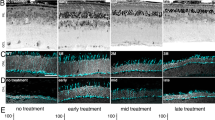
Abstract
Recent clinical trials of retinal pigment epithelium gene (RPE65) supplementation therapy in Leber congenital amaurosis type 2 patients have demonstrated improvements in rod and cone function, but it may be some years before the effects of therapy on photoreceptor survival become apparent. The Rpe65-deficient dog is a very useful pre-clinical model in which to test efficacy of therapies, because the dog has a retina with a high degree of similarity to that of humans. In this study, we evaluated the effect of RPE65 gene therapy on photoreceptor survival in order to predict the potential benefit and limitations of therapy in patients. We examined the retinas of Rpe65-deficient dogs after RPE65 gene therapy to evaluate the preservation of rods and cone photoreceptor subtypes. We found that gene therapy preserves both rods and cones. While the moderate loss of rods in the Rpe65-deficient dog retina is slowed by gene therapy, S-cones are lost extensively and gene therapy can prevent that loss, although only within the treated area. Although LM-cones are not lost extensively, cone opsin mislocalization indicates that they are stressed, and this can be partially reversed by gene therapy. Our results suggest that gene therapy may be able to slow cone degeneration in patients if intervention is sufficiently early and also that it is probably important to treat the macula in order to preserve central function.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Redmond TM, Yu S, Lee E, Bok D, Hamasaki D, Chen N et al. Rpe65 is necessary for production of 11-cis-vitamin A in the retinal visual cycle. Nat Genet 1998; 20: 344–351.
Redmond TM, Poliakov E, Yu S, Tsai JY, Lu Z . Gentleman S. Mutation of key residues of RPE65 abolishes its enzymatic role as isomerohydrolase in the visual cycle. Proc Natl Acad Sci USA 2005; 102: 13658–13663.
Redmond TM, Poliakov E, Kuo S, Chander P . Gentleman S. RPE65, visual cycle retinol isomerase, is not inherently 11-cis-specific: support for a carbocation mechanism of retinol isomerization. J Biol Chem 2010; 285: 1919–1927.
Moiseyev G, Chen Y, Takahashi Y, Wu BX, Ma JX . RPE65 is the isomerohydrolase in the retinoid visual cycle. Proc Natl Acad Sci USA 2005; 102: 12413–12418.
Jin M, Li S, Moghrabi WN, Sun H, Travis GH . Rpe65 is the retinoid isomerase in bovine retinal pigment epithelium. Cell 2005; 122: 449–459.
Mata NL, Radu RA, Clemmons RC, Travis GH . Isomerization and oxidation of vitamin a in cone-dominant retinas: a novel pathway for visual-pigment regeneration in daylight. Neuron 2002; 36: 69–80.
Wang JS, Estevez ME, Cornwall MC, Kefalov VJ . Intra-retinal visual cycle required for rapid and complete cone dark adaptation. Nat Neurosci 2009; 12: 295–302.
Wang JS, Kefalov VJ . An alternative pathway mediates the mouse and human cone visual cycle. Curr Biol 2009; 19: 1665–1669.
Wang JS, Kefalov VJ . The cone-specific visual cycle. Prog Retin Eye Res 2011; 30: 115–128.
Seeliger MW, Grimm C, Stahlberg F, Friedburg C, Jaissle G, Zrenner E et al. New views on RPE65 deficiency: the rod system is the source of vision in a mouse model of Leber congenital amaurosis. Nat Genet 2001; 29: 70–74.
Znoiko SL, Crouch RK, Moiseyev G, Ma JX . Identification of the RPE65 protein in mammalian cone photoreceptors. Invest Ophthalmol Vis Sci 2002; 43: 1604–1609.
Rohrer B, Lohr HR, Humphries P, Redmond TM, Seeliger MW, Crouch RK . Cone opsin mislocalization in Rpe65−/− mice: a defect that can be corrected by 11-cis retinal. Invest Ophthalmol Vis Sci 2005; 46: 3876–3882.
Feathers KL, Lyubarsky AL, Khan NW, Teofilo K, Swaroop A, Williams DS et al. Nrl-knockout mice deficient in Rpe65 fail to synthesize 11-cis retinal and cone outer segments. Invest Ophthalmol Vis Sci 2008; 49: 1126–1135.
Tang PH, Wheless L, Crouch RK . Regeneration of Photopigment Is Enhanced in Mouse Cone Photoreceptors Expressing RPE65 Protein. J Neurosci 2011; 31: 10403–10411.
Lorenz B, Gyurus P, Preising M, Bremser D, Gu S, Andrassi M et al. Early-onset severe rod-cone dystrophy in young children with RPE65 mutations. Invest Ophthalmol Vis Sci 2000; 41: 2735–2742.
Paunescu K, Wabbels B, Preising MN, Lorenz B . Longitudinal and cross-sectional study of patients with early-onset severe retinal dystrophy associated with RPE65 mutations. Graefes Arch Clin Exp Ophthalmol 2005; 243: 417–426.
Thompson DA, Gyurus P, Fleischer LL, Bingham EL, McHenry CL, Apfelstedt-Sylla E et al. Genetics and phenotypes of RPE65 mutations in inherited retinal degeneration. Invest Ophthalmol Vis Sci 2000; 41: 4293–4299.
Aguirre GK, Komaromy AM, Cideciyan AV, Brainard DH, Aleman TS, Roman AJ et al. Canine and human visual cortex intact and responsive despite early retinal blindness from RPE65 mutation. PLoS Med 2007; 4: e230.
Jacobson SG, Aleman TS, Cideciyan AV, Heon E, Golczak M, Beltran WA et al. Human cone photoreceptor dependence on RPE65 isomerase. Proc Natl Acad Sci USA 2007; 104: 15123–15128.
Jacobson SG, Aleman TS, Cideciyan AV, Roman AJ, Sumaroka A, Windsor EA et al. Defining the residual vision in leber congenital amaurosis caused by RPE65 mutations. Invest Ophthalmol Vis Sci 2009; 50: 2368–2375.
Jacobson SG, Cideciyan AV, Aleman TS, Sumaroka A, Windsor EA, Schwartz SB et al. Photoreceptor layer topography in children with leber congenital amaurosis caused by RPE65 mutations. Invest Ophthalmol Vis Sci 2008; 49: 4573–4577.
Caruso RC, Aleman TS, Cideciyan AV, Roman AJ, Sumaroka A, Mullins CL et al. Retinal disease in Rpe65-deficient mice: comparison to human leber congenital amaurosis due to RPE65 mutations. Invest Ophthalmol Vis Sci 2010; 51: 5304–5313.
Van Hooser JP, Aleman TS, He YG, Cideciyan AV, Kuksa V, Pittler SJ et al. Rapid restoration of visual pigment and function with oral retinoid in a mouse model of childhood blindness. Proc Natl Acad Sci USA 2000; 97: 8623–8628.
Samardzija M, Tanimoto N, Kostic C, Beck S, Oberhauser V, Joly S et al. In conditions of limited chromophore supply rods entrap 11-cis-retinal leading to loss of cone function and cell death. Hum Mol Genet 2009; 18: 1266–1275.
Gu SM, Thompson DA, Srikumari CR, Lorenz B, Finckh U, Nicoletti A et al. Mutations in RPE65 cause autosomal recessive childhood-onset severe retinal dystrophy. Nat Genet 1997; 17: 194–197.
Jacobson SG, Aleman TS, Cideciyan AV, Sumaroka A, Schwartz SB, Windsor EA et al. Identifying photoreceptors in blind eyes caused by RPE65 mutations: Prerequisite for human gene therapy success. Proc Natl Acad Sci USA 2005; 102: 6177–6182.
Simonelli F, Ziviello C, Testa F, Rossi S, Fazzi E, Bianchi PE et al. Clinical and molecular genetics of Leber’s congenital amaurosis: a multicenter study of Italian patients. Invest Ophthalmol Vis Sci 2007; 48: 4284–4290.
Lorenz B, Poliakov E, Schambeck M, Friedburg C, Preising MN, Redmond TM . A comprehensive clinical and biochemical functional study of a novel RPE65 hypomorphic mutation. Invest Ophthalmol Vis Sci 2008; 49: 5235–5242.
Maeda T, Cideciyan AV, Maeda A, Golczak M, Aleman TS, Jacobson SG et al. Loss of cone photoreceptors caused by chromophore depletion is partially prevented by the artificial chromophore pro-drug, 9-cis-retinyl acetate. Hum Mol Genet 2009; 18: 2277–2287.
Pasadhika S, Fishman GA, Stone EM, Lindeman M, Zelkha R, Lopez I et al. Differential macular morphology in patients with RPE65-, CEP290-, GUCY2D-, and AIPL1-related Leber congenital amaurosis. Invest Ophthalmol Vis Sci 2010; 51: 2608–2614.
Cideciyan AV, Aleman TS, Boye SL, Schwartz SB, Kaushal S, Roman AJ et al. Human gene therapy for RPE65 isomerase deficiency activates the retinoid cycle of vision but with slow rod kinetics. Proc Natl Acad Sci USA 2008; 105: 15112–15117.
Bainbridge JW, Smith AJ, Barker SS, Robbie S, Henderson R, Balaggan K et al. Effect of gene therapy on visual function in Leber’s congenital amaurosis. N Engl J Med 2008; 358: 2231–2239.
Cideciyan AV, Hauswirth WW, Aleman TS, Kaushal S, Schwartz SB, Boye SL et al. Human RPE65 gene therapy for Leber congenital amaurosis: persistence of early visual improvements and safety at 1 year. Hum Gene Ther 2009; 20: 999–1004.
Cideciyan AV, Hauswirth WW, Aleman TS, Kaushal S, Schwartz SB, Boye SL et al. Vision 1 year after gene therapy for Leber’s congenital amaurosis. N Engl J Med 2009; 361: 725–727.
Hauswirth WW, Aleman TS, Kaushal S, Cideciyan AV, Schwartz SB, Wang L et al. Treatment of leber congenital amaurosis due to RPE65 mutations by ocular subretinal injection of adeno-associated virus gene vector: short-term results of a phase I trial. Hum Gene Ther 2008; 19: 979–990.
Maguire AM, High KA, Auricchio A, Wright JF, Pierce EA, Testa F et al. Age-dependent effects of RPE65 gene therapy for Leber’s congenital amaurosis: a phase 1 dose-escalation trial. Lancet 2009; 374: 1597–1605.
Maguire AM, Simonelli F, Pierce EA, Pugh EN, Mingozzi F, Bennicelli J et al. Safety and efficacy of gene transfer for Leber’s congenital amaurosis. N Engl J Med 2008; 358: 2240–2248.
Simonelli F, Maguire AM, Testa F, Pierce EA, Mingozzi F, Bennicelli JL et al. Gene therapy for Leber’s congenital amaurosis is safe and effective through 1.5 years after vector administration. Mol Ther 2010; 18: 643–650.
Bemelmans AP, Kostic C, Crippa SV, Hauswirth WW, Lem J, Munier FL et al. Lentiviral gene transfer of RPE65 rescues survival and function of cones in a mouse model of Leber congenital amaurosis. PLoS Med 2006; 3: e347.
Pang J, Boye SE, Lei B, Boye SL, Everhart D, Ryals R et al. Self-complementary AAV-mediated gene therapy restores cone function and prevents cone degeneration in two models of Rpe65 deficiency. Gene Therapy 2010; 17: 815–826.
Kostic C, Crippa SV, Pignat V, Bemelmans AP, Samardzija M, Grimm C et al. Gene therapy regenerates protein expression in cone photoreceptors in Rpe65(R91W/R91W) mice. PLoS One 2011; 6: e16588.
Mowat FM, Petersen-Jones SM, Williamson H, Williams DL, Luthert PJ, Ali RR et al. Topographical characterization of cone photoreceptors and the area centralis of the canine retina. Mol Vis 2008; 14: 2518–2527.
Narfstrom K, Wrigstad A, Nilsson SE . The Briard dog: a new animal model of congenital stationary night blindness. Br J Ophthalmol 1989; 73: 750–756.
Narfstrom K, Wrigstad A, Ekesten B, Nilsson SE . Hereditary retinal dystrophy in the Briard dog: clinical and hereditary characteristics. Progr Vet Comp Ophthalmol 1994; 4: 85–92.
Narfstrom K . Retinal dystrophy or ‘congenital stationary night blindness’ in the Briard dog. Vet Ophthalmol 1999; 2: 75–76.
Veske A, Nilsson SE, Narfstrom K, Gal A . Retinal dystrophy of Swedish briard/briard-beagle dogs is due to a 4-bp deletion in RPE65. Genomics 1999; 57: 57–61.
Acland GM, Aguirre GD, Ray J, Zhang Q, Aleman TS, Cideciyan AV et al. Gene therapy restores vision in a canine model of childhood blindness. Nat Genet 2001; 28: 92–95.
Acland GM, Aguirre GD, Bennett J, Aleman TS, Cideciyan AV, Bennicelli J et al. Long-term restoration of rod and cone vision by single dose rAAV-mediated gene transfer to the retina in a canine model of childhood blindness. Mol Ther 2005; 12: 1072–1082.
Wrigstad A, Narfstrom K, Nilsson SE . Slowly progressive changes of the retina and retinal pigment epithelium in Briard dogs with hereditary retinal dystrophy. A morphological study. Doc Ophthalmol 1994; 87: 337–354.
Wrigstad A, Nilsson SE, Narfstrom K . Ultrastructural changes of the retina and the retinal pigment epithelium in Briard dogs with hereditary congenital night blindness and partial day blindness. Exp Eye Res 1992; 55: 805–818.
Aguirre GD, Baldwin V, Pearce-Kelling S, Narfstrom K, Ray K, Acland GM . Congenital stationary night blindness in the dog: common mutation in the RPE65 gene indicates founder effect. Mol Vis 1998; 4: 23.
Hernandez M, Pearce-Kelling SE, Rodriguez FD, Aguirre GD, Vecino E . Altered expression of retinal molecular markers in the canine RPE65 model of Leber congenital amaurosis. Invest Ophthalmol Vis Sci 2010; 51: 6793–6802.
Gearhart PM, Gearhart C, Thompson DA, Petersen-Jones SM . Improvement of visual performance with intravitreal administration of 9-cis-retinal in Rpe65-mutant dogs. Arch Ophthalmol 2010; 128: 1442–1448.
Annear MJ, Bartoe JT, Barker SE, Smith AJ, Curran PG, Bainbridge JW et al. Gene therapy in the second eye of RPE65-deficient dogs improves retinal function. Gene Therapy 2011; 18: 53–61.
Le Meur G, Stieger K, Smith AJ, Weber M, Deschamps JY, Nivard D et al. Restoration of vision in RPE65-deficient Briard dogs using an AAV serotype 4 vector that specifically targets the retinal pigmented epithelium. Gene Therapy 2007; 14: 292–303.
Zhang H, Fan J, Li S, Karan S, Rohrer B, Palczewski K et al. Trafficking of membrane-associated proteins to cone photoreceptor outer segments requires the chromophore 11-cis-retinal. J Neurosci 2008; 28: 4008–4014.
Fan J, Rohrer B, Frederick JM, Baehr W, Crouch RK . Rpe65−/− and Lrat−/− mice: comparable models of leber congenital amaurosis. Invest Ophthalmol Vis Sci 2008; 49: 2384–2389.
Erdman C, Emerson JW . bcp: An R package for performing a Bayesian analysis of change-point problems. J Stat Softw 2007; 23: 1–13.
Curcio CA, Allen KA, Sloan KR, Lerea CL, Hurley JB, Klock IB et al. Distribution and morphology of human cone photoreceptors stained with anti-blue opsin. J Comp Neurol 1991; 312: 610–624.
Jacobson SG, Cideciyan AV, Ratnakaram R, Heon E, Schwartz SB, Roman AJ et al. Gene Therapy for Leber Congenital Amaurosis Caused by RPE65 Mutations: Safety and Efficacy in 15 Children and Adults Followed Up to 3 Years. Arch Ophthalmol 2012; 130: 9–24.
Znoiko SL, Rohrer B, Lu K, Lohr HR, Crouch RK, Ma JX . Downregulation of cone-specific gene expression and degeneration of cone photoreceptors in the Rpe65−/− mouse at early ages. Invest Ophthalmol Vis Sci 2005; 46: 1473–1479.
Szel A, Rohlich P, Mieziewska K, Aguirre G, van Veen T . Spatial and temporal differences between the expression of short- and middle-wave sensitive cone pigments in the mouse retina: a developmental study. J Comp Neurol 1993; 331: 564–577.
Cepko CL . The patterning and onset of opsin expression in vertebrate retinae. Curr Opin Neurobiol 1996; 6: 542–546.
Pang JJ, Chang B, Hawes NL, Hurd RE, Davisson MT, Li J et al. Retinal degeneration 12 (rd12): a new, spontaneously arising mouse model for human Leber congenital amaurosis (LCA). Mol Vis 2005; 11: 152–162.
Bumsted K, Hendrickson A . Distribution and development of short-wavelength cones differ between Macaca monkey and human fovea. J Comp Neurol 1999; 403: 502–516.
Kunchithapautham K, Coughlin B, Crouch RK, Rohrer B . Cone outer segment morphology and cone function in the Rpe65−/− Nrl−/− mouse retina are amenable to retinoid replacement. Invest Ophthalmol Vis Sci 2009; 50: 4858–4864.
Zhang T, Zhang N, Baehr W, Fu Y . Cone opsin determines the time course of cone photoreceptor degeneration in Leber congenital amaurosis. Proc Natl Acad Sci USA 2011; 108: 8879–8884.
Kolesnikov AV, Tang PH, Parker RO, Crouch RK, Kefalov VJ . The mammalian cone visual cycle promotes rapid M/L-cone pigment regeneration independently of the interphotoreceptor retinoid-binding protein. J Neurosci 2011; 31: 7900–7909.
Samardzija M, von Lintig J, Tanimoto N, Oberhauser V, Thiersch M, Reme CE et al. R91W mutation in Rpe65 leads to milder early-onset retinal dystrophy due to the generation of low levels of 11-cis-retinal. Hum Mol Genet 2008; 17: 281–292.
Leveillard T, Mohand-Said S, Lorentz O, Hicks D, Fintz AC, Clerin E et al. Identification and characterization of rod-derived cone viability factor. Nat Genet 2004; 36: 755–759.
Gao GP, Qu G, Faust LZ, Engdahl RK, Xiao W, Hughes JV et al. High-titer adeno-associated viral vectors from a Rep/Cap cell line and hybrid shuttle virus. Hum Gene Ther 1998; 9: 2353–2362.
Petersen-Jones SM, Bartoe JT, Fischer AJ, Scott M, Boye SL, Chiodo V et al. AAV retinal transduction in a large animal model species: comparison of a self-complementary AAV2/5 with a single-stranded AAV2/5 vector. Mol Vis 2009; 15: 1835–1842.
Li A, Zhu X, Brown B, Craft CM . Gene expression networks underlying retinoic acid-induced differentiation of human retinoblastoma cells. Invest Ophthalmol Vis Sci 2003; 44: 996–1007.
Pinheiro J, Bates D, DebRoy S, Sarkar D . and the R Development Core Team. nlme: linear and nonlinear mixed effects models. 2012, R package version 3.1-104 http://cran.r-project.org/web/packages/nlme/citation.html.
Team RDC . R: a language and environment for statistical computing,. 2012, http://www.r-project.org/.
Barry D, Hartigan JA . A Bayesian-analysis for change point problems. J Am Stat Assoc 1993; 88: 309–319.
Acknowledgements
This work was funded by the British Retinitis Pigmentosa Society, Midwest Eye Banks and Transplantation Center Research Program, The Hal and Jean Glassen Memorial Foundation, Myers-Dunlap Endowment for Canine Health and MSU College of Veterinary Medicine Purebred Dog Endowment Fund. RRA and JWB are investigators at The NIHR Center for Ophthalmology at UCL and Moorfields Eye Hospital. We acknowledge Janice Querubin for providing veterinary technician expertise and Cheri Johnson for assistance with canine reproduction. We thank Cheryl Sisk and Ray Figuera for assisting with cryostat use.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on Gene Therapy website
Rights and permissions
About this article
Cite this article
Mowat, F., Breuwer, A., Bartoe, J. et al. RPE65 gene therapy slows cone loss in Rpe65-deficient dogs. Gene Ther 20, 545–555 (2013). https://doi.org/10.1038/gt.2012.63
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/gt.2012.63
Keywords
This article is cited by
-
Dual-AAV split prime editor corrects the mutation and phenotype in mice with inherited retinal degeneration
Signal Transduction and Targeted Therapy (2023)
-
Serotype-specific transduction of canine joint tissue explants and cultured monolayers by self-complementary adeno-associated viral vectors
Gene Therapy (2023)
-
Lentiviral mediated RPE65 gene transfer in healthy hiPSCs-derived retinal pigment epithelial cells markedly increased RPE65 mRNA, but modestly protein level
Scientific Reports (2020)
-
Adaptations for amphibious vision in sea otters (Enhydra lutris): structural and functional observations
Journal of Comparative Physiology A (2020)
-
Investigation of SLA4A3 as a candidate gene for human retinal disease
Journal of Negative Results in BioMedicine (2016)