Abstract
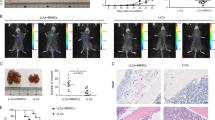
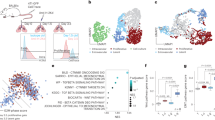
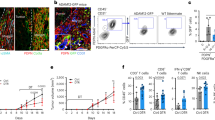
Cancer progression from microscopic dormant tumors into disseminated disease involves tumor angiogenesis, and is commonly referred to as the ‘angiogenic switch’. CD11b+Gr1+ immature myeloid cells (IMCs) were reported to promote angiogenesis and tumor progression. Here, we studied a model of tumor dormancy, in which Lewis Lung Carcinoma tumor cells were inoculated intra-abdominally into C57Bl/6J mice. Dormancy versus expansive growth was determined by the site of tumor implantation (lower vs upper abdomen). Global gene expression of IMCs was evaluated in different stages of recruitment, starting in the bone marrow, followed by the peripheral blood and finally in the vascular versus dormant tumors. We first demonstrated a ∼3 fold enrichment of IMCs within vascular tumors as compared with dormant tumors, correlating with tumor-infiltrating CD31+ endothelial cells. Although their migration from the PB into dormant tumors led to differential expression of a relatively small number of genes, recruitment of IMCs into the upper tumors was associated with a profound transcriptional response. Importantly, a large set of proangiogenic genes were significantly upregulated in IMCs derived from vascular tumors compared with those derived from dormant tumors. We therefore, suggest that proangiogenic versus nonangiogenic transcriptional patterns is associated with the ability of IMCs to promote tumor angiogenesis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 6 digital issues and online access to articles
$119.00 per year
only $19.83 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Harach HR, Franssila KO, Wasenius VM . Occult papillary carcinoma of the thyroid. a ‘normal’ finding in Finland. a systematic autopsy study. Cancer 1985; 56: 531–538.
Nielsen M, Thomsen JL, Primdahl S, Dyreborg U, Andersen JA . Breast cancer and atypia among young and middle-aged women: a study of 110 medicolegal autopsies. Br J Cancer 1987; 56: 814–819.
Aguirre-Ghiso JA . Models, mechanisms and clinical evidence for cancer dormancy. Nat Rev Cancer 2007; 7: 834–846.
Black WC, Welch HG . Advances in diagnostic imaging and overestimations of disease prevalence and the benefits of therapy. N Engl J Med 1993; 328: 1237–1243.
Gimbrone MA, Leapman SB, Cotran RS, Folkman J . Tumor dormancy in vivo by prevention of neovascularization. J Exp Med 1972; 136: 261–276.
Hanahan D, Folkman J . Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell 1996; 86: 353–364.
Baeriswyl V, Christofori G . The angiogenic switch in carcinogenesis. Semin Cancer Biol 2009; 19: 329–337.
Folkman J, Kalluri R . Cancer without disease. Nature 2004; 427: 787.
Nolan DJ, Ciarrocchi A, Mellick AS, Jaggi JS, Bambino K, Gupta S et al. Bone marrow-derived endothelial progenitor cells are a major determinant of nascent tumor neovascularization. Genes Dev 2007; 21: 1546–1558.
Orimo A, Gupta PB, Sgroi DC, Arenzana-Seisdedos F, Delaunay T, Naeem R et al. Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell 2005; 121: 335–348.
De Palma M, Venneri MA, Galli R, Sergi Sergi L, Politi LS, Sampaolesi M et al. Tie2 identifies a hematopoietic lineage of proangiogenic monocytes required for tumor vessel formation and a mesenchymal population of pericyte progenitors. Cancer Cell 2005; 8: 211–226.
Fainaru O, Almog N, Yung CW, Nakai K, Montoya-Zavala M, Abdollahi A et al. Tumor growth and angiogenesis are dependent on the presence of immature dendritic cells. FASEB J; 24: 1411–1418.
Sica A, Bronte V . Altered macrophage differentiation and immune dysfunction in tumor development. J Clin Invest 2007; 117: 1155–1166.
Yang L, DeBusk LM, Fukuda K, Fingleton B, Green-Jarvis B, Shyr Y et al. Expansion of myeloid immune suppressor Gr+CD11b+ cells in tumor-bearing host directly promotes tumor angiogenesis. Cancer Cell 2004; 6: 409–421.
Shojaei F, Wu X, Malik AK, Zhong C, Baldwin ME, Schanz S et al. Tumor refractoriness to anti-VEGF treatment is mediated by CD11b+Gr1+ myeloid cells. Nat Biotechnol 2007; 25: 911–920.
Jablonska J, Leschner S, Westphal K, Lienenklaus S, Weiss S . Neutrophils responsive to endogenous IFN-beta regulate tumor angiogenesis and growth in a mouse tumor model. J Clin Invest; 120: 1151–1164.
Bergers G, Brekken R, McMahon G, Vu TH, Itoh T, Tamaki K et al. Matrix metalloproteinase-9 triggers the angiogenic switch during carcinogenesis. Nat Cell Biol 2000; 2: 737–744.
Okazaki T, Ebihara S, Asada M, Kanda A, Sasaki H, Yamaya M . Granulocyte colony-stimulating factor promotes tumor angiogenesis via increasing circulating endothelial progenitor cells and Gr1+CD11b+ cells in cancer animal models. Int Immunol 2006; 18: 1–9.
Shojaei F, Wu X, Zhong C, Yu L, Liang XH, Yao J et al. Bv8 regulates myeloid-cell-dependent tumour angiogenesis. Nature 2007; 450: 825–831.
Nozawa H, Chiu C, Hanahan D . Infiltrating neutrophils mediate the initial angiogenic switch in a mouse model of multistage carcinogenesis. Proc Natl Acad Sci USA 2006; 103: 12493–12498.
Fainaru O, Hantisteanu S, Hallak M . Immature myeloid cells accumulate in mouse placenta and promote angiogenesis. Am J Obstet Gynecol; 204: 544, e518–e523.
Kim JA, March K, Chae HD, Johnstone B, Park SJ, Cook T et al. Muscle-derived Gr1(dim)CD11b(+) cells enhance neovascularization in an ischemic hind limb mouse model. Blood; 116: 1623–1626.
Fainaru O, Adini A, Benny O, Adini I, Short S, Bazinet L et al. Dendritic cells support angiogenesis and promote lesion growth in a murine model of endometriosis. Faseb J 2008; 22: 522–529.
Zaslavsky A, Chen C, Grillo J, Baek KH, Holmgren L, Yoon SS et al. Regional control of tumor growth. Mol Cancer Res; 8: 1198–1206.
Chen P, Huang Y, Bong R, Ding Y, Song N, Wang X et al. Tumor-associated macrophages promote angiogenesis and melanoma growth via adrenomedullin in a paracrine and autocrine manner. Clin Cancer Res; 17: 7230–7239.
Muller H, Hu J, Popp R, Schmidt MH, Muller-Decker K, Mollenhauer J et al. Deleted in malignant brain tumors 1 is present in the vascular extracellular matrix and promotes angiogenesis. Arterioscler Thromb Vasc Biol; 32: 442–448.
Vazquez-Ortiz G, Pina-Sanchez P, Vazquez K, Duenas A, Taja L, Mendoza P et al. Overexpression of cathepsin F, matrix metalloproteinases 11 and 12 in cervical cancer. BMC Cancer 2005; 5: 68.
Van Obberghen-Schilling E, Tucker RP, Saupe F, Gasser I, Cseh B, Orend G . Fibronectin and tenascin-C: accomplices in vascular morphogenesis during development and tumor growth. Int J Dev Biol; 55: 511–525.
Powell JA, Mousa SA . Neutrophil-activating protein-2- and interleukin-8-mediated angiogenesis. J Cell Biochem 2007; 102: 412–420.
Zhang B, Tsang PC, Pate JL, Moses MA . A role for cysteine-rich 61 in the angiogenic switch during the estrous cycle in cows: regulation by prostaglandin F2alpha. Biol Reprod; 85: 261–268.
Zheng PS, Wen J, Ang LC, Sheng W, Viloria-Petit A, Wang Y et al. Versican/PG-M G3 domain promotes tumor growth and angiogenesis. FASEB J 2004; 18: 754–756.
Stone OA, Richer C, Emanueli C, van Weel V, Quax PH, Katare R et al. Critical role of tissue kallikrein in vessel formation and maturation: implications for therapeutic revascularization. Arterioscler Thromb Vasc Biol 2009; 29: 657–664.
Min JK, Park H, Choi HJ, Kim Y, Pyun BJ, Agrawal V et al. The WNT antagonist Dickkopf2 promotes angiogenesis in rodent and human endothelial cells. J Clin Invest; 121: 1882–1893.
Tan JX, Wang XY, Su XL, Li HY, Shi Y, Wang L et al. Upregulation of HYAL1 expression in breast cancer promoted tumor cell proliferation, migration, invasion and angiogenesis. PLoS ONE; 6: e22836.
Kigel B, Rabinowicz N, Varshavsky A, Kessler O, Neufeld G . Plexin-A4 promotes tumor progression and tumor angiogenesis by enhancement of VEGF and bFGF signaling. Blood; 118: 4285–4296.
Barresi V, Cerasoli S, Tuccari G . Correlative evidence that tumor cell-derived caveolin-1 mediates angiogenesis in meningiomas. Neuropathology 2008; 28: 472–478.
Harris LG, Pannell LK, Singh S, Samant RS, Shevde LA . Increased vascularity and spontaneous metastasis of breast cancer by hedgehog signaling mediated upregulation of cyr61. Oncogene; 31: 3370–3380.
Spinetti G, Fortunato O, Cordella D, Portararo P, Krankel N, Katare R et al. Tissue kallikrein is essential for invasive capacity of circulating proangiogenic cells. Circ Res; 108: 284–293.
Cheriyath V, Hussein MA . Osteopontin angiogenesis and multiple myeloma. Leukemia 2005; 19: 2203–2205.
Jung SY, Song HS, Park SY, Chung SH, Kim YJ . Pyruvate promotes tumor angiogenesis through HIF-1-dependent PAI-1 expression. Int J Oncol; 38: 571–576.
Labied S, Blacher S, Carmeliet P, Noel A, Frankenne F, Foidart JM et al. Transient reduction of placental angiogenesis in PAI-1-deficient mice. Physiol Genomics; 43: 188–198.
Yang G, Addai J, Wheeler TM, Frolov A, Miles BJ, Kadmon D et al. Correlative evidence that prostate cancer cell-derived caveolin-1 mediates angiogenesis. Hum Pathol 2007; 38: 1688–1695.
Langowski JL, Zhang X, Wu L, Mattson JD, Chen T, Smith K et al. IL-23 promotes tumour incidence and growth. Nature 2006; 442: 461–465.
Liu YH, Lin CY, Lin WC, Tang SW, Lai MK, Lin JY . Up-regulation of vascular endothelial growth factor-D expression in clear cell renal cell carcinoma by CD74: a critical role in cancer cell tumorigenesis. J Immunol 2008; 181: 6584–6594.
Kubota Y, Hirashima M, Kishi K, Stewart CL, Suda T . Leukemia inhibitory factor regulates microvessel density by modulating oxygen-dependent VEGF expression in mice. J Clin Invest 2008; 118: 2393–2403.
L’Esperance S, Popa I, Bachvarova M, Plante M, Patten N, Wu L et al. Gene expression profiling of paired ovarian tumors obtained prior to and following adjuvant chemotherapy: molecular signatures of chemoresistant tumors. Int J Oncol 2006; 29: 5–24.
Seyama K, Nukiwa T, Takahashi K, Takahashi H, Kira S . Amylase mRNA transcripts in normal tissues and neoplasms: the implication of different expressions of amylase isogenes. J Cancer Res Clin Oncol 1994; 120: 213–220.
Acknowledgements
We give special thanks to Professor Yoram Groner for his generous support and helpful discussions. This study was supported in part by research funding from Israel Science Foundation 142/09 (OF).
Author contributions: NP designed research, acquired the data, collected data, analyzed and interpreted data, performed statistical analysis, and wrote the manuscript; SH designed the research, acquired the data and edited the manuscript; OV performed microarray data analysis; MH designed the research and edited the manuscript; OF designed research, acquired the data, analyzed and interpreted data, and wrote the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on Genes and Immunity website
Supplementary information
Rights and permissions
About this article
Cite this article
Pencovich, N., Hantisteanu, S., Wurtzel, O. et al. Unique expression patterns associated with preferential recruitment of immature myeloid cells into angiogenic versus dormant tumors. Genes Immun 14, 90–98 (2013). https://doi.org/10.1038/gene.2012.59
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/gene.2012.59