Abstract
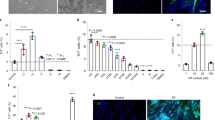
The Hedgehog (Hh) pathway is a crucial regulator of muscle development during embryogenesis. We have previously demonstrated that Sonic hedgehog (Shh) regulates postnatal myogenesis in the adult skeletal muscle both directly, by acting on muscle satellite cells, and indirectly, by promoting the production of growth factors from interstitial fibroblasts. Here, we show that in mdx mice, the murine equivalent of Duchenne muscular dystrophy in humans, progression of the dystrophic pathology corresponds to progressive inhibition of the Hh signaling pathway in the skeletal muscle. We also show that the upregulation of the Hh pathway in response to injury and during regeneration is significantly impaired in mdx muscle. Shh treatment increases the proliferative potential of satellite cells isolated from the muscles of mdx mice. This treatment also increases the production of proregenerative factors, such as insulin-like growth factor-1 and vascular endothelial growth factor, from fibroblasts isolated from the muscle of mdx mice. In vivo, overexpression of the Hh pathway using a plasmid encoding the human Shh gene promotes successful regeneration after injury in terms of increased number of proliferating myogenic cells and newly formed myofibers, as well as enhanced vascularization and decreased fibrosis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Borycki AG, Brunk B, Tajbakhsh S, Buckingham M, Chiang C, Emerson CP Jr . Sonic hedgehog controls epaxial muscle determination through Myf5 activation. Development 1999; 126: 4053–4063.
Gustafsson MK, Pan H, Pinney DF, Liu Y, Lewandowski A, Epstein DJ et al Myf5 is a direct target of long-range Shh signaling and Gli regulation for muscle specification. Genes Dev 2002; 16: 114–126.
Hu JK, McGlinn E, Harfe BD, Kardon G, Tabin CJ . Autonomous and non-autonomous roles of Hedgehog signaling in regulating limb muscle formation. Genes Dev 2012; 26: 2088–2102.
Anderson C, Williams VC, Moyon B, Daubas P, Tajbakhsh S, Buckingham ME et al Sonic hedgehog acts cell-autonomously on muscle precursor cells to generate limb muscle diversity. Genes Dev 2013; 26: 2103–2117.
Pola R, Ling LE, Aprahamian TR, Barban E, Bosch-Marce M, Curry C et al Postnatal recapitulation of embryonic hedgehog pathway in response to skeletal muscle ischemia. Circulation 2003; 108: 479–485.
Straface G, Aprahamian T, Flex A, Gaetani E, Biscetti F, Smith RC et al Sonic hedgehog regulates angiogenesis and myogenesis during post-natal skeletal muscle regeneration. J Cell Mol Med 2009; 13: 2424–2435.
Palladino M, Gatto I, Neri V, Straino S, Silver M, Tritarelli A et al Pleiotropic beneficial effects of sonic hedgehog gene therapy in an experimental model of peripheral limb ischemia. Mol Ther 2011; 19: 658–666.
Pola R, Ling LE, Silver M, Corbley MJ, Kearney M, Pepinsky RB et al The morphogen Sonic hedgehog is an indirect angiogenic agent upregulating two families of angiogenic growth factors. Nat Med 2001; 7: 706–711.
Kusano KF, Pola R, Murayama T, Curry C, Kawamoto A, Iwakura A et al Sonic hedgehog myocardial gene therapy: tissue repair through transient reconstitution of embryonic signaling. Nat Med 2005; 11: 1197–1204.
Elia D, Madhala D, Ardon E, Reshef R, Halevy O . Sonic hedgehog promotes proliferation and differentiation of adult muscle cells: involvement of MAPK/ERK and PI3K/Akt pathways. Biochim Biophys Acta 2007; 1773: 1438–1446.
Koleva M, Kappler R, Vogler M, Herwig A, Fulda S, Hahn H . Pleiotropic effects of sonic hedgehog on muscle satellite cells. Cell Mol Life Sci 2005; 62: 1863–1870.
Woo M, Tanabe Y, Ishii H, Nonaka I, Yokoyama M, Esaki K . Muscle fiber growth and necrosis in dystrophic muscles: a comparative study between dy and mdx mice. J Neurol Sci 1987; 82: 111–122.
Pastoret C, Sebille A . Mdx mice show progressive weakness and muscle deterioration with age. J Neurol Sci 1995; 129: 97–105.
Palladino M, Gatto I, Neri V, Stigliano E, Smith RC, Pola E et al Combined therapy with sonic hedgehog gene transfer and bone marrow-derived endothelial progenitor cells enhances angiogenesis and myogenesis in the ischemic skeletal muscle. J Vasc Res 2012; 49: 425–431.
Scholz D, Thomas S, Sass S, Podzuweit T . Angiogenesis and myogenesis as two facets of inflammatory post-ischemic tissue regeneration. Mol Cell Biochem 2003; 246: 57–67.
Tedesco FS, Dellavalle A, Diaz-Manera J, Messina G, Cossu G . Repairing skeletal muscle: regenerative potential of skeletal muscle stem cells. J Clin Invest 2010; 120: 11–19.
Murphy MM, Lawson JA, Mathew SJ, Hutcheson DA, Kardon G . Satellite cells, connective tissue fibroblasts and their interactions are crucial for muscle regeneration. Development 2011; 138: 3625–3637.
Montarras D, Morgan J, Collins C, Relaix F, Zaffran S, Cumano A et al Direct isolation of satellite cells for skeletal muscle regeneration. Science 2005; 309: 2064–2067.
Wang B, Miyagoe-Suzuki Y, Yada E, Ito N, Nishiyama T, Nakamura M et al Reprogramming efficiency and quality of induced pluripotent stem cells (iPSCs) generated from muscle-derived fibroblasts of mdx mice at different ages. PLoS Curr 2011; 3: RRN1274.
Borselli C, Hannah S, Benesch-Lee F, Shvartsman D, Christine C, Litchman JW et al Functional muscle regeneration with combined delivery of angiogenesis and myogenesis factors. Proc Natl Acad Sci USA 2010; 107: 3287–3292.
Emery AE . Population frequencies of inherited neuromuscular diseases—a world survey. Neuromuscul Disord 1991; 1: 19–29.
Matsumura M, Ohlendieck K, Ionasescu VV, Tomé FM, Nonaka I, Burghes AH et al The role of the dystrophin-glycoprotein complex in the molecular pathogenesis of muscular dystrophies. Neuromuscul Disord 1993; 3: 533–535.
Jejurikar SS, Kuzon WM Jr . Satellite cell depletion in degenerative skeletal muscle. Apoptosis 2003; 8: 573–578.
Kornegay JN, Childers MK, Bogan DJ, Bogan JR, Nghiem P, Wang J et al The paradox of muscle hypertrophy in muscular dystrophy. Phys Med Rehabil Clin N Am 2012; 23: 149–172.
Asai A, Sahani N, Kaneki M, Ouchi Y, Martyn JA, Yasuhara SE . Primary role of functional ischemia, quantitative evidence for the two-hit mechanism, and phosphodiesterase-5 inhibitor therapy in mouse muscular dystrophy. PLoS One 2007; 2: e806.
Thomas GD, Ye J, De Nardi C, Monopoli A, Ongini E, Victor RG . Treatment with a nitric oxide-donating NSAID alleviates functional muscle ischemia in the mouse model of Duchenne muscular dystrophy. PLoS One 2012; 7: e49350.
Verma M, Asakura Y, Hirai H, Watanabe S, Tastad C, Fong GH et al Flt-1 haploinsufficiency ameliorates muscular dystrophy phenotype by developmentally increased vasculature in mdx mice. Hum Mol Genet 2010; 19: 4145–4159.
Miyazaki D, Nakamura A, Fukushima K, Yoshida K, Takeda S, Ikeda S . Matrix metalloproteinase-2 ablation in dystrophin-deficient mdx muscles reduces angiogenesis resulting in impaired growth of regenerated muscle fibers. Hum Mol Genet 2011; 20: 1787–1799.
Palladino M, Gatto I, Neri V, Straino S, Smith RC, Silver M et al Angiogenic impairment of the vascular endothelium: a novel mechanism and potential therapeutic target in muscular dystrophy. Arterioscler Thromb Vasc Biol 2013; 33: 2867–2876.
Stanton BZ, Peng LF . Small-molecule modulators of the Sonic Hedgehog signaling pathway. Mol Biosyst 2010; 6: 44–54.
Alway SE, Pereira SL, Edens NK, Hao Y, Bennett BT . β-Hydroxy-β-methylbutyrate (HMB) enhances the proliferation of satellite cells in fast muscles of aged rats during recovery from disuse atrophy. Exp Gerontol 2013; 48: 973–984.
Acknowledgements
This work was supported by grant RBID08MAFS (FIRB-IDEAS) from the Italian Department of University and Research (MIUR) and by NIH grant 1R21HL089684.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Piccioni, A., Gaetani, E., Palladino, M. et al. Sonic hedgehog gene therapy increases the ability of the dystrophic skeletal muscle to regenerate after injury. Gene Ther 21, 413–421 (2014). https://doi.org/10.1038/gt.2014.13
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/gt.2014.13
This article is cited by
-
GLI3 regulates muscle stem cell entry into GAlert and self-renewal
Nature Communications (2022)
-
Development Aspects of Zebrafish Myotendinous Junction: a Model System for Understanding Muscle Basement Membrane Formation and Failure
Current Pathobiology Reports (2017)