study determined the influence of maternal intake of high sucrose (HS) during pregnancy on vessel tone, intracellular Ca2+ ([Ca2+]i), K+ channels, especially large-conductance Ca2+-activated K+ channels (BK), in mesenteric arteries in the offspring rats exposed to prenatal HS. Vessel tension and [Ca2+]i induced by angiotensin II were higher in the small mesenteric arteries of the HS offspring. In the vascular smooth muscle cells (VSMCs) from the HS offspring, electrophysiological studies showed depressed BK current density and depolarized membrane. Western blot showed altered expressions of BK α-subunits, AT1 and AT2 receptors in mesenteric arteries. The results suggest that decreased BK channel activity and depolarized membrane potential in the VSMCs partly contributed to the increased vessel tone and [Ca2+]i in the HS offspring, adding new information for understanding mechanisms in vascular malfunctions in fetal origins, and novel insights for early prevention and treatments against such vascular diseases.
Similar content being viewed by others
Introduction
Vascular smooth muscle cells (VSMCs) have important roles in the control of vessel tone. Among various K+ channels, large-conductance Ca2+-activated K+ channels (BK) are dominantly expressed in VSMCs and involved in repolarization and negative feedback in the regulation of vessel tone.1, 2, 3, 4, 5 Either opening or closing of BK channels affects resting membrane potential (RMP) and vessel tone remarkably.4, 5 Angiotensin II (Ang II) in renin-angiotensin system, as one of the most important vasoconstrictors, has critical roles in vascular regulation via increasing intracellular Ca2+ ([Ca2+]i).6, 7 One of the mechanisms by which Ang II controls vasoconstriction is linked to a direct inhibition of BK channels, contributing to depolarization and contraction.8
Growing evidence suggested that prenatal insults, including malnutrition, high salt diet, hypoxia and dehydration during pregnancy, could adversely affect cardiovascular conditions in offspring.9, 10, 11, 12, 13, 14 Our previous study has shown that maternal intake of high sucrose (HS) could increase blood glucose in the maternal rats, and affect fetal and offspring’s development,15 evidenced as increased fetal body weight. However, little is known about how maternal HS intake during pregnancy affects vascular functions in the offspring. The present study focused on BK channels and vessel tone in the offspring’s mesenteric arteries and mechanisms involved in the vascular problems in fetal origins.
Methods
Animals
Pregnant Sprague–Dawley rats from the Animal Center of Soochow University were randomly divided into two groups: (1) the control group fed with standard food and tap water and (2) the HS group provided with the same food with 20% sucrose solution since gestational day 1 as reported.15 After delivery, all maternal rats were provided tap water and standard rat food, and the baby rats were given breast feeding. After weaning, the offspring rats from both groups were given tap water and standard rat food for 5 months. All experimental procedures were approved by the Institutional Animal Care Committee and conformed to the Guide for the Care and Use of Laboratory Animals published by the United States National Institutes of Health (NIH Publication No. 85–23, 1996).
Experimental design
Preparation of mesenteric arteries
Rats were anesthetized with a fresh prepared mixture of ketamine (75 mg kg−1) and xylazine (5 mg kg−1) (Hengrui Medicine, Lianyungang, China) intraperitoneally. Adequate anesthesia was determined by loss of a pedal withdrawal reflex and any other reactions in response to pinching rat toe or ear. Fourth-order mesenteric arterial branches were isolated microscopically, removed without being stretched and placed into a Krebs–Henseleit solution (mmol l−1: NaCl 115.0, NaHCO3 25.0, KCl 4.6, NaH2PO4 1.2, MgCl2 1.2, CaCl2 2.5 and glucose 10.0; pH 7.4).
Measurement of vessel tone
Mesenteric arteries were cut into rings of 3.0 mm in length, mounted on a multimyograph system (Danish Myo Technology A/S, Midtjylland, Denmark) as described,16 and maintained in oxygenated Krebs–Henseleit solution (95% O2–5% CO2) at 37 °C. The rings were equilibrated for 60 min. Vessel tone induced by cumulatively increasing concentrations of Ang II (Sigma, St Louis, MO, USA) was normalized by the maximum contraction elicited by 60.0 mmol l−1 KCl. Signals were recorded by Power-Lab system (AD Instruments, Bella Vista, NSW, Australia) with Chart 5 software (AD Instruments).
Measurement of [Ca2+]i in mesenteric arteries
Experiment was carried out in a darkroom. [Ca2+]i in mesenteric arteries was monitored using fluorescent Ca2+ indicator, the acetoxymethyl ester of fura-2 (fura-2 AM; Calbiochem, San Diego, CA, USA) by a Radiance 2100 confocal system, as previously reported.17 In brief, vessels were superfused with modified Hanks’ buffered saline (37 °C) containing (mmol l−1) 137.0 NaCl, 4.2 NaHCO3, 3.0 Na2HPO4, 5.4 KCl, 0.4 KH2PO4, 1.3 CaCl2, 0.5 MgCl2, 0.8 MgSO4, 10.0 glucose and 5.0 HEPES (pH 7.4), and loaded with fura-2 AM (5 μmol l−1) for 120 min. [Ca2+]i levels in nmol l−1 were calculated qualitatively by fluorescence ratio of fura-2 AM at 340 and 380 nm wavelength.
Isolation of vascular myocytes
VSMCs were isolated enzymatically from dissected mesenteric arteries as reported.18 Vessels were cut into 1-mm strips in ice-cold physiological salt solution containing (mmol l−1) 137.0 NaCl, 5.6 KCl, 1.0 MgCl2, 0.42 Na2HPO4, 0.44 NaH2PO4, 4.2 NaHCO3 and 10.0 HEPES (pH 7.4). Dissected arteries were placed into physiological salt solution containing 4 mg ml−1 papain (Worthington Biochemical, Lakewood, NJ, USA) and 2 mg ml−1 dithioerythritol for 25 min at 37 °C; then transferred to physiological salt solution containing 1 mg ml−1 collagenase type F (Sigma) for 15 min at 37 °C. All enzymatic solutions contained 1 mg ml−1 bovine serum albumin. Separated VSMCs were obtained by gentle trituration with a fire-polished Pasteur pipette. The cells were suspended in physiological salt solution and stored at 4 °C for study within 6 h.
Electrophysiological measurement
Whole-cell K+ currents were recorded in conventional whole-cell configuration, voltage-clamp mode using an Axon Multiclamp 700B (Axon Instruments Inc, Foster City, CA, USA) with Clampex 10.1 and normalized to cell capacitance as picoampere per picofarad (pA/pF).19 Membrane potentials were measured in current-clamp configuration. Cells were continuously superfused with HEPES-buffered solution containing (mmol l−1) 135.0 NaCl, 4.0 KCl, 1.0 MgCl2, 2.0 CaCl2, 10.0 HEPES and 10.0 glucose (pH 7.4). Patch pipette tip resistance was 3–4 MΩ filled with pipette solution containing (mmol l−1) 150.0 KCl, 1.0 MgCl2, 0.5 EGTA and 10.0 HEPES (pH 7.4). BK currents were determined by using 1.0 mmol l−1 tetraethylammonium20, 21 (TEA; Sigma) and 100.0 nmol l−1 charybdotoxin22, 23 (Sigma).
Western blot analysis
Protein abundance of BK channel α-subunits, AT1 and AT2 receptors in mesenteric arteries were measured with western blot analysis normalized to β-actin as described.14, 24 Briefly, mesenteric arteries were homogenized. The primary antibodies were the rabbit polyclonal antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA) against BK channel α-subunits (1:500), AT1 (1:500) or AT2 (1:1000). The second antibody was horseradish peroxidase-conjugated goat anti-rabbit antibody (1:5000). Immunosignals were revealed using a scanner and the ratio of band intensity to β-actin was obtained to quantify the relative protein expression level.
Data analysis and statistics
Data were expressed as mean±s.e.m. Statistical significance (P<0.05) was determined by two-way analysis of variance or t-test, where appropriate. Curve fitting was performed with SigmaPlot 11 (Systat Software Inc, Chicago, IL, USA) and GraphPad Prism 5 (GraphPad Software Inc, La Jolla, CA, USA).
Results
Vessel tone and [Ca2+]i induced by Ang II
To determine the effect of prenatal HS on vascular tone, Ang II-increased contraction and [Ca2+]i in resistance-sized mesenteric arteries were measured. Cumulatively increasing concentrations of Ang II produced greater vessel tone and higher [Ca2+]i in mesenteric arteries in HS group than that in the control (Figure 1). Ang II-induced maximum contraction was 3.4±0.6% in the control (n=5) and 8.7±0.5% in the HS group (n=5, P<0.05; Figure 1a), and the maximum [Ca2+]i in the mesenteric arteries was 10.4±0.7% in the control (n=5) and 23.2±0.6% in the HS group (n=5, P<0.05; Figure 1b).
BK channels activity in mesenteric arteries
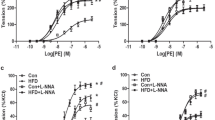
Whole-cell K+ current density in the HS VSMCs was significantly decreased compared with that in the control at the test potentials ranged from 0 to +60 mV (P<0.05), the maximum current density calculated at +60 mV was 36.9±0.8 pA/pF in the control (n=9) and 21.4±0.7 pA/pF in the HS (n=11) with a decrease by 42.0% (P<0.05; Figure 2). Exposure of the VSMCs to 1.0 mmol l−1 TEA (a blocker that can inhibit BK channels20, 21 with negligible effects on voltage-gated K+ channels (KV) at this concentration) or 100.0 nmol l−1 charybdotoxin (a potent and selective BK channel blocker22, 23) significantly depressed K+ currents in similar manner (Figure 2). I–V relationship displayed that whole-cell K+ current density at +60 mV inhibited by TEA and charybdotoxin in the VSMCs were 59.2% and 65.7% in the control (n=9), and 35.7% and 42.9% in the HS (n=11), respectively (Figure 2).
The effect of prenatal HS on whole-cell BK currents. (a, b) Typical K+ current traces recorded in the myocytes in the absence or presence of TEA or charybdotoxin (CTX). (c, d) I–V relationships showed the inhibitory effect of TEA or CTX on whole-cell K+ current density. Control: n=9, HS: n=11. *P<0.05. HS, high sucrose; TEA, tetraethylammonium.
Ang II-inhibited BK channels and depolarized cellular membrane
Ang II attenuated whole-cell K+ currents significantly in the control and HS VSMCs, the current density was decreased from +10 to +60 mV in the control (n=9, P<0.05), and from +30 to +60 mV in the HS (n=8, P<0.05), and the maximum current density was decreased at +60 mV by 46.0% in the control, and by 31.7% in the HS VSMCs (Figure 3).
The inhibitory effect of Ang II on whole-cell K+ currents. (a, b) Representative current traces before and after application of Ang II. (c, d) Resulting I–V curves showed the inhibitory effect on K+ current density by Ang II in the control and HS cells. Control: n=9, HS: n=8. *P<0.05. Ang II, angiotensin II; HS, high sucrose.
To test a possible involvement of BK channels in reduced whole-cell K+ currents, Ang II (100.0 nmol l−1) was employed with pretreatment of TEA (1.0 mmol l−1) in the control (n=8) and HS (n=8) cells (Figure 4). At voltages above 0 mV, there was an obvious reduction in K+ currents by TEA, subsequent application of Ang II showed a little reduction in K+ currents above +50 mV in the control (Figure 4c). For the HS cells, Ang II induced the similar response to TEA in the control (Figure 4d).
The effect of Ang II on whole-cell BK currents. (a, b) Representative traces of whole-cell K+ currents under baseline conditions, pretreated with TEA before Ang II. (c, d) I–V curves obtained in the control and HS cells. Control: n=8, HS: n=8. *P<0.05, #P<0.05. Ang II, angiotensin II; HS, high sucrose; TEA, tetraethylammonium.
Figure 5 showed RMP and the effect of Ang II on membrane potential in the control and HS VSMCs. The RMP was depolarized to −31.6±1.1 mV in the HS VSMCs (n=12) compared with −45.7±1.6 mV in the control (n=13, P<0.05). Ang II (100.0 nmol l−1) further depolarized cellular membrane to −26.0±1.3 mV in the control (n=13, P<0.05) and −20.2±1.4 mV in the HS VSMCs (n=12, P<0.05).
The effect of prenatal HS on membrane potential of VSMCs. (a, b) Typical traces of membrane potential in the control and HS myocytes. (c) Depolarization of RMP in HS rats compared with the controls under basal conditions, and further depolarization of membrane induced by Ang II in either group. Control: n=13, HS: n=12. *P<0.05, #P<0.05. Ang II, angiotensin II; HS, high sucrose; RMP, resting membrane potential.
Antagonist-altered Ang II-mediated inhibition of K+ currents
Losartan, as an antagonist of AT1 receptors, and PD123319, as an antagonist of AT2 receptors, were respectively used to testify the pathways of the inhibition effect of Ang II on K+ currents in control and HS cells. For the control, compared with the inhibitory effect of Ang II (n=9) on whole-cell K+ currents, application of Ang II with losartan (n=7) increased the K+ current density above +40 mV (P<0.05), whereas Ang II with PD123319 (n=6) further decreased the K+ current density above +20 mV (P<0.05; Figures 6a and c). For the HS group, compared with the inhibitory effect of Ang II (n=8) on whole-cell K+ currents, Ang II with losartan (n=6) did not change the K+ current density (P>0.05), whereas Ang II with PD123319 (n=5) further reduced the K+ current density significantly (P<0.05; Figures 6b and d).
The antagonists altered the inhibitory effect of Ang II on whole-cell K+ currents. (a, b) Representative traces of whole-cell K+ currents by application of Ang II pretreated with losartan or PD123319. (c) I–V curves showed that the inhibitory effect of Ang II (n=9) was alleviated by losartan (n=7) and enhanced by PD123319 (n=6) in the control. (d) I–V curves showed that the inhibitory effect of Ang II (n=8) was not altered by pretreatment with losartan (n=6), but further enhanced by PD123319 (n=5) in the HS cells. *P<0.05, #P<0.05. Ang II, angiotensin II; HS, high sucrose.
BK channel α-subunits and AT1/AT2 receptor expression in mesenteric arteries
Western blot analysis showed a significant reduction in protein expression of BK channel α-subunits in the mesenteric arteries from the HS group (Figure 7a). Compared with the control, BK α-subunits protein level was decreased by 54.6% in the HS group (n=5, P<0.05).
The effect of prenatal HS on protein abundance of BK channel α-subunits, AT1 and AT2 receptors in the mesenteric arteries. (a) Western blot analysis showed levels of BK channel α-subunits protein in the mesenteric arteries. (b) Levels of AT1 and AT2 receptors in the mesenteric arteries. Control: n=5, HS: n=5. *P<0.05. AT1: angiotensin II type 1 receptors; AT2: angiotensin II type 2 receptors. HS, high sucrose.
Analysis showed a significant increase in AT1 receptor protein and decrease in AT2 receptor protein in the mesenteric arteries of the HS group (Figure 7b). The AT1 level was increased by 172.2% in the HS group compared with that of the control (n=5, P<0.05), whereas AT2 level was decreased by 64.0% in the HS compared with that of the control (n=5, P<0.05).
Discussion
The present study demonstrated as the first time that expression and functions of BK channels could be depressed in the VSMCs of the rats prenatally exposed to high sugar. The novel findings include: (1) Ang II induced significantly higher vascular tone and intracellular Ca2+ in the mesenteric arteries of the offspring exposed to prenatal HS; (2) Prenatal exposure to HS affected whole-cell K+ current density and RMP in the VSMCs; and (3) BK current density in the HS group was reduced significantly. In addition, expression of BK channel α-subunits, AT1 and AT2 receptors in the mesenteric arteries of the HS offspring was altered. Together, the results indicate that intake of high sugar during pregnancy could increase vascular tone and intracellular Ca2+ in the mesenteric arteries, which might be partly related to inhibition of BK channels in the HS VSMCs.
The role of [Ca2+]i in the increased vascular tone in the HS offspring
In the present study, maternal feeding with HS during pregnancy could affect vascular functions in the adult offspring, evidenced as the increase of vessel tone in response to Ang II was significantly higher in the HS offspring.
Ang II controls vascular tone by influencing vasoconstriction via AT1 and AT2 receptors. A rapid increase in [Ca2+]i is a major determinant of vascular contraction.6, 25 Binding of Ang II to its receptors results in activation of downstream effectors, including hydrolyzing phosphatidylinositol 4,5-bisphosphate to generate inositol-1,4,5-triphosphate. Inositol-1,4,5-triphosphate in turn, acts on sarcoplasmic reticulum, leading to mobilization of intracellular Ca2+ and increase in [Ca2+]i.26 On the other hand, Ang II promotes Ca2+ influx.6, 7, 27 Ca2+ binds to calmodulin, causing smooth muscle cell contraction. In the present study, vessel contraction and intracellular Ca2+ levels in response to Ang II were significantly higher in the HS than that of the control, indicating that the Ang II-increased vessel tone was linked to intracellular Ca2+ changes. Fourth-order mesenteric arterial branches can be regarded as the representative of peripheral resistance arteries. The present study provided new information on possible mechanisms in the resistance arteries linked to increased vascular tension following exposure to prenatal HS. Previous studies demonstrated that endothelial cells also are important to vascular tension.28, 29 In our vessel studies, it is hard to exclude possibilities of involvement of endothelial cells in explaining the results, and further studies are needed.
Depolarization of membrane and elevated [Ca2+]i
Multiple factors, including membrane potential, can regulate [Ca2+]i in VSMCs. Membrane potential not only affects voltage-gated Ca2+ channels but also influences inositol-1,4,5-triphosphate-induced release of Ca2+ from intracellular stores and Ca2+ sensitivity of the contractile apparatus.26 In the present study, patch clamp test showed that RMP in the HS VSMCs was significantly depolarized compared with that in the control. In addition, Ang II could further depolarize the membrane in both the HS VSMCs and the control, indicating that the enhanced vessel tension and elevated [Ca2+]i might be partly related to depolarization of membrane in the HS cells. Cellular membrane potential and [Ca2+]i are closely related to vascular tone.6, 7, 30, 31 Thus, whether the altered membrane potential could be a major influence of the elevated [Ca2+]i in the present study is worth future investigation.
BK channels in control of membrane potential and vascular tone
K+ channels in VSMCs have a key role in control of membrane potential and vascular tone.4, 5, 32 Opening of K+ channels in VSMCs results in membrane hyperpolarization and closing of voltage-dependent Ca2+ channels and therefore induces vasodilatation. Conversely, closing of K+ channels reduces K+ efflux and depolarizes cell membrane, thereby leading to opening of voltage-gated Ca2+ channels and vasoconstriction.4, 27, 32 Our patch clamp study demonstrated a significantly reduced whole-cell K+ current density in the HS VSMCs, indicating declined K+ channel’s activity. It is well known that BK currents are the major components in K+ currents in VSMCs.2, 5, 32 Thus, the present study focused on BK channels in the VSMCs from the HS offspring that has not been investigated.
BK channels, activated by both elevation of [Ca2+]i and depolarization of membrane, serve as a key determinant in negative feedback against depolarization and increased vascular tone because of the large conductance and high density in vascular smooth muscles.1, 2, 3, 4, 5 In general, K+ channels, especially BK, are involved in the control of vascular tone. In the present study, the TEA- or charybdotoxin-sensitive current component attributed to opening of BK channels was significantly lower in the HS cells than that of the control, indicating impaired activity of BK channels.3, 4, 5, 33 The decreased activity of BK channels in VSMCs could depolarize the membrane leading to an increase of vascular tone. Our western blot experiments showed that BK channel α-subunit expression in the HS mesenteric arteries was significantly reduced, which might be linked to depressed BK current density and membrane depolarization in the HS VSMCs. This also provides new supportive information that the changes of the BK channels, either in their protein expression levels or functional status, may have an important role in the mechanisms of the increased intracellular Ca2+ related to vessel tension in the resistance vasculature of the HS group.
In the present study, Ang II significantly increased vascular tone and [Ca2+]i in the HS mesenteric arteries. Furthermore, electrophysiological measurements demonstrated downregulation of intrinsic suppressed whole-cell K+ currents in the HS VSMCs by Ang II. The data suggest that Ang II-increased intracellular Ca2+ determines vessel contraction, and alteration of K+ channels in the VSMCs may change vessel responses to Ang II. Depolarization due to inhibition of BK currents could activate L-Ca2+ channels, leading to an elevated [Ca2+]i and vessel contraction.2, 3, 4, 5, 34 The present study showed that Ang II mainly inhibited BK channels in both the control and HS VSMCs. This means that in response to Ang II, the inhibited expression of BK channels or their activity weakened the feedback via BK channels in the regulation of vascular tone in the HS offspring by affecting membrane potential and intracellular Ca2+. In light of this, BK channels could be viewed as targets for future investigation on angiotensin-mediated vascular diseases in fetal origins in prevention and treatments.
Ang II-inhibited K+ channels via the AT1/AT2 pathways
Most vascular effects of Ang II are mediated via AT1 receptors. Although physiological or pathophysiological effects of AT2 receptors in control of vascular tone are unclear, several studies suggested that AT2 receptors antagonized the effects of AT1 receptors by inhibiting its signaling pathways via activation of tyrosine or serine/threonine phosphatases,35, 36 suggesting that Ang II induces vasoconstriction by binding to AT1 receptors, and vasodilatation by binding to AT2 receptors.37, 38 In the present study, AT1 receptor antagonist losartan alleviated the inhibitory effect of Ang II, whereas block of AT2 receptors with PD123319 enhanced the inhibitory effect in the control VSMCs. Interestingly, for the HS VSMCs, losartan did not significantly affect the inhibitory effect of Ang II, whereas PD123319 could further enhance the inhibitory effect of Ang II. Furthermore, our experiments demonstrated increased expression of AT1 receptors and decreased expression of AT2 receptors in the HS mesenteric arteries. Together, the results indicated that alteration of AT1/AT2 receptors might have a role in the cellular phenomenon observed in this study. An imbalance of AT1/AT2 receptor ratio in the vasculature may affect Ang II-mediated vascular tone.
In the present study, from vessel tension testing to electrophysiological recording on the cells, we found the influence of prenatal over intake of sucrose on the functional development of the vessel systems. We realized limitation of the approaches used in the study in explaining the mechanisms as to how prenatal insults affect cardiovascular systems. Epigenetic influence could be one of the explanations, and which deserves further investigation.
In summary, the data demonstrated that BK channel activity was reduced and vascular tone enhanced in small mesenteric arteries from the HS rats, supporting the hypothesis that intake of high sugar during pregnancy affects vascular tone and [Ca2+]i in the offspring. Moreover, the altered expression of BK channels and AT1/AT2 receptors and their activities in the HS mesenteric arteries may affect membrane potential of VSMCs, which might be partly contributed to the elevation of [Ca2+]i and increased vessel tone induced by Ang II in the HS offspring. Although whether vascular endothelium involved in those changes needs further studies, the present data added new information for further understanding the ionic channel mechanisms affecting vessel tone in fetal origins, and provided new insights for early prevention and treatments against such vascular diseases.
References
Latorre R, Oberhauser A, Labarca P, Alvarez O . Varieties of calcium-activated potassium channels. Annu Rev Physiol 1989; 51: 385–399.
Latorre R, Brauchi S . Large conductance Ca2+-activated K+ (BK) channel: activation by Ca2+ and voltage. Biol Res 2006; 39: 385–401.
Ko EA, Han J, Jung ID, Park WS . Physiological roles of K+ channels in vascular smooth muscle cells. J Smooth Muscle Res 2008; 44: 65–81.
Jackson WF . Ion channels and vascular tone. Hypertension 2000; 35: 173–178.
Nelson MT, Quayle JM . Physiological roles and properties of potassium channels in arterial smooth muscle. Am J Physiol 1995; 268: C799–C822.
Touyz RM, Schiffrin EL . Role of calcium influx and intracellular calcium stores in angiotensin II-mediated calcium hyper-responsiveness in smooth muscle from spontaneously hypertensive rats. J Hypertens 1997; 15: 1431–1439.
Nieves-Cintrón M, Amberg GC, Navedo MF, Molkentin JD, Santana LF . The control of Ca2+ influx and NFATc3 signaling in arterial smooth muscle during hypertension. Proc Natl Acad Sci USA 2008; 105: 15623–15628.
Toro L, Amador M, Stefani E . ANG II inhibits calcium-activated potassium channels from coronary smooth muscle in lipid bilayers. Am J Physiol 1990; 258: H912–H915.
Mao C, Shi L, Xu F, Zhang L, Xu Z . Development of fetal brain renin-angiotensin system and hypertension programmed in fetal origins. Prog Neurobiol 2009; 87: 252–263.
Barker DJ, Osmond C, Forsen TJ, Kajantie E, Eriksson JG . Maternal and social origins of hypertension. Hypertension 2007; 50: 565–571.
Poston L . Influences of maternal nutritional status on vascular function in the offspring. Curr Drug Targets 2007; 8: 914–922.
Porter JP, King SH, Honeycutt AD . Prenatal high-salt diet in the Sprague-Dawley rat programs blood pressure and heart rate hyperresponsiveness to stress in adult female offspring. Am J Physiol Regul Integr Comp Physiol 2007; 293: R334–R342.
Zhang L . Prenatal hypoxia and cardiac programming. J Soc Gynecol Investig 2005; 12: 2–13.
Guan J, Mao C, Xu F, Geng C, Zhu L, Wang A, Xu Z . Prenatal dehydration alters renin-angiotensin system associated with angiotensin-increased blood pressure in young offspring. Hypertens Res 2009; 32: 1104–1111.
Wu L, Mao C, Liu Y, Shi A, Xu F, Zhang L, Xu Z . Altered dipsogenic responses and expression of angiotensin receptors in the offspring exposed to prenatal high sucrose. Peptides 2011; 32: 104–111.
Neidhold S, Eichhorn B, Kasper M, Ravens U, Kaumann AJ . The function of alpha- and beta-adrenoceptors of the saphenous artery in caveolin-1 knockout and wild-type mice. Br J Pharmacol 2007; 150: 261–270.
Kip SN, Hunter LW, Ren Q, Harris PC, Somlo S, Torres VE, Sieck GC, Qian Q . [Ca2+]i reduction increases cellular proliferation and apoptosis in vascular smooth muscle cells: relevance to the ADPKD phenotype. Circ Res 2005; 96: 873–880.
Eichhorn B, Muller G, Leuner A, Sawamura T, Ravens U, Morawietz H . Impaired vascular function in small resistance arteries of LOX-1 overexpressing mice on high-fat diet. Cardiovasc Res 2009; 82: 493–502.
Pérez GJ, Bonev AD, Patlak JB, Nelson MT . Functional coupling of ryanodine receptors to KCa channels in smooth muscle cells from rat cerebral arteries. J Gen Physiol 1999; 113: 229–238.
Langton PD, Nelson MT, Huang Y, Standen NB . Block of calcium-activated potassium channels in mammalian arterial myocytes by tetraethylammonium ions. Am J Physiol 1991; 260: H927–H934.
Brayden JE, Nelson MT . Regulation of arterial tone by activation of calcium-dependent potassium channels. Science 1992; 256: 532–535.
Anderson CS, MacKinnon R, Smith C, Miller C . Charybdotoxin block of single Ca2+-activated K+ channels. Effects of channel gating, voltage, and ionic strength. J Gen Physiol 1988; 91: 317–333.
Miller C, Moczydlowski E, Latorre R, Phillips M . Charybdotoxin a protein inhibitor of single Ca2+-activated K+ channels from mammalian skeletal muscle. Nature 1985; 313: 316–318.
Choudhury S, Garg SK, Singh TU, Mishra SK . Functional and molecular characterization of maxi K+ -channels (BKCa) in buffalo myometrium. Anim Reprod Sci 2011; 126: 173–178.
Rembold CM . Regulation of contraction and relaxation in arterial smooth muscle. Hypertension 1992; 20: 129–137.
Ushio-Fukai M, Griendling KK, Akers M, Lyons PR, Alexander RW . Temporal dispersion of activation of phospholipase C-beta1 and -gamma isoforms by angiotensin II in vascular smooth muscle cells. Role of alphaq/11, alpha12, and beta gamma G protein subunits. J Biol Chem 1998; 273: 19772–19777.
Touyz RM, Wu XH, He G, Park JB, Chen X, Vacher J, Rajapurohitam V, Schiffrin EL . Role of c-Src in the regulation of vascular contraction and Ca2+ signaling by angiotensin II in human vascular smooth muscle cells. J Hypertens 2001; 19: 441–449.
Sandow SL, Senadheera S, Grayson TH, Welsh DG, Murphy TV . Calcium and endothelium-mediated vasodilator signaling. Adv Exp Med Biol 2012; 740: 811–831.
Lamas S, Rodríguez-Puyol D . Endothelial control of vasomotor tone: the kidney perspective. Semin Nephrol 2012; 32: 156–166.
Smirnov SV, Aaronson PI . Ca2+ currents in single myocytes from human mesenteric arteries: evidence for a physiological role of L-type channels. J Physiol (Lond) 1992; 457: 455–475.
Kubo T, Taguchi K, Ueda M . L-type calcium channels in vascular smooth muscle cells from spontaneously hypertensive rats: effects of calcium agonist and antagonist. Hypertens Res 1998; 21: 33–37.
Jackson WF . Potassium channels in the peripheral microcirculation. Microcirculation 2005; 12: 113–127.
Burnham MP, Johnson IT, Weston AH . Reduced Ca2+-dependent activation of large-conductance Ca2+-activated K+ channels from arteries of Type 2 diabetic Zucker diabetic fatty rats. Am J Physiol Heart Circ Physiol 2006; 290: H1520–H1527.
Turner RW, Anderson D, Zamponi GW . Signaling complexes of voltage-gated calcium channels. Channels (Austin) 2011; 5: 440–448.
Bedecs K, Elbaz N, Sutren M, Masson M, Susini C, Strosberg AD, Nahmias C . Angiotensin II type 2 receptors mediate inhibition of mitogen-activated protein kinase cascade and functional activation of SHP-1 tyrosine phosphatase. Biochem J 1997; 325: 449–454.
Munzenmaier DH, Greene AS . Opposing actions of angiotensin II on microvascular growth and arterial blood pressure. Hypertension 1996; 27: 760–765.
Pulgar VM, Yamashiro H, Rose JC, Moore LG . Role of the AT2 receptor in modulating the angiotensin II contractile response of the uterine artery at mid-gestation. J Renin Angiotensin Aldosterone Syst 2011; 12: 176–183.
Cat AN, Touyz RM . A new look at the renin-angiotensin system—focusing on the vascular system. Peptides 2011; 32: 2141–2150.
Acknowledgements
This work was supported partly by 2012CB947600, National Natural Science Foundation of China (81030006 and 30973211, 81070540 and 30902018); and grants (BK2009122, CX09B-031Z and 2011BAZ03151, as well as Jiangsu Province’s Key Discipline/Laboratory of Medicine and ‘ChuangXin TuanDui’ grant, China).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Li, S., Fang, Q., Zhou, A. et al. Intake of high sucrose during pregnancy altered large-conductance Ca2+-activated K+ channels and vessel tone in offspring’s mesenteric arteries. Hypertens Res 36, 158–165 (2013). https://doi.org/10.1038/hr.2012.146
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/hr.2012.146
Keywords
This article is cited by
-
Primary Pediatric Hypertension: Current Understanding and Emerging Concepts
Current Hypertension Reports (2017)
-
Ca2+-regulated lysosome fusion mediates angiotensin II-induced lipid raft clustering in mesenteric endothelial cells
Hypertension Research (2016)
-
Chronic fetal exposure to caffeine altered resistance vessel functions via RyRs-BKCa down-regulation in rat offspring
Scientific Reports (2015)
-
Chronic hypoxia in pregnancy affected vascular tone of renal interlobar arteries in the offspring
Scientific Reports (2015)
-
High-sucrose diets in pregnancy alter angiotensin II-mediated pressor response and microvessel tone via the PKC/Cav1.2 pathway in rat offspring
Hypertension Research (2014)