Abstract
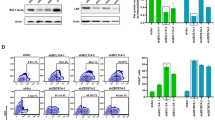
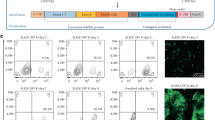
Regulated transgene expression may reduce transgene-specific and genotoxic risks associated with gene therapy. To prove this concept, we have investigated the suitability of doxycycline (Dox)-inducible human cytidine deaminase (hCDD) overexpression from lentiviral vectors to mediate effective myeloprotection while circumventing the lymphotoxicity observed with constitutive CDD activity. Rapid Dox-mediated transgene induction associated with a 6–17-fold increase in drug resistance was observed in 32D and primary murine bone marrow (BM) cells. Moreover, robust Dox-regulated transgene expression in the entire haematopoietic system was demonstrated for primary and secondary recipients of hCDD-transduced R26-M2rtTA transgenic BM cells. Furthermore, mice were significantly protected from myelosuppressive chemotherapy as evidenced by accelerated recovery of granulocytes (1.9±0.6 vs 1.3±0.3, P=0.034) and platelets (883±194 vs 584±160 103 per μl, P=0.011). Minimal transgene expression in the non-induced state and no overt cellular toxicities including lymphotoxicity were detected. Thus, using a relevant murine transplant model our data provide conclusive evidence that drug-resistance transgenes can be expressed in a regulated fashion in the lymphohaematopoietic system, and that Dox-inducible systems may be used to reduce myelotoxic side effect of anticancer chemotherapy or to avoid side effects of high constitutive transgene expression.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Aiuti A, Slavin S, Aker M, Ficara F, Deola S, Mortellaro A et alCorrection of ADA-SCID by stem cell gene therapy combined with nonmyeloablative conditioning. Science 2002; 296: 2410–2413.
Boztug K, Schmidt M, Schwarzer A, Banerjee PP, Diez IA, Dewey RA et al. Stem-cell gene therapy for the Wiskott-Aldrich syndrome. N Engl J Med 363: 1918–1927.
Cavazzana-Calvo M, Hacein-Bey S, de Saint Basile G, Gross F, Yvon E, Nusbaum P et al. Gene therapy of human severe combined immunodeficiency (SCID)-X1 disease. Science 2000; 288: 669–672.
Ott MG, Schmidt M, Schwarzwaelder K, Stein S, Siler U, Koehl U et al. Correction of X-linked chronic granulomatous disease by gene therapy, augmented by insertional activation of MDS1-EVI1, PRDM16 or SETBP1. Nat Med 2006; 12: 401–409.
Hacein-Bey-Abina S, Garrigue A, Wang GP, Soulier J, Lim A, Morillon E et al. Insertional oncogenesis in 4 patients after retrovirus-mediated gene therapy of SCID-X1. J Clin Invest 2008; 118: 3132–3142.
Woods NB, Bottero V, Schmidt M, von Kalle C, Verma IM . Gene therapy: therapeutic gene causing lymphoma. Nature 2006; 440: 1123.
Wicke DC, Meyer J, Buesche G, Heckl D, Kreipe H, Li Z et al. Gene therapy of MPL deficiency: challenging balance between leukemia and pancytopenia. Mol Ther 2010; 18: 343–352.
Gentner B, Visigalli I, Hiramatsu H, Lechman E, Ungari S, Giustacchini A et al. Identification of hematopoietic stem cell-specific miRNAs enables gene therapy of globoid cell leukodystrophy. Sci Transl Med 2010; 2: 58ra84.
Mayo KE, Warren R, Palmiter RD . The mouse metallothionein-I gene is transcriptionally regulated by cadmium following transfection into human or mouse cells. Cell 1982; 29: 99–108.
Wurm FM, Gwinn KA, Kingston RE . Inducible overproduction of the mouse c-myc protein in mammalian cells. Proc Natl Acad Sci USA 1986; 83: 5414–5418.
Kenneth NS, Rocha S . Regulation of gene expression by hypoxia. Biochem J 2008; 414: 19–29.
Rivera VM, Clackson T, Natesan S, Pollock R, Amara JF, Keenan T et al. A humanized system for pharmacologic control of gene expression. Nat Med 1996; 2: 1028–1032.
Roscilli G, Rinaudo CD, Cimino M, Sporeno E, Lamartina S, Ciliberto G et al. Long-term and tight control of gene expression in mouse skeletal muscle by a new hybrid human transcription factor. Mol Ther 2002; 6: 653–663.
Vegeto E, Allan GF, Schrader WT, Tsai MJ, McDonnell DP, O'Malley BW . The mechanism of RU486 antagonism is dependent on the conformation of the carboxy-terminal tail of the human progesterone receptor. Cell 1992; 69: 703–713.
Gossen M, Bujard H . Tight control of gene expression in mammalian cells by tetracycline-responsive promoters. Proc Natl Acad Sci USA 1992; 89: 5547–5551.
Gossen M, Freundlieb S, Bender G, Muller G, Hillen W, Bujard H . Transcriptional activation by tetracyclines in mammalian cells. Science 1995; 268: 1766–1769.
Das AT, Zhou X, Vink M, Klaver B, Verhoef K, Marzio G et al. Viral evolution as a tool to improve the tetracycline-regulated gene expression system. J Biol Chem 2004; 279: 18776–18782.
Heinz N, Schambach A, Galla M, Maetzig T, Baum C, Loew R et al. Retroviral and transposon-based tet-regulated all-in-one vectors with reduced background expression and improved dynamic range. Hum Gene Ther 22: 166–176.
Urlinger S, Baron U, Thellmann M, Hasan MT, Bujard H, Hillen W . Exploring the sequence space for tetracycline-dependent transcriptional activators: novel mutations yield expanded range and sensitivity. Proc Natl Acad Sci USA 2000; 97: 7963–7968.
Trobridge GD, Kiem HP . Large animal models of hematopoietic stem cell gene therapy. Gene Therapy 2010; 17: 939–948.
Moritz T, Williams DA . Marrow protection—transduction of hematopoietic cells with drug resistance genes. Cytotherapy 2001; 3: 67–84.
Allay JA, Persons DA, Galipeau J, Riberdy JM, Ashmun RA, Blakley RL et al. In vivo selection of retrovirally transduced hematopoietic stem cells. Nat Med 1998; 4: 1136–1143.
Capiaux GM, Budak-Alpdogan T, Alpdogan O, Bornmann W, Takebe N, Banerjee D et al. Protection of hematopoietic stem cells from pemetrexed toxicity by retroviral gene transfer with a mutant dihydrofolate reductase-mutant thymidylate synthase fusion gene. Cancer Gene Ther 2004; 11: 767–773.
Meisel R, Bardenheuer W, Strehblow C, Sorg UR, Elmaagacli A, Seeber S et al. Efficient protection from methotrexate toxicity and selection of transduced human hematopoietic cells following gene transfer of dihydrofolate reductase mutants. Exp Hematol 2003; 31: 1215–1222.
Sorrentino BP, Brandt SJ, Bodine D, Gottesman M, Pastan I, Cline A et al. Selection of drug-resistant bone marrow cells in vivo after retroviral transfer of human MDR1. Science 1992; 257: 99–103.
Hildinger M, Schilz A, Eckert HG, Bohn W, Fehse B, Zander A et al. Bicistronic retroviral vectors for combining myeloprotection with cell-surface marking. Gene Therapy 1999; 6: 1222–1230.
Schiedlmeier B, Schilz AJ, Kuhlcke K, Laufs S, Baum C, Zeller WJ et al. Multidrug resistance 1 gene transfer can confer chemoprotection to human peripheral blood progenitor cells engrafted in immunodeficient mice. Hum Gene Ther 2002; 13: 233–242.
Jansen M, Sorg UR, Ragg S, Flasshove M, Seeber S, Williams DA et al. Hematoprotection and enrichment of transduced cells in vivo after gene transfer of MGMT(P140K) into hematopoietic stem cells. Cancer Gene Ther 2002; 9: 737–746.
Milsom MD, Jerabek-Willemsen M, Harris CE, Schambach A, Broun E, Bailey J et al. Reciprocal relationship between O6-methylguanine-DNA methyltransferase P140K expression level and chemoprotection of hematopoietic stem cells. Cancer Res 2008; 68: 6171–6180.
Neff T, Beard BC, Peterson LJ, Anandakumar P, Thompson J, Kiem HP . Polyclonal chemoprotection against temozolomide in a large-animal model of drug resistance gene therapy. Blood 2005; 105: 997–1002.
Zielske SP, Reese JS, Lingas KT, Donze JR, Gerson SL . In vivo selection of MGMT(P140K) lentivirus-transduced human NOD/SCID repopulating cells without pretransplant irradiation conditioning. J Clin Invest 2003; 112: 1561–1570.
Bardenheuer W, Lehmberg K, Rattmann I, Brueckner A, Schneider A, Sorg UR et al. Resistance to cytarabine and gemcitabine and in vitro selection of transduced cells after retroviral expression of cytidine deaminase in human hematopoietic progenitor cells. Leukemia 2005; 19: 2281–2288.
Momparler RL, Eliopoulos N, Bovenzi V, Letourneau S, Greenbaum M, Cournoyer D . Resistance to cytosine arabinoside by retrovirally mediated gene transfer of human cytidine deaminase into murine fibroblast and hematopoietic cells. Cancer Gene Ther 1996; 3: 331–338.
Neff T, Blau CA . Forced expression of cytidine deaminase confers resistance to cytosine arabinoside and gemcitabine. Exp Hematol 1996; 24: 1340–1346.
Rattmann I, Kleff V, Sorg UR, Bardenheuer W, Brueckner A, Hilger RA et al. Gene transfer of cytidine deaminase protects myelopoiesis from cytidine analogs in an in vivo murine transplant model. Blood 2006; 108: 2965–2971.
Zufferey R, Dull T, Mandel RJ, Bukovsky A, Quiroz D, Naldini L et al. Self-inactivating lentivirus vector for safe and efficient in vivo gene delivery. J Virol 1998; 72: 9873–9880.
Toy G, Austin WR, Liao HI, Cheng D, Singh A, Campbell DO et al. Requirement for deoxycytidine kinase in T and B lymphocyte development. Proc Natl Acad Sci USA 107: 5551–5556.
Stentoft J . The toxicity of cytarabine. Drug Saf 1990; 5: 7–27.
van Pelt K, de Haan G, Vellenga E, Daenen SM . Administration of low-dose cytarabine results in immediate S-phase arrest and subsequent activation of cell cycling in murine stem cells. Exp Hematol 2005; 33: 226–231.
Brennig S, Rattmann I, Lachmann N, Schambach A, Williams DA, Moritz T . In vivo enrichment of cytidine deaminase gene-modified hematopoietic cells by prolonged cytosine-arabinoside application. Cytotherapy 2012; 14: 451–460.
Modlich U, Navarro S, Zychlinski D, Maetzig T, Knoess S, Brugman MH et al. Insertional transformation of hematopoietic cells by self-inactivating lentiviral and gammaretroviral vectors. Mol Ther 2009; 17: 1919–1928.
Cattoglio C, Facchini G, Sartori D, Antonelli A, Miccio A, Cassani B et al. Hot spots of retroviral integration in human CD34+ hematopoietic cells. Blood 2007; 110: 1770–1778.
Schroder AR, Shinn P, Chen H, Berry C, Ecker JR, Bushman F . HIV-1 integration in the human genome favors active genes and local hotspots. Cell 2002; 110: 521–529.
Montini E, Cesana D, Schmidt M, Sanvito F, Ponzoni M, Bartholomae C et al. Hematopoietic stem cell gene transfer in a tumor-prone mouse model uncovers low genotoxicity of lentiviral vector integration. Nat Biotechnol 2006; 24: 687–696.
Lachmann N, Jagielska J, Heckl D, Brennig S, Pfaff N, Maetzig T et al. MicroRNA-150-regulated vectors allow lymphocyte-sparing transgene expression in hematopoietic gene therapy. Gene Therapy 2011, e-pub ahead of print 6 October 2011; doi: 10.1038/gt.2011.148.
Favre D, Blouin V, Provost N, Spisek R, Porrot F, Bohl D et al. Lack of an immune response against the tetracycline-dependent transactivator correlates with long-term doxycycline-regulated transgene expression in nonhuman primates after intramuscular injection of recombinant adeno-associated virus. J Virol 2002; 76: 11605–11611.
Latta-Mahieu M, Rolland M, Caillet C, Wang M, Kennel P, Mahfouz I et al. Gene transfer of a chimeric trans-activator is immunogenic and results in short-lived transgene expression. Hum Gene Ther 2002; 13: 1611–1620.
Mackall CL, Fleisher TA, Brown MR, Andrich MP, Chen CC, Feuerstein IM et al. Age, thymopoiesis, and CD4+ T-lymphocyte regeneration after intensive chemotherapy. N Engl J Med 1995; 332: 143–149.
Gregori S, Tomasoni D, Pacciani V, Scirpoli M, Battaglia M, Magnani CF et al. Differentiation of type 1 T regulatory cells (Tr1) by tolerogenic DC-10 requires the IL-10-dependent ILT4/HLA-G pathway. Blood 116: 935–944.
Benabdellah K, Cobo M, Munoz P, Toscano MG, Martin F . Development of an all-in-one lentiviral vector system based on the original TetR for the easy generation of Tet-ON cell lines. PLoS One 6: e23734.
Szulc J, Wiznerowicz M, Sauvain MO, Trono D, Aebischer P . A versatile tool for conditional gene expression and knockdown. Nat Methods 2006; 3: 109–116.
Dull T, Zufferey R, Kelly M, Mandel RJ, Nguyen M, Trono D et al. A third-generation lentivirus vector with a conditional packaging system. J Virol 1998; 72: 8463–8471.
Maetzig T, Brugman MH, Bartels S, Heinz N, Kustikova OS, Modlich U et al. Polyclonal fluctuation of lentiviral vector-transduced and expanded murine hematopoietic stem cells. Blood 117: 3053–3064.
Heckl D, Wicke DC, Brugman MH, Meyer J, Schambach A, Busche G et al. Lentiviral gene transfer regenerates hematopoietic stem cells in a mouse model for Mpl-deficient aplastic anemia. Blood 117: 3737–3747.
Zhang CC, Lodish HF . Murine hematopoietic stem cells change their surface phenotype during ex vivo expansion. Blood 2005; 105: 4314–4320.
Acknowledgements
We thank Matthias Ballmaier (PhD) and his team from the Core-Facility Cell-Sorting of Hannover Medical School for cell sorting, and Doreen Lüttge (Hannover Medical School) for excellent technical assistance. Furthermore, we thank Georg Kensah (Hannover Medical School) for the help in performing the timelapse video studies. This work was supported by grants from the Deutsche Forschungsgemeinschaft: Cluster of Excellence REBIRTH (Exc 62/1), SPP1230 Grant MO 886/3-1 (UM and TM) and by the EU framework programme grant PERSIST (CHB).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on Gene Therapy website
Rights and permissions
About this article
Cite this article
Lachmann, N., Brennig, S., Pfaff, N. et al. Efficient in vivo regulation of cytidine deaminase expression in the haematopoietic system using a doxycycline-inducible lentiviral vector system. Gene Ther 20, 298–307 (2013). https://doi.org/10.1038/gt.2012.40
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/gt.2012.40
Keywords
This article is cited by
-
The CpG-sites of the CBX3 ubiquitous chromatin opening element are critical structural determinants for the anti-silencing function
Scientific Reports (2017)
-
Chemoprotection of murine hematopoietic cells by combined gene transfer of cytidine deaminase (CDD) and multidrug resistance 1 gene (MDR1)
Journal of Experimental & Clinical Cancer Research (2015)
-
Deoxycytidine-kinase knockdown as a novel myeloprotective strategy in the context of fludarabine, cytarabine or cladribine therapy
Leukemia (2015)
-
Induced neural stem/precursor cells for fundamental studies and potential application in neurodegenerative diseases
Neuroscience Bulletin (2015)
-
R88-APOBEC3Gm Inhibits the Replication of Both Drug-resistant Strains of HIV-1 and Viruses Produced From Latently Infected Cells
Molecular Therapy - Nucleic Acids (2014)