Abstract
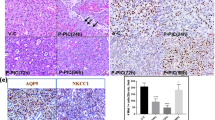
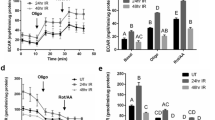
Previously (Shan et al, 2005), we reported that adenoviral vector-mediated transfer of the human aquaporin-1 (hAQP1) cDNA to minipig parotid glands following irradiation (IR) transiently restored salivary flow to near normal levels. This study evaluated a serotype 2, adeno-associated viral (AAV2) vector for extended correction of IR (single dose; 20 Gy)-induced, parotid salivary hypofunction in minipigs. At 16 weeks following the IR parotid salivary flow decreased by 85–90%. AAV2hAQP1 administration at week 17 transduced only duct cells and resulted in a dose-dependent increase in salivary flow to ∼35% of pre-IR levels (to ∼1 ml per 10 min) after 8 weeks (peak response). Administration of a control AAV2 vector or saline was without effect. Little change was observed in clinical chemistry and hematology values after AAV2hAQP1 delivery. Vector-treated animals generated high anti-AAV2 neutralizing antibody titers by week 4 (∼1:1600) and significant elevations in salivary (∼15%), but not serum, granulocyte macrophage colony-stimulating factor levels. Following vector administration, salivary [Na+] was dramatically increased, from ∼10 to ∼55 mM (at 4 weeks) and finally to 39 mM (8 weeks). The findings demonstrate that localized delivery of AAV2hAQP1 to IR-damaged parotid glands leads to increased fluid secretion from surviving duct cells, and may be useful in providing extended relief of salivary hypofunction in previously irradiated patients.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ . Cancer statistics 2009. CA Cancer J Clin 2009; 59: 225–249.
Vissink A, Jansma J, Spijkervet FK, Burlage FR, Coppes RP . Oral sequelae of head and neck radiotherapy. Crit Rev Oral Biol Med 2003; 14: 199–212.
Vissink A, Burlage FR, Spijkervet FK, Jansma J, Coppes RP . Prevention and treatment of the consequences of head and neck radiotherapy. Crit Rev Oral Biol Med 2003; 14: 213–225.
Langendijk JA, Doornaert P, Verdonck-de Leeuw IM, Leemans CR, Aaronson NK, Slotman BJ . Impact of late treatment-related toxicity on quality of life among patients with head and neck cancer treated with radiotherapy. J Clin Oncol 2008; 26: 3770–3776.
Ho KF, Farnell DJ, Routledge JA, Burns MP, Sykes AJ, Slevin NJ et al. Developing a CTCAEs patient questionnaire for late toxicity after head and neck radiotherapy. Eur J Cancer 2009; 45: 1992–1998.
Brizel DM, Overgaard J . Does amifostine have a role in chemoradiation treatment? Lancet Oncol 2003; 4: 378–381.
Vergeer MR, Doornaert P, Reitveld DH, Leemans CR, Slotman BJ, Langendijk JA . Intensity-modulated radiotherapy reduces radiation-induced morbidity and improves health-related quality of life: results of a non-randomized prospective study using a standardized follow-up program. Int J Radiat Oncol Biol Phys 2009; 74: 1–8.
Cotrim AP, Sowers A, Mitchell JB, Baum BJ . Prevention of irradiation-induced salivary hypofunction by microvessel protection in mouse salivary glands. Mol Ther 2007; 15: 2101–2106.
Epperly MW, Melendez JA, Zhang X, Nie S, Pearce L, Peterson J et al. Mitochondrial targeting of a catalase transgene product by plasmid liposomes increases radioresistance in vitro and in vivo. Radiat Res 2009; 171: 588–595.
Cox JD, Steitz J, Pajak TF . Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European organization for research and treatment of cancer. Int J Radiat Oncol Biol Phys 1995; 31: 1341–1346.
Baum BJ, Zheng C, Cotrim AP, McCullagh L, Goldsmith CM, Brahim JS et al. Aquaporin-1 gene transfer to correct radiation-induced salivary hypofunction. In: Beitz E (eds). Aquaporins Handbook of Experimental Pharmacology. Springer-Verlag: Berlin Heidelberg, 2009; 190: 403–418.
Preston GM, Agre P . Isolation of the cDNA for erythrocyte integral membrane protein of 28 kilodaltons: member of an ancient channel family. Proc Natl Acad Sci USA 1991; 88: 11110–11114.
Delporte C, O’Connell BC, He X, Lancaster HE, O’Connell AC, Agre P et al. Increased fluid secretion after adenoviral-mediated transfer of the aquaporin-1 cDNA to irradiated rat salivary glands. Proc Natl Acad Sci USA 1997; 94: 3268–3273.
Shan Z, Li J, Zheng C, Liu X, Fan Z, Zhang C et al. Increased fluid secretion after adenoviral-mediated transfer of the human aquaporin-1 cDNA to irradiated miniature pig parotid glands. Mol Ther 2005; 11: 444–451.
Zheng C, Goldsmith CM, Mineshiba F, Chiorini JA, Kerr A, Wenk ML et al. Toxicity and biodistribution of a first-generation recombinant adenoviral vector, encoding aquaporin-1, after retroductal delivery to a single rat submandibular gland. Hum Gene Ther 2006; 17: 1122–1133.
Kagami H, Atkinson JC, Michalek SM, Handelman B, Yu S, Baum BJ et al. Repetitive adenovirus administration to the parotid gland: role of immunological barriers and induction of oral tolerance. Hum Gene Ther 1998; 9: 305–313.
Braddon VR, Chiorini JA, Wang S, Kotin RM, Baum BJ . Adenoassociated virus-mediated transfer of a functional water channel into salivary epithelial cells in vitro and in vivo. Hum Gene Ther 1998; 9: 2777–2785.
Voutetakis A, Kok MR, Zheng C, Bossis I, Wang J, Cotrim AP et al. Reengineered salivary glands are stable endogenous bioreactors for systemic gene therapeutics. Proc Natl Acad Sci USA 2004; 101: 3053–3058.
Voutetakis A, Zheng C, Mineshiba F, Cotrim AP, Goldsmith CM, Schmidt M et al. Adeno-associated virus serotype 2-mediated gene transfer to the parotid glands of nonhuman primates. Hum Gene Ther 2007; 18: 142–150.
Cotrim AP, Hyodo F, Matsumoto K-I, Sowers AL, Cook JA, Baum BJ et al. Differential radiation protection of salivary glands versus tumor by Tempol with accompanying tissue assessment of tempol by magnetic resonance imaging. Clin Cancer Res 2007; 13: 4928–4933.
Li J, Shan Z, Ou G, Liu X, Zhang C, Baum BJ et al. Structural and functional characteristics of irradiation damage to parotid glands in the miniature pig. Int J Radiat Oncol Biol Phys 2005; 62: 1510–1516.
Hai B, Yan X, Voutetakis A, Zheng C, Cotrim AP, Shan Z et al. Long-term transduction of miniature pig parotid glands using serotype 2 adeno-associated viral vectors. J Gene Med 2009; 11: 506–514.
Li J, Nielsen S, Dai Y, Lazowski KW, Christensen EI, Tabak LA et al. Examination of rat salivary glands for the presence of the aquaporin CHIP. Pflugers Arch 1994; 428: 455–460.
Manno CS, Pierce GF, Arruda VR, Glader B, Ragni M, Rasko JJ et al. Successful transduction of liver in hemophilia by AAV-Factor IX and limitations imposed by the host immune response. Nat Med 2006; 12: 342–347.
Voutetakis A, Zheng C, Cotrim AP, Mineshiba F, Afione S, Roescher N et al. AAV5-mediated gene transfer to the parotid glands of non-human primates. Gene Ther 2010; 17: 50–60.
Nakamoto T, Srivastava A, Romanenko VG, Ovitt CE, Perez-Cornejo P, Arreola J et al. Functional and molecular characterization of the fluid secretion mechanism in human parotid acinar cells. Am J Physiol Regul Integr Comp Physiol 2007; 292: R2380–R2390.
Catalan MA, Nakamoto T, Melvin JE . The salivary gland fluid secretion mechanism. J Med Invest 2009; 56 (Suppl): 192–196.
Voutetakis A, Zheng C, Wang J, Goldsmith CM, Afione S, Chiorini JA et al. Gender differences in serotype 2 adeno-associated virus biodistribution after administration to rodent salivary glands. Hum Gene Ther 2007; 18: 1109–1118.
Kok MR, Voutetakis A, Yamano S, Wang J, Cotrim A, Katano H et al. Immune responses following salivary gland administration of recombinant adeno-associated virus serotype 2 vectors. J Gene Med 2005; 7: 432–441.
Li J, Zheng C, Zhang X, Liu X, Zhang C, Goldsmith CM et al. Developing a convenient large animal model for gene transfer to salivary glands in vivo. J Gene Med 2004; 6: 55–63.
Yan JX, Wait R, Berkelman T, Harry RA, Westbrook JA, Wheeler CH, Dunn MJ . A modified silver staining protocol for visualization of proteins compatible with matrix-assisted laser desorption/ionization and electrospray ionization-mass spectrometry. Electrophoresis 2000; 21: 3666–3672.
Katano H, Kok MR, Cotrim AP, Yamano S, Schmidt M, Afione S et al. Enhanced transduction of mouse salivary glands with AAV5-based vectors. Gene Ther 2006; 13: 594–601.
Acknowledgements
This work was supported by grants from the National Basic Research Program of China (2007CB947304 and 2010CB944801), the Funding Project for Academic Human Resources Development in Institutions of Higher Learning under the jurisdiction of Beijing Municipality (PHR20090510, 2006 ID 0301200094 and KM200510025002), National Excellent PhD Theses Award grant from Ministry of Education, China (200778) and the Intramural Research Program of the National Institute of Dental and Craniofacial Research.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on Gene Therapy website
Rights and permissions
About this article
Cite this article
Gao, R., Yan, X., Zheng, C. et al. AAV2-mediated transfer of the human aquaporin-1 cDNA restores fluid secretion from irradiated miniature pig parotid glands. Gene Ther 18, 38–42 (2011). https://doi.org/10.1038/gt.2010.128
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/gt.2010.128
Keywords
This article is cited by
-
A Mathematical Model of Salivary Gland Duct Cells
Bulletin of Mathematical Biology (2022)
-
Cancer nanotechnology: current status and perspectives
Nano Convergence (2021)
-
Calcium Dynamics and Water Transport in Salivary Acinar Cells
Bulletin of Mathematical Biology (2021)
-
Delivery of human erythropoietin gene with an adeno-associated virus vector through parotid glands to treat renal anaemia in a swine model
Gene Therapy (2017)
-
Gene therapy for radioprotection
Cancer Gene Therapy (2015)