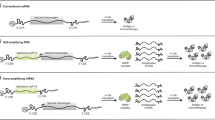
Abstract
The use of DNA to deliver vaccine antigens offers many advantages, including ease of manufacture and cost. However, most DNA vaccines are plasmids and must be grown in bacterial culture, necessitating elements that are either unnecessary for effective gene delivery (for example, bacterial origins of replication) or undesirable (for example, antibiotic resistance genes). Removing these elements may improve the safety profile of DNA for the delivery of vaccines. Here, we describe a novel, double-stranded, linear DNA construct produced by an enzymatic process that solely encodes an antigen expression cassette, comprising antigen, promoter, polyA tail and telomeric ends. We compared these constructs (called ‘Doggybones’ because of their shape) with conventional plasmid DNA. Using luciferase-expressing constructs, we demonstrated that expression levels were equivalent between Doggybones and plasmids both in vitro and in vivo. When mice were immunized with DNA constructs expressing the HIV envelope protein gp140, equivalent humoral and cellular responses were induced. Immunizations with either construct type expressing hemagluttinin were protective against H1N1 influenza challenge. This is the first example of an effective DNA vaccine, which can be produced on a large scale by enzymatic processes.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Tang DC, DeVit M, Johnston SA Genetic immunization is a simple method for eliciting an immune response. Nature 1992; 356: 152–154.
Gurunathan S, Klinman DM, Seder RA DNA vaccines: immunology, application, and optimization. Ann Rev Immunol 2000; 18: 927–974.
Evensen O, Leong JA DNA vaccines against viral diseases of farmed fish. Fish Shellfish Immunol 2013; 35: 1751–1758.
Liu MA DNA vaccines: an historical perspective and view to the future. Immunol Rev 2011; 239: 62–84.
Ferraro B, Morrow MP, Hutnick NA, Shin TH, Lucke CE, Weiner DB Clinical applications of DNA vaccines: current progress. Clin Infect Dis 2011; 53: 296–302.
Klinman DM, Klaschik S, Tross D, Shirota H, Steinhagen F FDA guidance on prophylactic DNA vaccines: analysis and recommendations. Vaccine 2010; 28: 2801–2805.
Belakova J, Horynova M, Krupka M, Weigl E, Raska M DNA vaccines: are they still just a powerful tool for the future? Arch Immunol Ther Exp 2007; 55: 387–398.
Luke JM, Vincent JM, Du SX, Gerdemann U, Leen AM, Whalen RG et al. Improved antibiotic-free plasmid vector design by incorporation of transient expression enhancers. Gene Ther 2011; 18: 334–343.
Luke J, Carnes AE, Hodgson CP, Williams JA Improved antibiotic-free DNA vaccine vectors utilizing a novel RNA based plasmid selection system. Vaccine 2009; 27: 6454–6459.
Mairhofer J, Cserjan-Puschmann M, Striedner G, Nöbauer K, Razzazi-Fazeli E, Grabherr R Marker-free plasmids for gene therapeutic applications—Lack of antibiotic resistance gene substantially improves the manufacturing process. J Biotechnol 2010; 146: 130–137.
van der Heijden I, Gomez-Eerland R, van den Berg JH, Oosterhuis K, Schumacher TN, Haanen JB et al. Transposon leads to contamination of clinical pDNA vaccine. Vaccine 2013; 31: 3274–3280.
Prather KJ, Sagar S, Murphy J, Chartrain M Industrial scale production of plasmid DNA for vaccine and gene therapy: plasmid design, production, and purification. Enzyme Microb Tech 2003; 33: 865–883.
Darquet AM, Cameron B, Wils P, Scherman D, Crouzet J A new DNA vehicle for nonviral gene delivery: supercoiled minicircle. Gene Therapy 1997; 4: 1341–1349.
Moreno S, Lopez-Fuertes L, Vila-Coro AJ, Sack F, Smith CA, Konig SA et al. DNA immunisation with minimalistic expression constructs. Vaccine 2004; 22: 1709–1716.
Kay MA, He CY, Chen ZY A robust system for production of minicircle DNA vectors. Nat Biotechnol 2010; 28: 1287–1289.
Hill V (Inventor; Touchlight Genetics Ltd, assignee). Production of Closed Linear DNA. WO 2010086626 A1, 5 August 2010.
Heinrich J, Schultz J, Bosse M, Ziegelin G, Lanka E, Moelling K Linear closed mini DNA generated by the prokaryotic cleaving-joining enzyme TelN is functional in mammalian cells. J Mol Med 2002; 80: 648–654.
Huang X, Jin W, Hu K, Luo S, Du T, Griffin GE et al. Highly conserved HIV-1 gp120 glycans proximal to CD4-binding region affect viral infectivity and neutralizing antibody induction. Virology 2012; 423: 97–106.
Kopycinski J, Cheeseman H, Ashraf A, Gill D, Hayes P, Hannaman D et al. A DNA-based candidate HIV vaccine delivered via in vivo electroporation induces CD4 responses toward the alpha4beta7-binding V2 loop of HIV gp120 in healthy volunteers. Clin Vaccine Immunol 2012; 19: 1557–1559.
Rosazza C, Buntz A, Riess T, Woll D, Zumbusch A, Rols MP . Intracellular tracking of single plasmid DNA-particles after delivery by electroporation. Mol Ther 2013; 21 12: 2217–2226.
Yin W, Xiang P, Li Q Investigations of the effect of DNA size in transient transfection assay using dual luciferase system. Anal Biochem 2005; 346: 289–294.
Zhang G, Ludtke JJ, Thioudellet C, Kleinpeter P, Antoniou M, Herweijer H et al. Intraarterial delivery of naked plasmid DNA expressing full-length mouse dystrophin in the mdx mouse model of duchenne muscular dystrophy. Hum Gene Ther 2004; 15: 770–782.
von Groll A, Levin Y, Barbosa MC, Ravazzolo AP . Linear DNA low efficiency transfection by liposome can be improved by the use of cationic lipid as charge neutralizer. Biotechnol Prog 2006; 22: 1220–1224.
Darquet AM, Rangara R, Kreiss P, Schwartz B, Naimi S, Delaere P et al. Minicircle: an improved DNA molecule for in vitro and in vivo gene transfer. Gene Therapy 1999; 6: 209–218.
Madeira C, Rodrigues CA, Reis MS, Ferreira FF, Correia RE, Diogo MM et al. Nonviral gene delivery to neural stem cells with minicircles by microporation. Biomacromolecules 2013; 14: 1379–1387.
Chen ZY, He CY, Ehrhardt A, Kay MA Minicircle DNA vectors devoid of bacterial DNA result in persistent and high-level transgene expression in vivo. Mol Ther 2003; 8: 495–500.
Endmann A, Baden M, Weisermann E, Kapp K, Schroff M, Kleuss C et al. Immune response induced by a linear DNA vector: influence of dose, formulation and route of injection. Vaccine 2010; 28: 3642–3649.
Mann JF, McKay PF, Arokiasamy S, Patel RK, Klein K, Shattock RJ Pulmonary delivery of DNA vaccine constructs using deacylated PEI elicits immune responses and protects against viral challenge infection. J Control Release 2013; 170: 452–459.
Mann JF, McKay PF, Arokiasamy S, Patel RK, Tregoning JS, Shattock RJ Mucosal application of gp140 encoding DNA polyplexes to different tissues results in altered immunological outcomes in Mice. PLoS One 2013; 8: e67412.
Zhang C, Gao S, Jiang W, Lin S, Du F, Li Z et al. Targeted minicircle DNA delivery using folate-poly(ethylene glycol)-polyethylenimine as non-viral carrier. Biomaterials 2010; 31: 6075–6086.
Vaysse L, Gregory LG, Harbottle RP, Perouzel E, Tolmachov O, Coutelle C Nuclear-targeted minicircle to enhance gene transfer with non-viral vectors in vitro and in vivo. J Gene Med 2006; 8: 754–763.
Hutnick NA, Myles DJ, Bian CB, Muthumani K, Weiner DB . Selected approaches for increasing HIV DNA vaccine immunogenicity in vivo. Curr Opin Virol 2011; 1: 233–240.
Lopez-Fuertes L, Perez-Jimenez E, Vila-Coro AJ, Sack F, Moreno S, Konig SA et al. DNA vaccination with linear minimalistic (MIDGE) vectors confers protection against Leishmania major infection in mice. Vaccine 2002; 21: 247–257.
Smith HA Regulatory considerations for nucleic acid vaccines. Vaccine 1994; 12: 1515–1519.
Donnelly L, Curran RM, Tregoning JS, McKay PF, Cole T, Morrow RJ et al. Intravaginal immunization using the recombinant HIV-1 clade-C trimeric envelope glycoprotein CN54gp140 formulated within lyophilized solid dosage forms. Vaccine 2011; 29: 4512–4520.
Elleman CJ, Barclay WS The M1 matrix protein controls the filamentous phenotype of influenza A virus. Virology 2004; 321: 144–153.
Tregoning JS, Yamaguchi Y, Wang B, Mihm D, Harker JA, Bushell ES et al. Genetic susceptibility to the delayed sequelae of neonatal respiratory syncytial virus infection is MHC dependent. J Immunol 2010; 185: 5384–5391.
Acknowledgements
We would like to thank Qin Hu (St George’s University of London) for the MWS2 gp140 gene sequence, Ruth Elderfield and Wendy Barclay (Imperial College London) for the influenza hemagluttinin gene sequence and influenza virus and Paul Rothwell, Jon Extance, Karen Oliver and Kinga Karbowniczek (Touchlight Genetics Ltd.) for the generation of the Doggybone material. This work was funded in part by Touchlight Genetics Ltd. and the Exgenomes project under the Seventh Framework program for funding. EK is funded by an MRC CASE studentship MR/J006548/1. We are indebted to Dormeur Investment Service Ltd. for funding of equipment used in this project.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The study was funded in part by Touchlight Genetics, who were involved in all stages of the study conduct and analysis. Dr Caproni and Dr Porter are employees of Touchlight Genetics. The corresponding author had final responsibility to submit for publication.
Additional information
Supplementary Information accompanies this paper on Gene Therapy website
Supplementary information
Rights and permissions
About this article
Cite this article
Walters, A., Kinnear, E., Shattock, R. et al. Comparative analysis of enzymatically produced novel linear DNA constructs with plasmids for use as DNA vaccines. Gene Ther 21, 645–652 (2014). https://doi.org/10.1038/gt.2014.37
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/gt.2014.37
This article is cited by
-
DNA synthesis technologies to close the gene writing gap
Nature Reviews Chemistry (2023)
-
Production of lentiviral vectors using novel, enzymatically produced, linear DNA
Gene Therapy (2019)
-
Resolving systematic errors in widely used enhancer activity assays in human cells
Nature Methods (2018)
-
Linear doggybone DNA vaccine induces similar immunological responses to conventional plasmid DNA independently of immune recognition by TLR9 in a pre-clinical model
Cancer Immunology, Immunotherapy (2018)